ABSTRACT
The long-term persistence of hepatitis B surface antibody (anti-HBs) after hepatitis B vaccination among adults harboring isolated hepatitis B core antibody (anti-HBc) is not yet clarified. The present study aimed to assess the immunogenicity and persistence of antibodies in adults 8 years after vaccination. A total of 309 participants including 94 participants in the isolated anti-HBs group and 215 in the control group were recruited in this study. All subjects received three doses of hepatitis B vaccine (20 μg) at 0, 1, and 12 months, followed by testing for serological responses 1 month after the third vaccination. Subsequently, 154 participants were excluded because their anti-HBs data of 8 y after the first vaccination were missing. The prevalence of isolated anti-HBc was about 11.5%, the positive seroprotection rate was 72%, and the geometric mean titer (GMT) value of anti-HBs titer was 24.55 mIU/mL in the isolated anti-HBc group 8 y after three doses of vaccination. No significant difference was detected in the positive seroprotection rate (P = .434) and the GMT values of anti-HBs titers (P = .674) between the isolated anti-HBc and control groups after 8 y. In conclusion, isolated anti-HBc-positive subjects could achieve satisfactory long-term immune effects after hepatitis B vaccination. The GMT values of anti-HBs titers were lower than those of the control group at 1 month, but no significant difference was detected after 8 years.
Introduction
Hepatitis B infection is a major public health concern worldwide. According to the World Health Organization (WHO) report, about 2 billion individuals were infected with hepatitis B virus, 257 million had chronic hepatitis B infection, and viral hepatitis caused 1.34 million deaths in 2015. The number of deaths due to viral hepatitis is continually increasing.Citation1,Citation2 China has the world’s largest burden of HBV infection. At present, estimations suggest that in China there are 86 million HBV carriers, including 32 million chronic Hepatitis B patients.Citation3 Hepatitis B vaccination is the most economical and effective measure to prevent and control hepatitis B infection. Hepatitis B vaccination of newborns was integrated into the national immunization program of China in 1992, and newborns were encouraged to be vaccinated at their own expense. In 2002, all newborn vaccinations in China were free of charge. At present, China’s newborn hepatitis B vaccine full coverage rate had reached 99.58%.Citation4 It reduces the prevalence of infection to 1.3% in children.Citation2
However, it was difficult to reduce the incidence of hepatitis B in adults. More social activities and some special occupational exposure made the risk of hepatitis B infection in adults increased. Our previous study showed that the prevalence of hepatitis B surface antigen (HBsAg) among participants aged 15–59 y was 8.12%, which confirms that hepatitis B vaccination is essential to prevent HBV infection in adults.Citation5 For hepatitis B vaccine was self-financed and voluntary in adults, the current coverage rate was only 14%.Citation6 Although several studies have focused on hepatitis B vaccination in adults, only a few have investigated the effectiveness in the subset of individuals with isolated hepatitis B core antibody (anti-HBc).Citation7–9
Isolated anti-HBc is defined by the presence of anti-HBc in the absence of HBsAg and hepatitis B surface antibody (anti-HBs). The positive result for anti-HBc alone may be due to a nonspecific or cross-reaction with other agents or the window period after a recent HBV infection.Citation10 It can also be detected in patients with occult HBV infection, especially immunocompromised patients.Citation11 The prevalence of isolated anti-HBc varied from 1% to 11.9% in normal individuals;Citation12–14 whether an isolated anti-HBc individual requires vaccination is yet controversial. Some studies suggested that vaccination is used for the prevention of HBV reactivation.Citation15,Citation16 One study pointed out that hepatitis B vaccination may be used as a diagnostic tool for clarifying the situation of the subjects with isolated anti-HBc.Citation17
Anti-HBc was not a protective antibody and there was a high percentage of false positive in anti-HBC test;Citation18 hepatitis B vaccine was still recommended for high-risk groups with single positive anti-HBC. Our previous study suggested that isolated anti-HBc individuals exhibited a decreased serological response to HBV vaccination as compared to normal individuals at 1 month after vaccination.Citation19 Currently, there is no study on the long-term persistence of isolated anti-HBc antibodies in adults. Thus, the present study aimed to assess the immunogenicity and persistence of antibodies 8 years after vaccination in these isolated anti-HBc adults.
