ABSTRACT
There is currently a lack of sufficient data on human papillomavirus (HPV)-attributable cervical carcinoma in China. Accordingly, we aimed to determine the prevalence and genotype distribution of HPV among women with cervical lesions in Shenzhen, in order to evaluate the potential benefit of HPV vaccination programs and inform cervical cancer control policies. We enrolled 5,255 patients who were admitted to the University of Chinese Academy of Sciences Shenzhen Hospital from January 2017 to December 2019. The HPV prevalence and genotype distribution were analyzed using the 21 HPV GenoArray diagnostic assay. A total of 937/5,255 patients showed HPV-positivity (prevalence rate 17.83%), of whom 85.81% (804/937) had high-risk HPV infection. HPV52 was the most prevalent genotype (4.72%, 248/5,255), followed by HPV58 (3.04%, 160/5,255), and HPV16 (2.72%, 143/5,255). The HPV prevalence rates among women with a normal cervix, low-grade intraepithelial lesions, high-grade intraepithelial lesions, invasive cervical cancer, and other characteristics were 15.63% (50/320), 58.65% (61/104), 80.00% (44/55), 88.57% (31/35), and 15.84% (751/4,741), respectively. HPV16, HPV18, and HPV52 accounted for the majority of cervical lesions, and the infection rates of HPV16 and HPV18 gradually increased with intraepithelial lesion progression (both P < .001). Our study found that HPV16, HPV52, and HPV18 played important roles in the occurrence and development of cervical lesions. This finding has the potential to guide the formulation of HPV screening and vaccination programs and preventive strategies for HPV-attributable cancer in this region.
Introduction
Cervical cancer (CC) was the second most frequently occurring cancer among women in 2018, worldwide, with an estimated incidence of 570,000 cases, and the World Health Organization (WHO) reported a mortality value of 311,000 deaths, with China and India together contributing to more than a third of all such cases.Citation1 CC and its precursors are commonly caused by persistent human papillomavirus (HPV) infection.Citation2,Citation3 HPV is a small double-stranded DNA virus, which belongs to Papillomaviridae, a large family with a tropism for squamous epithelium. Till date, more than 200 HPV genotypes have been identified, and these have been clinically divided into high-risk and low-risk genotypes based on the degree of tissue infection.Citation4 HPV16 and HPV18, the most commonly noted high-risk genotypes, were previously found to be responsible for almost three quarters of all CC cases, worldwide, with high-risk HPV genotypes HPV31, HPV45, and HPV52 accounting for the remaining cases.Citation2,Citation3
HPV vaccines are effective against HPV16, HPV18, and some other high-risk genotypes, and are the optimal prevention strategies for the control of the prevalence of CC and other HPV-associated diseases in women.Citation5,Citation6 Currently, there are three HPV vaccines licensed against HPV infections. Bivalent HPV vaccine (2vHPV) and quadrivalent HPV vaccine (4vHPV) are the first-generation HPV vaccines, and both of them protect against HPV16 and HPV18, while 4vHPV also protects against HPV6 and HPV11. The second-generation nonviolent HPV vaccine (9vHPV), which addresses the four HPV types in 4vHPV plus five additional oncogenic types (HPV31, 33, 45, 52, and 58), was licensed by the Food and Drug Administration (FDA) of the United States in 2014 and has the potential to prevent up to 93% of cervical cancers.Citation7 However, due to multiple factors, including the lack of comprehensive CC screening programs and treatment of lesions found on screening, absence of access to HPV vaccines due to the high costs, limited healthcare services for the delivery of these vaccines, as well as the vaccine being considered low-priority, CC remains the most widespread form of cancer induced by HPV and the second most commonly observed type of malignancy affecting women.Citation8–11 Although HPV vaccines have been on the national immunization schedule in 92 countries, the overall HPV prevalence is still 12% in women, globally, with a majority of these cases occurring in low to lower-middle-income countries.Citation9
Appropriate cancer control strategies and HPV vaccination programs are formulated based on a comprehensive understanding of the HPV-attributable disease burden. However, the prevalence and genotype distribution of HPV infection vary geographically in China, and the HPV‐attributable cancer burden is still not well characterized.Citation12 Gaining an understanding of the behaviors of HPV type-specific infection can boost not only the implementation and evaluation of vaccination strategies, but also CC screening, and can provide useful data for the cost-effective evaluation of these intervention programs. We sought to perform a study (including 5,255 women) to identify the most prevalent genotypes of HPV and the correlation between HPV genotypes and cervical pathology status, as presented in Shenzhen city, with the aim of guiding the formulation of HPV screening and vaccination programs and preventive strategies for HPV‐attributable cancer.
