ABSTRACT
Whooping cough continues to be an important public health issue despite high levels of vaccination coverage with acellular pertussis vaccine. Young unimmunized infants represent the most vulnerable group with the highest rates of complications and death. As infant-specific pertussis epidemiologic data, especially among neonates, in Italy were limited, a retrospective observational study of hospitalizations for whooping cough in Italian infants aged <12 months between 2007 and 2018 was conducted to address this knowledge gap. The temporal trend of rates, also stratified for age classes according to the expected age for the administration of vaccine doses, were analyzed by the slope of the regression line. The mean age at the time of admission was 92 d (±64). A clear seasonal pattern in the occurrence of pertussis hospitalizations with a summer peak was observed. Infants younger than 3 months old had the highest hospitalization rates (169 x 100000 infants on average), with a significant rising trend of 9 x 100000 infants on average per year. Limiting the analysis to Bordetella pertussis-related hospitalizations such trend was even more evident. In the other age classes, hospitalization rates were considerably lower and gradually decreased with increasing age. This study demonstrated that pediatric populations, too young to be protected by vaccination, had a greater risk of contracting pertussis. Thus, it is necessary to promote additional immunization strategies besides one booster dose in adolescents, including vaccination during pregnancy.
Introduction
Pertussis (whooping cough) is a highly contagious, respiratory disease caused by Bordetella pertussis (BP).Citation1 It continues as a public health concern threat given its reemergence despite high vaccination coverage.Citation2
The reasons for such resurgence are not fully elucidated but it may be attributed to various factors including increased awareness, improved diagnosis methods, genetic changes in circulating BP, and increased bacterial circulation among adolescents and adults related to the waning of vaccine-induced immunity.Citation3,Citation4 In particular, these epidemiological changes have made adolescents and adults a reservoir for BP and the source of infection to the unvaccinated newborns.Citation3 In addition, while adolescents and adults tend to have a prolonged illness characterized by cough but without other major symptoms, young unimmunized infants represent the most vulnerable group with the highest rates of morbidity, hospitalization, complications, intensive care unit (ICU) admission, and mortality.Citation1,Citation5 History of prematurity and an age of less than 3 months emerged as risk factors for the development of fatal pertussis.Citation5 An increased white blood cells (WBC) count at presentation represents a warning signal for fatal cases, although no particular value of leukocytosis accurately predicts death or survival.Citation5
In the pre-vaccine era, epidemic cycles reoccur every 2 to 5 y.Citation6 This cyclic pattern continued to occur in the whole-cell pertussis vaccine (wP) era and is still occurring in the present acellular pertussis vaccine (aP) era.Citation6 Thus, the circulation of BP has not been controlled by immunization, and young infants continue to be at risk for severe pertussis and pertussis deaths.Citation6
In Italy, the Ministry of Health recommendations on pertussis immunization provide for a two-dose primary series with aP at 3 and 5 months of age and a booster at 11 months; a pre-school booster dose between 5 and 6 y and a further booster between 11 and 18 y.Citation7 Despite a high vaccination coverage, a retrospective study reviewing the Italian national hospital discharge form database showed that, in the period 2002–2016, most hospitalizations (63.39%) involved subjects <1 y of age.Citation8
As infant-specific pertussis epidemiologic data, especially among neonates, in Italy were limited, the present study was designed to address this knowledge gap.
Materials and methods
A retrospective observational study of hospitalizations for whooping cough in Italian infants aged <12 months was conducted. All records with primary or secondary diagnoses of pertussis between 2007 and 2018 were extracted from the Hospital Discharge Database of Ministry of Health, based on the following codes of the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM): 033.0 (whooping cough due to BP), 033.1 (whooping cough due to Bordetella parapertussis), 033.8 (whooping cough due to other specified organisms), 033.9 (whooping cough, unspecified organism), and 484.3 (whooping cough, unspecified species with pneumonia).
Descriptive statistics were calculated, including frequencies and percentages for categorical variables (sex, age classes, nationality, geographical location, seasonality, deaths, type of diagnosis, and ICD9-CM codes), and mean with standard deviations (SDs) for continuous variables (age and length of hospital stay).