Materials and methods
Study procedures
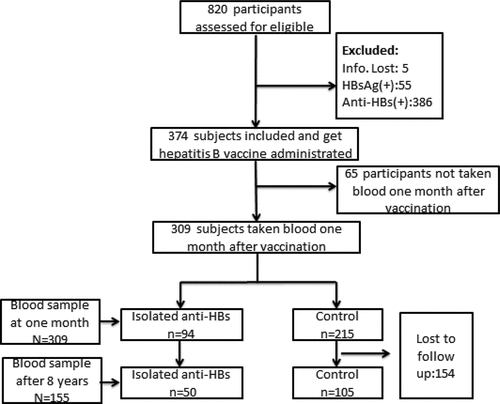
This study was carried out in Nanxun County in Zhejiang Province from May 2010 to November 2018. Participants aged 15–48 years were recruited and asked to complete a questionnaire that included information about gender, date of birth, HBV vaccination history, telephone number, and home address. All the participants were tested for HBsAg, anti-HBs, and anti-HBc. Originally, the study recruited 820 participants. Those with anti-HBs(+), HBsAg(+), or incomplete information were excluded. Next, 374 participants were vaccinated with a recombinant hepatitis B vaccine (dose 20 μg, Huabei Biotech, China). The vaccinations were carried out at 0–1–12-month vaccination schedule. Finally, 309 participants were recruited, who received the full vaccination course (three doses); also, a blood sample was withdrawn 1 month after the first course. 154/309 participants were unavailable to follow-up, while blood samples were obtained from the remaining 155 patients in November 2018, i.e., 8 y after vaccination.
The exclusion criteria were as follows: organ transplantation, renal dialysis, participants with hepatitis C and acquired immune deficiency syndrome, vaccination contraindication, and previous vaccination against HBV. All the participants provided informed consent according to the study protocol and were willing to receive the HBV vaccine. Informed consent was signed by the guardian for persons under 18 y. Institutional Review Board approval was obtained from Research Ethics Committee of Zhejiang Provincial Center for Disease Control and prevention (approved number: T-043-R).
Laboratory testing
Frozen serum samples were sent to Adicon Clinical Laboratories Inc. in Hang Zhou for the quantification of HBsAg, anti-HBs, and anti-HBc by chemiluminescence microparticle immunoassay (CMIA). An Architect-i2000SR (Abbott, USA) analyzer was used to perform the CMIA. The following signal-to-noise ratios were considered to indicate positivity: HBsAg ≥0.05 IU/mL and anti-HBc levels ≥1 S/CO. A previous study suggested that anti-HBs levels of 2–9.9 mIU/mL could not be considered negative in participants with a history of vaccination or resolved infection.Citation20 In the current study, those who had been vaccinated against HBV were excluded. Hence, the level of anti-HBs ≥10 mIU/mL was considered positive and defined as having a protective effect against HBV infection. A low response was defined as follows: 10 mIU/mL≤anti-HBs<100 mIU/mL, while a normal response was defined as follows: 100 mIU/mL≤anti-HBs<1000 mIU/mL; a high response was defined as follows: anti-HBs≥1000 mIU/mL after the third dose of vaccine.
Statistical evaluation
Statistical analyses were performed using SPSS version 19.0 (SPSS Inc., IL, USA). Continuous variables were expressed as means ± standard deviation and categorical variables as frequencies and proportions. Comparisons of the positive seroprotection rate were conducted by chi-square test method and the anti-HBs geometric mean titer (GMT) level was assessed with t-test. Age was adjusted by logistic regression or linear regression. Comparisons of the immune response proportion between the two groups were analyzed by rank-sum test. P-value <0.05 indicated a statistically significant difference.