Materials and methods
Participants
A total of 5,255 outpatients treated for gynecological diseases, aged between 17 and 60 years (median age: 35.5 years), at the University of Chinese Academy of Sciences Shenzhen Hospital from January 2017 to December 2019 were included in this study. The subjects met the following requirements: long-term permanent resident of Shenzhen city, and more than 1 year of sexual history. Exclusion criteria were pregnancy, previous surgical procedures on cervix, systemic infection or autoimmune diseases, sexual activity, or receive surgery for uterine diseases within 3 days. This study was conducted in accordance with the Declaration of Helsinki and a protocol approved by the University of Chinese Academy of Sciences, Shenzhen Hospital (Guangming).
Histopathological diagnosis
All patients first underwent routine cervical exams. Patients with cervical lesions, or HPV infection or other morphologic abnormalities by cytological screening (ThinPrep cytological test, TCT), underwent histological (biopsy) and histopathological procedures for final diagnosis. The biopsy specimens obtained from these patients were fixed, embedded, and stained. Pathological identification was performed according to the 2014 WHO classification criteria (fourth Edition) and described as follows: a) Normal cervix: negative for malignancy, intraepithelial lesions, inflammation, and other benign lesions, b) Low-grade squamous intraepithelial lesion (LSIL) corresponding to cervical intraepithelial neoplasia grade (CIN) I (low dysplasia), c) High-grade squamous intraepithelial lesion (HSIL) corresponding to CIN II (moderate dysplasia) and CIN III (severe dysplasia), d) invasive cervical cancer (ICC), and e) Others, pertaining to the presence of inflammatory conditions, including cervical hypertrophy, hyperplasia, chronic cervicitis, bleeding, and erosion, or the use of surgery or hysteromyoma treatment, etc.
HPV genotype testing
HPV genotyping detection was performed using 21 HPV GenoArray Diagnostic Kit (Guangdong Kaipu Biotechnology Co., Ltd, China). The 21 HPV GenoArray Diagnostic Kit is a qualitative polymerase chain reaction (PCR)-based test used for the detection and determination of 21 specific HPV DNAs in cervical specimens. The 21 HPV genotypes are divided into 15 high-risk genotypes (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66 and 68) and 6 low-risk genotypes (HPV 6,11,42,43,44, CP8304), and the specific detection steps were followed in strict adherence to instructions, as described previously.Citation13,Citation14 Briefly, the HPV DNA extracted from cervical samples was amplified using PCR, and then hybridized with specific HPV probes located inside the membrane strip, followed by the use of the enzyme immunoassay method to obtain colorimetric results. Positive results appeared as a clearly visible violet dot, and HPV genotypes were determined by the dot distribution of the membrane strip.
Statistical analysis
Analyses were performed using SPSS 25.0 statistical software (Chicago, USA). The infection rates were expressed as percentage and compared based on the disease group using a Chi-square test. An independent sample F/t test was used for comparisons among groups to evaluate the changes in the HPV prevalence; statistical significance was defined as p < .05.
Results
HPV prevalence and genotypes
A total of 5,255 female patients were enrolled in this study, and the overall prevalence of HPV infection was 17.83% (937/5255). Of the 937 patients, 85.81% (804/937) were identified as having the high-risk HPV genotypes. HPV52 was the most commonly observed genotype (4.72%, 248/5255), followed by HPV58 (3.04%, 160/5255), HPV16 (2.72%, 143/5255), HPV51 (1.67%, 88/5255), and HPV53 (1.50%, 79/5255) ().