Results
Baseline characteristics
A total of 820 participants were initially enrolled for screening, of whom, 309 were finally included. Among those screened, 511 participants were excluded because 55 were positive for HBsAg, 386 were positive for anti-HBs, blood was not withdrawn from 65 at 1 month after vaccination, and 5 participants had incomplete data. Finally, 309 participants, including 94 participants in the isolated anti-HBs group and 215 in the control group, were recruited in this study. Of these, 154 were excluded as their anti-HBs data of 8 years after the first vaccination were missing ().
The mean age of the participants was 32.87 ± 8.25 (range: 15–48) years. The mean age of the isolated anti-HBc group was 36.39 ± 7.10 years, while that of the control group was 31.33 ± 8.26 years, indicating a significant difference between the two groups (P < .001). The cohort consisted of 41 males (43.6%) in the isolated anti-HBc group and 89 males (41.4%) in the control group, showing no significant difference (P = .659).
After 8 years of follow-up, 155 participants could still be contacted (). There were 19 males (38.0%) in the isolated anti-HBc group and 35 males (33.3%) in the control group. No significant difference was noted between the isolated anti-HBc group and the control group with respect to sex in the followed cohort (P = .569). The mean age of the isolated anti-HBc group was 37.84 ± 6.32 years, while that of the control group was 32.89 ± 8.02 years. The difference was statistically significant with respect to age (P < .001). The proportion of isolated anti-HBc showed a significant age-dependent increase 5.3% (age 15–25 y), 39.4% (age 26–35 y), and 55.3% (age 36–48 y).
Table 1. Baseline information about the two groups
Immune response to the hepatitis B vaccine 1 month and 8 years later
In , the positive seroprotection rate was 91.5% at 1 month after the third vaccination and 72.0% after 8 years in the isolated anti-HBs group (P = .002). The positive seroprotection rate was 95.8% 1 month after the third vaccination and 65.7% after 8 years in the control group (P < .001). Conversely, no significant differences were detected between the two groups at 1 month after the third vaccination (P = .125) or after 8 years (P = .434). Furthermore, after adjusting for age, no significant differences were observed between the two groups at 1 month after the third vaccination (P = .223) or after 8 years (P = .294).
Table 2. Comparison of the positive seroprotection rate after 1 month and 8 years between the two groups
Significant differences were detected in the anti-HBs GMT 8 years later as compared to 1 month after the third vaccination in both isolated anti-HBsand control groups (P < .001). At 1-month after the third vaccination, the anti-HBs GMT of the subjects in the isolated anti-HBc group was 380.19 mIU/mL (95% confidence interval (CI): 223.87–660.69) as compared to 870.96 mIU/mL (95% CI: 645.65–1202.26) in the control group; the difference between the two groups was statistically significant (P = .005). However, no significant difference was detected 8 years later between the two groups 24.55 mIU/mL (95% CI: 11.22–53.70) vs. 30.20 mIU/mL (95% CI: 17.78–50.12) (P = .674) (). After adjusting for age, significant differences were detected between the two groups at 1 month after the third vaccination (P = .006), while no significant difference was noted after 8 years (P = .890).
Table 3. Comparison of the GMT of anti-HBs between the two groups after 1 month and 8 years
Comparison in the different immune responses of the two groups to hepatitis B vaccine after 8 years
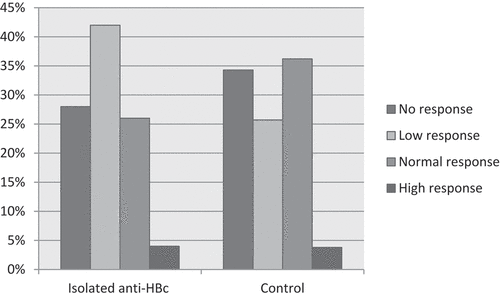
The subset of participants with different immune responses, i.e., isolated anti-HBc and control groups are shown in . The proportion of no-response, low-response, normal-response and high-response in the isolated anti-HBc group was 28.0%, 42.0%, 26.0%, and 4.0%, while in the control group was 34.3%, 25.7%, 36.2%, and 3.8%. No significant difference was detected in the proportion of immune response between the isolated anti-HBc and control groups (P = .804).