Table 1. Distribution of human papillomavirus genotypes according to the final diagnoses among the 5,255 patients
Twenty-one HPV genotypes were detected a total of 1,253 times in 937 HPV-positive patients. Of all the infections, single genotype infection was the most commonly observed, with a prevalence rate of 74.28% (696/937). The single low-risk HPV and high-risk HPV infection rates were 12.17% (114/937) and 62.11% (582/937), respectively, while the prevalence of multiple infections (≥2 genotypes) was 25.72% (241/937) and that of double infection was 19.21% (162/937) ().
Table 2. Single and multiple infection status in patients with HPV infection
HPV genotypes in relation to the severity of cervical lesions
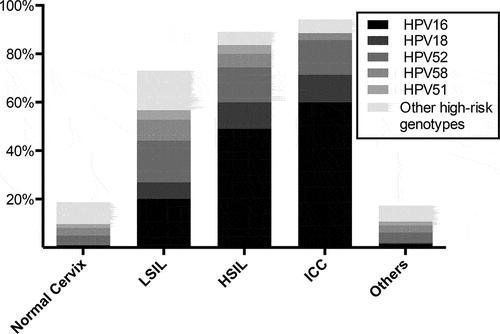
Of the 5,255 patients, 6.09% (320/5,255) had a normal cervix, 1.98% (104/5,255) had LSIL, 1.05% (55/5,255) had HSIL, 0.67% (35/5,255) had ICC. Moreover, 90.22% (4741/5,255) had other characteristics typically involved chronic cervicitis, cervical erosion, cervical hypertrophy, bleeding, nabothian cysts, cervical polyp, cervical hypertrophy, and erosion (Table S1). Our results showed HPV infection prevalence rates of 15.63% (50/320), 58.65% (61/104), 80.00% (44/55), 88.57% (31/35), and 15.84% (751/4,741), respectively, among those with a normal cervix, LSIL, HSIL, ICC, and others (). The most commonly observed HPV genotypes in patients with a normal cervix (normal cervix group) were HPV52 (4.06%, 13/320), HPV58 (3.11%, 10/320), and HPV33 (2.19%, 7/320). However, the most prevalent HPV genotypes were HPV16 (20.19%, 21/104), and HPV52 (17.34%, 18/104) in patients with LSIL, and HPV16 (49.09%, 27/55), HPV 52 (14.55%, 8/55) and HPV18 (10.91%, 6/55) in those with HSIL. In the 35 patients with ICC, HPV16, HPV52, and HPV18 were also the most prevalent HPV genotypes, with prevalence rates of 60.00% (21/35), 14.59% (5/35), and 11.43% (4/35), respectively.
Moreover, although HPV52 and HPV58 were the most prevalent subtypes in the 5,255 cases, their prevalence rates decreased with the severity of intraepithelial lesions, from 17.31% to 8.65% in patients with LSIL to 14.29% and 2.86% in those with ICC, respectively (). However, HPV16, HPV18, and HPV52 accounted for the majority of cases with severe cervical lesions (74.55% in HSIL patients and 85.71% in ICC patients) (). The majority of HSIL cases were attributed to HPV16 (49.09%), followed by HPV52 (14.55%) and HPV18 (10.91%). HPV16 also accounted for most of the ICC cases, with a prevalence rate of 60.00%, followed by HPV52 (14.29%) and HPV18 (11.43%). Interestingly, with intraepithelial lesion progression, the infection rates of HPV16 and HPV18 gradually increased (both p < .001).
HPV infection in different age groups
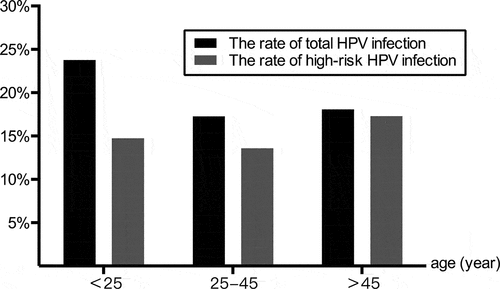
The total rate of HPV infection in women aged <25 years was 23.77% (87/366), which was significantly higher than that observed in the age of 25–45 years group (714/4,137, 17.26%) and >45 years group (136/752, 18.08%) (χ2 = 9.938, p = .007). However, as shown in , the rates of high-risk HPV infection in women aged <25 years, 25–45 years, and >45 years were 14.75% (54/366), 13.58% (562/4137), and 17.29% (130/752), respectively, and the difference between the three groups was statistically significant (χ2 = 7.262, p = .026).