Discussion
In the current study, the prevalence of isolated anti-HBc was about 11.5%, while it was identified in 1–10% of the population in areas with low HBV prevalence, such as Europe and the USA.Citation13 In one Korean study, the prevalence in the general population was 8.9%Citation11 and about 10.8% in the other study.Citation21 The prevalence was higher in men than women and increased with age (0.7% in the <20-y-old age group; 1.9% in the 21–40-y-old age group; 7.4% in the 41–60-y-old age group; 17.1% in the 61–80-year-old age group; 24.2% in the >80-y-old age group).Citation14 A retrospective study of Korean American subjects found that the rate of isolated anti-HBc was significantly higher in males (13.00%) than females (8.94%) (P < .01). The evaluation of the proportion of isolated anti-HBc showed a significant age-dependent increase in the percentage of isolated anti-HBc individuals: 2.1% (age 21–30 y); 6.9% (age 31–40 y); 15.4% (age 41–50 y); 40% (age 51–60 y); 45.8% (age 61–70 y); and 54.6% (age 71–91 y).Citation21 This phenomenon was similar in the current study 5.3% (age 15–25 y), 39.4% (age 26–35 y), and 55.3% (age 36–48 y), causing a significant difference in the age between the isolated anti- HBc and control groups; however, no difference was noted in the male proportion, which could be attributed to the age of cohort (15–48 y). Positivity for anti-HBc alone may be due to: (1) an aspecific reaction; (2) cross-reaction with other agents; (3) the window period after a recent HBV infection; (4) past HBV infection with undetectable levels of anti-HBs; (5) HBV chronic carriership with undetectable levels of HBsAg in the blood. The prevalence of isolated anti-HBc was high in China, and it focused on adults. Hence, vaccinating is important because these adults may with false-positive results or carriers of HBV, especially those who have not received hepatitis B immunization from the childhood. As such, the long-term immunity effects of isolated anti-HBc in adults need to be determined.
The positive seroprotection rate was 91.5% at 1-month after three doses of vaccination and 72% after 8 years in the isolated anti-HBc group. The response to hepatitis B vaccine in isolated anti-HBc subjects ranges from 70% to 92% in various studies.Citation7,Citation8,Citation22,Citation23 One research reported that anti-HBs seroconversion was achieved in the anti-HBc-positive subjects after full vaccination, and this rate was similar to that of the control subjects (89.5% vs. 96.6%, P = .067) in a study of health-care workers in Korea.Citation24 However, the mean titer was significantly lower in the isolated anti-HBc-positive group after three rounds of vaccinations (392.9 ± 122.7 vs. 669.3 ± 38.1 mIU/mL, P < .001). These findings were in accordance with those from the study which was conducted on a cohort of subjects from Taipei.Citation25 Similar to the current study, all the previous studies suggested that the geometric mean anti-HBs level was significantly lower in the isolated anti-HBc group and minor differences were observed in the positive seroprotection rate.Citation19 Interestingly, one study demonstrated that anti-HBc-positive subjects were 12.2-fold likely to fail seroconversion in HIV and HCV as compared to normal persons (95% CI: 3.2–46.4, P < .001).Citation26 Thus, discrepancies in response rate to hepatitis B vaccination among isolated anti-HBc subjects can be explained by the age, epidemiological differences (prevalence of HBV infection), or vaccination history of the subject.