Discussion
In the present study, we reported on the correlation between HPV genotypes and cervical pathology status among women with cervical lesions presenting in Shenzhen city and found that the overall prevalence of HPV infection was 17.83%, with 85.81% of these cases showing the high-risk genotypes. HPV52, HPV16, and HPV58 were the three subtypes with the highest prevalence, whereas HPV16 was the most commonly observed carcinogenic HPV subtype in patients with intraepithelial lesions, followed by HPV52 and HPV18. Moreover, HPV16 and HPV18 were associated with premalignant lesion severity in the cervix. This study would demonstrate the understanding of the prevalence and distribution of the HPV genotypes in the female population with cervical lesions presenting in Shenzhen, China, and will assist in developing the formulation of preventive strategies for cervical carcinoma and HPV vaccination programs.
Persistent high-risk HPV infection is considered a driving factor for the progression of precancerous lesions to ICC. Early detection, prevention, and treatment are key to reducing the associated morbidity and mortality. In our study, the overall HPV prevalence rate was 17.83% (937/5,255) in Shenzhen city. However, HPV infection rates are known to vary across different regions in China (7.3% in Guangdong,Citation15 9.9% in Beijing,Citation16 10.3% in Liaoning,Citation17 18.7% in Shaanxi,Citation18 and 24.7% in XinjiangCitation19), and across different countries (8.0% to 27.3%).Citation9,Citation20-22 Demographic factors such as ethnicity, socioeconomic status, health conditions, and education level may contribute to the uneven distribution of HPV incidence.Citation23 A total of 21 HPV genotypes including 15 high-risk types and six low-risk types were detected in this study. The most prevalent HPV subtypes were HPV52, HPV58, and HPV16, which supported the viewpoint that HPV52 and HPV58 are highly prevalent among individuals in eastern Asia.Citation24 Similar to our results, another study also found that HPV-52 was the most prevalent HPV genotype in women in Guangdong province.Citation25 However, our results are inconsistent with those of a worldwide retrospective cross-sectional study, in which the top eight HPV genotypes related to ICC were HPV16, 18, 31, 33, 35, 45, 52 and 58 in America, Europe, and Africa.Citation24 Therefore, current knowledge on the distribution of HPV subtypes in a given region remains to be further verified through studies with large sample sizes; this may have great value in the formulation of screening, treatment, prevention, and vaccine development strategies for HPV-attributable diseases.
Previous studies have demonstrated that the pathogenic ability of HPV differs across its genotypes and that HPV16 and HPV18 are the two most commonly observed genotypes associated with intraepithelial lesion severity.Citation26,Citation27 For better preparedness against future precancerous lesions and ICC, virological HPV screening was recommended by the International Agency for Research on Cancer in 2017.Citation28 In addition, according to the American Congress of Obstetricians and Gynecologists and American Society for Colposcopy and Cervical Pathology guidelines, patients with HPV16 and HPV18 infection should be examined by colposcopy even if the cytology is negative for malignancy.Citation29 In the present study, the prevalence rates of HPV16, HPV18, and HPV52 infections in cases with a normal cervix were significantly lower than those observed in cases with intraepithelial lesions, consistent with several previous studies from China and western countries.Citation3,Citation26,Citation27,Citation30 As the most commonly occurring genotype among all grades of cervical lesions,Citation24,Citation27,Citation31 the prevalence of HPV16 significantly increased with intraepithelial lesion severity, from 20.19% in LSIL patients to 60.00% in patients with ICC. This suggests that HPV16 is the subtype with the strongest carcinogenic potential in Shenzhen city and that its prevalence increases with intraepithelial lesion severity. Comparatively, although HPV18 was not the most prevalent genotype, it was the third most commonly observed genotype in patients with cervical intraepithelial changes following HPV16 and HPV52. Previous reports have indicated that HPV16 is highly correlated to both adenocarcinoma and squamous-cell carcinoma, whereas HPV18 is predominantly a risk factor for the development of adenocarcinoma.Citation32 Further investigation is required to confirm the relationship between the types of CC and HPV genotypes. Inconsistent with the results observed in western countries,Citation20,Citation24 we demonstrated that HPV52 occurred commonly in Chinese women with or without cervical lesions.Citation12,Citation25,Citation26 However, no significant differences in the rates of HPV52 infection between cases with LSIL, HSIL, and ICC were found. This result illustrates that, while HPV52 may be associated with cervical intraepithelial lesions, its contribution to the carcinogenesis from LSIL to ICC is small.
Biological and epidemiological investigations have shown that population type and age may impact the rate of HPV infection and genotype distribution.Citation9,Citation15,Citation24 Young, sexually active women show the highest rates of HPV infection, and HPV vaccination provides the best preventive effect in women who are not yet sexually active.Citation28 According to the present study, the rate of HPV infection was the highest among women aged between 25 and 45 years, with the highest rate noted among those aged <25 years; this may be related to the small number of people included in the <25 years old age group. In addition, the rapid development of the economy, changes in sexual concept, and participation in unsafe sexual behaviors may have resulted in younger age at first sexual activity and high levels of sexual activity among women in Shenzhen city, increasing the risk of infection.Citation33,Citation34 Consistent with previous studies,Citation27,Citation35 the rate of high-risk HPV infection in women aged >45 years was statistically significant higher than that among those aged <25 years and 25–45 years. A previous study showed that the incidence of HPV infection has steadily increased with age in China, and the incidence of CC peaked in the 45–54 years group.Citation36 Nonetheless, early detection and active prevention are still important principles in CC prevention and treatment.
The HPV vaccine is a prophylactic vaccine that prevents HPV infection, and the WHO recommends its administration to girls aged 9 to 14 years as the most cost-effective public health measure against cervical cancer and other HPV-associated diseases.Citation28 As of 2019, the HPV vaccine has been on the national immunization schedules in 92 countries, globally, showing outstanding results in some countries.Citation5,Citation6 Since 2008, the rate of immunization using 2vHPV vaccines for girls aged 12 to 13 years has exceeded 90% in Scotland, and the prevalence of HPV infection has decreased by 89%.Citation6 The Australian federal government started providing free HPV vaccination for girls aged 12 to 13 years in 2007, and this was expanded to boys in 2013, resulting in a major decrease in the HPV infection rate, from 22.7% in 2005 to 1.1% in 2015.Citation5 In China, 2vHPV, 4vHPV, and 9vHPV vaccines were approved by the China Food and Drug Administration for marketing in July 2016, May 2017, and April 2018, respectively. However, previous studies showed that the coverage rates of HPV vaccines among young women in China were still low,Citation37–40 and the main reasons for this may be related to confusion about the choice of vaccine type, optimal age for vaccination, insufficient vaccine supply, as well as vaccination procedures and cost. In addition, women’s levels of awareness and attitudes toward the etiology of cervical lesions, and HPV and its vaccines greatly influence the rate of vaccination and participation in screening.Citation40,Citation41 Our study showed that HPV16, HPV52, and HPV18 played important roles in the occurrence and development of intraepithelial lesions in Shenzhen city; infection with all these subtypes can only be prevented with the use of the 9vHPV vaccine. However, insufficient vaccine supply and the high cost seriously limit the promotion and application of the 9vHPV vaccine. Clarifying the infection rates and epidemiological patterns of HPV is of great significance for the prevention and control of HPV-attributable cancer, and useful for the design of vaccination policies in order to improve vaccine coverage.
There are several limitations in this study, one of which is the absence of behavioral information. Previous studies showed that behavioral information, including smoking, sexual activity, and dietary patterns, plays key roles in the development of HPV infection and cervical cancer.Citation27,Citation42-44 In our further research, a study about the correlation between behavioral information and cervical cancer will be performed. Moreover, in consideration of the selected women mostly with gynecological diseases, our findings cannot be generalized to the entire female population in Shenzhen, which may limit the use of the current data for epidemiological purposes. Further large sample population-based surveys based on the entire female population are in demand.