Similar to previous studies of the normal population, the anti-HBs levels declined progressively with time after a primary hepatitis B immunization schedule in adults.Citation5,Citation27 A recent study showed sustained immune memory and long-term protection 20–30 y after a complete primary hepatitis B vaccination course during adulthood.Citation28 No long-term immunity effect has been reported in the isolated anti-HBc population. Similar to the control group, we found that the positive seroprotection rate and anti-HBs GMT in the isolated anti-HBc group decreased with time. Moreover, no significant differences were observed in the positive seroprotection rate and the level of anti-HBs GMT between the two groups after 8 years. This phenomenon suggested that people with isolated anti-HBc can gain adequate long-term immunity.
Why can the subjects of isolated anti-HBc group obtain the same long-term immune effects as the control group? One hypothesis was that most of the isolated anti-HBc group subjects with false-positive results. The second hypothesis was that those may prior exposured to hepatitis B virus can also benefit from vaccination. Nevertheless, the HBV DNA and HBeAg were not tested, and hence, we could not categorize the adult subjects with isolated anti-HBc. Recent studies suggested that 1–2% of anti-HBc reactive units contain a low level of HBV DNA.Citation17,Citation29 In the follow-up study, we will observe the effect of vaccination of those isolated anti-HBc subjects with occult infection. The present study also has other limitations, first, because of insufficient funding, only anti-HBs were collected after 8 y, the status of other hepatitis B serological marks including HBsAg, hepatitis B e antigen (HBeAg), hepatitis B e antibody (anti-HBe), and anti-HBc were unknown. Second, as young people like to work in big cities, the rate of loss to follow-up was high, and a significant difference was detected in the age between the two groups. This may affect the power of the trial; however, a similar result was obtained when data were analyzed after adjusting for age.
In conclusion, the positive rates and anti-HBs levels of the isolated anti-HBc group declined progressively after a primary hepatitis B immunization in a time-dependent manner. The anti-HBs-positive seroprotection rate was 72.0%, and the GMT was 24.55 mIU/mL at 8 years after vaccination. Isolated anti-HBc-positive subjects achieved satisfactory immune effects post-hepatitis B vaccination. The GMT values of anti-HBs titer were lower than those of the control group at 1 month; however, no significant difference was detected after 8 years.
Disclosure of potential conflicts of interest
The authors declared no potential conflicts of interest.
Acknowledgments
The authors thank the NanXun Center for Disease Control and Prevention and other relevant personnel for their contributions to this study.
Additional information
Funding
References
- World Health Organization. Global hepatitis report, 19 Apr 2017 [ OL]. [cited 2020 May 5]. https://www.who.int/publications-detail/global-hepatitis-report-2017.
- World Health Organization. Hepatitis B [OL]. 18 Jul 2019. [cited 2020 May 5]. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
- Wang FZ, Zheng W, Su XJ, Miao N, Zhang GM, Yin ZD. Achievements and prospects for hepatitis B prevention and control in China. Chin J Vacc Immun. 2019;25:487–92. (In Chinese).
- Jing W, Liu J, Liu M. Eliminating mother-to-child transmission of HBV: progress and challenges in China. Front Med PMID: 31974872. 2020;14(1):21–29. doi:10.1007/s11684-020-0744-2.
- Ren W, Ren JJ, Wu Z, Shen LZ, Shan H, Dai XW, Li J,Liu Y,Qiu Y, Yao J, et al. Long-term persistence of anti-HBs after hepatitis B vaccination among adults: 8-year results. Hum Vaccin Immunother. 2020;16(3):687–92. doi:10.1080/21645515.2019.1666612. PMID: 31526223.
- Liang XF, Bi SL, Yang WZ, Wang LD, Cui G, Cui FQ, Zhang Y, Liu J, Gong X, Chen Y, et al. Reprint of: epidemiological serosurvey of Hepatitis B in China–declining HBV prevalence due to Hepatitis B vaccination. Vaccine. 2013;31(Suppl 9):J21–8. doi:10.1016/j.vaccine.2013.08.012. PMID: 23948229.