In conclusion, our study yielded extensive results on the prevalence and distribution of the HPV genotypes in the female population of Shenzhen city, China, and found that HPV16, HPV52, and HPV18 played important roles in the occurrence and development of intraepithelial lesions. Our results may offer guidance for the establishment of HPV vaccination programs and preventive strategies for cervical carcinoma in this region.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (58.9 KB)Acknowledgments
The authors would like to thank all pathologists of the University of Chinese Academy of Sciences Shenzhen Hospital for help on the diagnosis of cervical intraepithelial lesions and cervical carcinoma.
Supplementary material
Supplemental data for this article can be accessed online at http://dx.doi.org/10.1080/21645515.2020.1805993.
Additional information
Funding
References
- Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, Bray F. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health. 2020;8:e191–e203. doi:10.1016/S2214-109X(19)30482-6.
- Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88:63–73. doi:10.1038/sj.bjc.6600688.
- Ma L, Cong X, Shi M, Wang XH, Liu HY, Bian ML. Distribution of human papillomavirus genotypes in cervical lesions. Exp Ther Med. 2017;13:535–41. doi:10.3892/etm.2016.4000.
- Soto D, Song C, McLaughlin-Drubin ME. Epigenetic alterations in human papillomavirus-associated cancers. Viruses. 2017;9. doi:10.3390/v9090248.
- Patel C, Brotherton JM, Pillsbury A, Jayasinghe S, Donovan B, Macartney K, Marshall H. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonavalent vaccine prevent? Euro Surveill. 2018;23. doi:10.2807/1560-7917.ES.2018.23.41.1700737.
- Kavanagh K, Pollock KG, Cuschieri K, Palmer T, Cameron RL, Watt C, Bhatia R, Moore C, Cubie H, Cruickshank M, Robertson C.Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis. 2017;17:1293–302. doi:10.1016/S1473-3099(17)30468-1.
- Brotherton JML, Tabrizi SN, Phillips S, Pyman J, Cornall AM, Lambie N, Anderson L, Cummings M, Payton D, Scurry JP, et al. Looking beyond human papillomavirus (HPV) genotype 16 and 18: defining HPV genotype distribution in cervical cancers in Australia prior to vaccination. Int J Cancer. 2017;141(8):1576–84. doi:10.1002/ijc.30871.
- Siu JY-M, Fung TKF, Leung LH-M. Social and cultural construction processes involved in HPV vaccine hesitancy among Chinese women: a qualitative study. Int J Equity Health. 2019;18. doi:10.1186/s12939-019-1052-9.
- Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–99. doi:10.1086/657321.
- Yin Y. HPV vaccination in China needs to be more cost-effective. The Lancet. 2017;390:1735–36. doi:10.1016/S0140-6736(17)32606-5.
- Ortiz RR, Smith A, Coyne-Beasley T. A systematic literature review to examine the potential for social media to impact HPV vaccine uptake and awareness, knowledge, and attitudes about HPV and HPV vaccination. Hum Vaccin Immunother. 2019;15:1465–75. doi:10.1080/21645515.2019.1581543.
- Liu SS, Chan KY, Leung RC, Chan KK, Tam KF, Luk MH, Lo SST, Fong DYT, Cheung ANY, Lin ZQ, et al. Prevalence and risk factors of human papillomavirus (HPV) infection in southern Chinese women - a population-based study. PLoS One. 2011;6:e19244. doi:10.1371/journal.pone.0019244.
- Lin M, Yang LY, Li LJ, Wu JR, Peng YP, Luo ZY. Genital human papillomavirus screening by gene chip in Chinese women of Guangdong province. Aust N Z J Obstet Gynaecol. 2008;48:189–94. doi:10.1111/j.1479-828X.2008.00844.x.
- Wang X, Ji Y, Li J, Dong H, Zhu B, Zhou Y, Wang J, Zhou X, Wang Y, Peppelenbosch MP, et al. Prevalence of human papillomavirus infection in women in the autonomous region of Inner Mongolia: A population-based study of a Chinese ethnic minority. J Med Virol. 2018;90:148–56. doi:10.1002/jmv.24888.
- Jing L, Zhong X, Zhong Z, Huang W, Liu Y, Yang G, Zhang X, Zou J, Jing C, Wei X, et al. Prevalence of human papillomavirus infection in Guangdong Province, China: a population-based survey of 78,355 women. Sex Transm Dis. 2014;41:732–38. doi:10.1097/OLQ.0000000000000201.
- Li C, Wu M, Wang J, Zhang S, Zhu L, Pan J, Zhang W. A population-based study on the risks of cervical lesion and human papillomavirus infection among women in Beijing, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 2010;19:2655–64. doi:10.1158/1055-9965.EPI-10-0212.
- Xue H, Lin X, Li T, Yan X, Guo K, Zhang Y. Prevalence and genotype distribution of human papillomavirus infection in asymptomatic women in Liaoning province, China. J Med Virol. 2015;87:1248–53. doi:10.1002/jmv.24029.
- Li J, Wang YY, Tian XF, Nan X, Yan T, Wang P, Fu YL, Wang GQ. HPV genotype analysis for women in Shaanxi Province of China. Genet Mol Res. 2016;15. doi:10.4238/gmr15047178.
- Du R, Chen ZF, Li XH, Ding Y, Zhang Y. Human papillomavirus infection among Uyghur women with cervical intraepithelial neoplasia in Xinjiang area. Eur J Gynaecol Oncol. 2015;36:564–68.
- Wheeler CM, Hunt WC, Cuzick J, Langsfeld E, Pearse A, Montoya GD, Robertson M, Shearman CA, Castle PE. A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int J Cancer. 2013;132:198–207. doi:10.1002/ijc.27608.
- Murillo R, Herrero R, Sierra MS, Forman D. Cervical cancer in central and South America: burden of disease and status of disease control. Cancer Epidemiol. 2016;44(Suppl 1):S121–S30. doi:10.1016/j.canep.2016.07.015.
- Quek SC, Lim BK, Domingo E, Soon R, Park J-S, Vu TN, Tay EH, Le QT, Kim Y-T, Vu BQ. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical intraepithelial neoplasia across 5 countries in Asia. Int J Gynecological Cancer. 2013;23:148–56. doi:10.1097/IGC.0b013e31827670fd.
- Duan R, Qiao Y, Clifford G, Zhao F. Cancer burden attributable to human papillomavirus infection by sex, cancer site, age, and geographical area in China. Cancer Med. 2019;9:374–84. doi:10.1002/cam4.2697.
- Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927–35. doi:10.1002/ijc.25396.
- Chen Q, Luo ZY, Lin M, Lin QL, Chen CY, Yang C, Xie L-X, Li H, Zheng J-K, Yang L-Y, et al. Prevalence and genotype distribution of human papillomavirus infections in women attending hospitals in Chaozhou of Guangdong province. Asian Pac J Cancer Prev. 2012;13:1519–24. doi:10.7314/APJCP.2012.13.4.1519.
- Long W, Yang Z, Li X, Chen M, Liu J, Zhang Y, Sun X. HPV-16, HPV-58, and HPV-33 are the most carcinogenic HPV genotypes in Southwestern China and their viral loads are associated with severity of premalignant lesions in the cervix. Virol J. 2018;15:e94. doi:10.1186/s12985-018-1003-x.
- Wolday D, Derese M, Gebressellassie S, Tsegaye B, Ergete W, Gebrehiwot Y, Caplan O, Wolf DG, Maayan S. HPV genotype distribution among women with normal and abnormal cervical cytology presenting in a tertiary gynecology referral Clinic in Ethiopia. Infect Agent Cancer. 2018;13(1):28. doi:10.1186/s13027-018-0201-x.
- World Health Organization. Electronic address swi. Human papillomavirus vaccines: WHO position paper, May 2017-Recommendations. Vaccine. 2017;35:5753–55. doi:10.1016/j.vaccine.2017.05.069.
- Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FAR, Moriarty AT, Waxman AG, Wilbur DC. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–72. doi:10.3322/caac.21139.
- Clifford GM, Gallus S, Herrero R, Munoz N, Snijders PJ, Vaccarella S, Anh P, Ferreccio C, Hieu NT, Matos E, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366(9490):991–98. doi:10.1016/S0140-6736(05)67069-9.