- Sunbul M, Leblebicioglu H, Esen S, Eroglu C, Barut S. Response to hepatitis B vaccine in HBsAg/anti-HBs negative and anti-HBc positive subjects. Scand J Infect Dis PMID: 10879605. 2000;32(3):315–16. doi:10.1080/00365540050165983.
- Kabir A, Keshvari M, Kashani AH, Alavian SM. Predicting response to HBV vaccination in people with positive anti-HBc but negative HBsAg and anti-HBs. Hum Vaccin PMID: 18398305. 2008;4(5):379–83. doi:10.4161/hv.4.5.6010.
- Sunbul M, Leblebicioglu H, Esen S, Eroglu C, Barut S. Response to hepatitis B vaccine in HBsAg/anti-HBs negative and anti-HBc positive subjects. Scand J Infect Dis PMID: 10879605. 2000;32(3):315–16. doi:10.1080/00365540050165983.
- Greub G, Frei PC. Presence of low levels of anti-HBs antibody in so-called ‘anti-HBc alone’ subjects. Liver PMID:11903881. 2001;21(6):380–83. doi:10.1034/j.1600-0676.2001.210603.x.
- Moretto F, Catherine FX, Esteve C, Blot M, Piroth L. Isolated Anti-HBc: significance and management. J Clin Med PMID:31940817. 2020;9(1):202. doi:10.3390/jcm9010202.
- Lok AS, Lai CL, Wu PC. Prevalence of isolated antibody to hepatitis B core antigen in an area endemic for hepatitis B virus infection: implications in hepatitis B vaccination programs. Hepatology PMID:2968954. 1988;8(4):766–70. doi:10.1002/hep.1840080411.
- Grob P, Jilg W, Bornhak H, Gerken G, Gerlich W, Gunther S, Hess G, Hudig H, Kitchen A, Margolis H, et al. Serological pattern “anti-HBc alone”: report on a workshop. J Med Virol. 2000;62(4):450–55. doi:10.1002/1096-9071(200012)62:4<450::aid-jmv9>3.0.co;2-y. PMID:11074473.
- Kang SY, Kim MH, Lee WI. The prevalence of “anti-HBc alone” and HBV DNA detection among anti-HBc alone in Korea. J Med Virol PMID: 20648604. 2010;82(9):1508–14. doi:10.1002/jmv.21862.
- Morsica G, Bagaglio S, Spagnuolo V, Castagna A, Serio C, Galli A, Liviana DT, Andrea A, Alexander P. Immune response to hepatitis B vaccination in HIV-positive individuals with isolated antibodies against hepatitis B core antigen: results of a prospective Italian study. Plos One. 2017;12(9):e0184128. doi:10.1371/journal.pone.0184128. PMID:28863182.
- Piroth L, Launay O, Michel ML, Bourredjem A, Miailhes P, Ajana F, Catherine C, David Z, Marre JW, Dani N, et al. Vaccination against Hepatitis B Virus (HBV) in HIV-1-infected patients with isolated anti-HBV core antibody: the ANRS HB EP03 CISOVAC prospective study. J Infect Dis. 2016;213(11):1735–42. doi:10.1093/infdis/jiw011. PMID:26768256.
- Bahari A, Izadi S, Bari Z, Khosravi S, Baghaei B, Saneimoghadam E, Firouzi F, Espiari A, Esmaeilzadeh A, Mokhtarifar A, et al. Significance of response to hepatitis B recombinant vaccine in subjects with isolated antibody to hepatitis B core antigen. Middle East J Dig Dis. 2015;7(4):233–40. PMID: 26609352.