- Zhang L, Bi Q, Deng H, Xu J, Chen J, Zhang M, Mu X. Human papillomavirus infections among women with cervical lesions and cervical cancer in Eastern China: genotype-specific prevalence and attribution. BMC Infect Dis. 2017;17(1):107. doi:10.1186/s12879-017-2223-1.
- Bulk S, Berkhof J, Bulkmans NW, Zielinski GD, Rozendaal L, van Kemenade FJ, Snijders PJF, Meijer CJLM. Preferential risk of HPV16 for squamous cell carcinoma and of HPV18 for adenocarcinoma of the cervix compared to women with normal cytology in The Netherlands. Br J Cancer. 2006;94(1):171–75. doi:10.1038/sj.bjc.6602915.
- Yan Y, Yan M, Luan RS, Zhang XX, Xu SK. Study on the sexual behavior and its influencing factors among college students in Shenzhen, Guangdong. Chin J Sch Health. 2016;37:1784–86.
- Wagner M, Bennetts L, Patel H, Welner S, de Sanjose S, Weiss TW. Global availability of data on HPV genotype-distribution in cervical, vulvar and vaginal disease and genotype-specific prevalence and incidence of HPV infection in females. Infect Agent Cancer. 2015;10:13. doi:10.1186/s13027-015-0008-y.
- Wang S, Wei H, Wang N, Zhang S, Zhang Y, Ruan Q, Jiang W, Xiao Q, Luan X, Qian X. The prevalence and role of human papillomavirus genotypes in primary cervical screening in the northeast of China. BMC Cancer. 2012;12(1):160. doi:10.1186/1471-2407-12-160.
- Song B, Ding C, Chen W, Sun H, Zhang M, Chen W. Incidence and mortality of cervical cancer in China, 2013. Chinese J Cancer Res. 2017;29:471–76. doi:10.21147/j..1000-9604.2017.06.01.
- Santhanes D, Yong CP, Yap YY, Saw PS, Chaiyakunapruk N, Khan TM. Factors influencing intention to obtain the HPV vaccine in South East Asian and Western Pacific regions: A systematic review and meta-analysis. Sci Rep. 2018;8:3640. doi:10.1038/s41598-018-21912-x.
- Zhang SK, Pan XF, Wang SM, Yang CX, Gao XH, Wang ZZ, Li M, Ren Z-F, Zheng -Q-Q, Ma W, et al. Knowledge of human papillomavirus vaccination and related factors among parents of young adolescents: a nationwide survey in China. Ann Epidemiol. 2015;25:231–35. doi:10.1016/j.annepidem.2014.12.009.
- Wang Z, Wang J, Fang Y, Gross DL, Wong MCS, Wong ELY, Lau JTF. Parental acceptability of HPV vaccination for boys and girls aged 9-13years in China - A population-based study. Vaccine. 2018;36:2657–65. doi:10.1016/j.vaccine.2018.03.057.
- Lin W, Wang Y, Liu Z, Chen B, Yuan S, Wu B, Gong L. Inequalities in awareness and attitude towards HPV and its vaccine between local and migrant residents who participated in cervical cancer screening in Shenzhen, China. Cancer Res Treat. 2020;52:207–17. doi:10.4143/crt.2019.053.
- Lim JN, Ojo AA. Barriers to utilisation of cervical cancer screening in Sub Sahara Africa: a systematic review. Eur J Cancer Care (Engl). 2017;26. doi:10.1111/ecc.12444.
- Wang X, Tian X, Liu F, Zhao Y, Sun M, Chen D, Lu C, Wang Z, Shi X, Zhang Q. Detection of HPV DNA in esophageal cancer specimens from different regions and ethnic groups: a descriptive study. BMC Cancer. 2010;10(1):19. doi:10.1186/1471-2407-10-19.
- Trottier H, Ferreira S, Thomann P, Costa MC, Sobrinho JS, Prado JC, Rohan TE, Villa LL, Franco EL. et al. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res. 2010;70:8569–77. doi:10.1158/0008-5472.CAN-10-0621.
- Barchitta M, Maugeri A, Quattrocchi A, Agrifoglio O, Scalisi A, Agodi A. The association of dietary patterns with high-risk human papillomavirus infection and cervical Cancer: A cross-sectional study in Italy. Nutrients. 2018;10. doi:10.3390/nu10040469.