- Zhang L, Yan B, Lv J, Liu JY, Wu WL, Feng Y, Xu AQ. Antibody response to hepatitis B vaccine is independently associated with hepatitis B breakthrough infection among adults: results from a three-year follow-up study in China. Vaccine. 2018;36(16):2207–12. doi:10.1016/j.vaccine.2018.02.039. PMID:29548609.
- Yao J, Ren W, Chen YD, Jiang ZG, Shen LZ, Shan H, Dai XW, Li J, Liu Y, Qiu Y, et al. Responses to hepatitis B vaccine in isolated anti-HBc positive adults. Hum Vaccin Immunother. 2016;12(7):1847–51. doi:10.1080/21645515.2016.1139256. PMID: 27065099.
- McMahon BJ, Dentinger CM, Bruden D, Lisa B, Anthory EF, Beth PB, Thomas WH, et al. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009;200(9):1390–96. doi:10.1086/606119. PMID:19785526.
- Hyun CS, Lee S, Ventura WR. The prevalence and significance of isolated hepatitis B core antibody (anti-HBc) in endemic population. BMC Res Notes PMID: 31060623. 2019;12(1):251. doi:10.1186/s13104-019-4287-z.
- Koh HJ, Kim SD, Choi JH, Kim SR, Lee JS. A study of immune response to hepatitis B vaccine & HBV DNA in isolated anti-HBc positive subjects. J Prev Med Public Health. 2005;38(2):170–74. PMID: 16315754.
- Silva AE, McMahon BJ, Parkinson AJ, Sjogren MH, Hoofnagle JH, Di BAM. Hepatitis B virus DNA in persons with isolated antibody to hepatitis B core antigen who subsequently received hepatitis B vaccine. Clin Infect Dis. 1998;26(4):895–97. doi:10.1086/513918. PMID: 9564471
- Shim J, Kim KY, Kim BH, Chun H, Lee M, Hwangbo Y, Jae YJ, Seok HD, Hyo JK, Young WC, et al. Anti-hepatitis B core antibody is not required for prevaccination screening in healthcare workers. Vaccine. 2011;29(8):1721–26. doi:10.1016/j.vaccine.2010.11.044. PMID: 21147128.
- Chan CY, Lee SD, Tsai YT, Lo KJ. Hepatitis B vaccination alone is not adequate for the categorizing of adult subjects with isolated anti-HBc. J Gastroenterol Hepatol PMID:7787166. 1995;10(2):192–97. doi:10.1111/j.1440-1746.1995.tb01077.x.
- Chang JJ, Mohtashemi N, Bhattacharya D. Significance and management of isolated Hepatitis B core antibody (Anti-HBc) in HIV and HCV: strategies in the DAA Era. Curr HIV/AIDS Rep PMID:29572624. 2018;15(2):172–81. doi:10.1007/s11904-018-0379-y.
- Li H, GJ L, Chen QY, Fang ZL, Wang XY, Tan C, Yang QL, Wang FZ, Zhang S, Bi SL, et al. Long-term effectiveness of plasma-derived hepatitis B vaccine 22-28 years after immunization in a hepatitis B virus endemic rural area: is an adult booster dose needed. Epidemiol Infect. 2017;145(5):887–94. doi:10.1017/S0950268816003046. PMID:28065199.
- Van Damme P, Dionne M, Leroux-Roels G, Meeren O, Paolo E, Salaun B, Pemmaraju SK, Nicalas F. Persistence of HBsAg-specific antibodies and immune memory two to three decades after hepatitis B vaccination in adults. J Viral Hepat. 2019;26(9):1066–75. doi:10.1111/jvh.13125. PMID: 31087382.
- Katz L, Strong DM, Tegtmeier G, Stramer S. Performance of an algorithm for the reentry of volunteer blood donors deferred due to false-positive test results for antibody to hepatitis B core antigen. Transfusion (Paris) PMID:18647367. 2008;48(11):2315–22. doi:10.1111/j.1537-2995.2008.01844.x.