Hospitalization rates, expressed per 100000 infants, were calculated using the National resident population aged <12 months provided by the Italian Institute of Statistics (ISTAT).Citation9 The temporal trend of rates, also stratified for age classes (<3 months, 3–4 months, 5–10 months, and >10 months, according to the expected age for the administration of vaccine doses), were analyzed by the slope of the regression line. Data for vaccination coverage with pertussis vaccines at age 24 months were obtained from the Ministry of Health database.Citation7 A p-value of <0.05 was the criterion for statistical significance. Stata 15 (StataCorp, College Station, Texas, USA) was used for data analysis.
Data provided by the Ministry of the Health did not contain any patient identifiers and was therefore completely anonymous. Hence, notification of the study to Ethics Committees was not applicable, nor was informed consent of patients required.
Availability of data and materials
Hospital discharge records are available at the National Archive of HDRs data, Ministry of Health, General Directorate of Healthcare Planning, VI Office.
Results
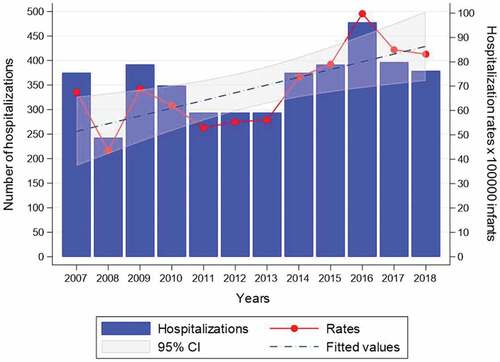
Between 1 January 2007 and 31 December 2018, there were 4262 whooping cough hospitalizations in Italian infants aged <12 months (), 77.9% (3322/4262) of which with pertussis-related codes in principal diagnosis. Overall, the majority of cases (2604/4262, 61.1%) were infants aged <3 months. The mean age at the time of admission was 92 d (±64) and the mean length of hospital stay was 7 d (±7). Over the study period, there were 10 recorded deaths among pertussis-related admissions, all of them in infants aged <3 months. A clear seasonal pattern in the occurrence of pertussis hospitalizations with a summer peak (38.1%, 1624/4262) was observed.
Table 1. Characteristics of infants (<12 months old) hospitalized for pertussis in 2007–2018, in Italy
The overall hospitalization rate, per 100000 infants, was 68.92 (95% CI: 58.73–79.12), with a peak in 2016 (100 x 100000 infants), when 470 infant admissions were recorded. The analysis showed a clear and significant increasing trend of hospitalization rates (β coefficient = 3.18, p = .009) ().
Figure 1. Yearly frequency distribution and hospitalization rates for pertussis admissions in Italian infants aged <12 months, 2007–2018. Trend test. Number of hospitalizations: β = 8.71, p = .104; hospitalization rates: β = 3.18, p = .009
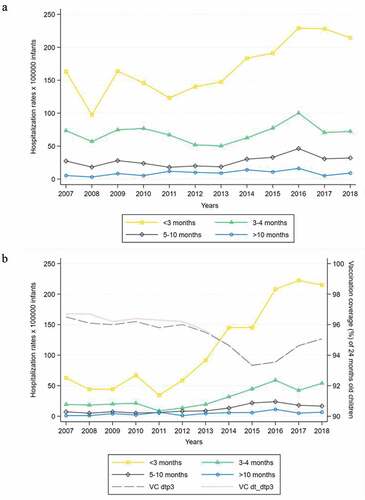
When stratified by age (), <3 months old children had the highest hospitalization rates, with an average value for the study period equal to 169 x 100000 infants. In the other age classes, hospitalization rates were considerably lower and gradually decreased with increasing age. A significant rising trend was recorded for hospitalization rates during the first 3 months of life (β coefficient = 9.12, p = .002), of 9 x 100000 infants on average per year. The other age classes showed a substantially stable and not significant trend during the study period. Limiting the analysis to BP-related hospitalizations (), such trends were even more evident, with an average annual increase of twice that recorded for all etiologies in <3 months old infants (β coefficient = 18.11, p < .001). A slightly decreasing trend of vaccination coverage of 24 months old children with pertussis vaccines was found (dtp3: β coefficient = −0.23, p = .003; dt_dtp3: β coefficient = −0.27, p = .001), although with average value >95% (95.3% for dtp3 and 95.4% for dt_dtp3). Considering the other possible etiologies, a significant decreasing trend emerged for whooping cough of unspecified organism in the first 3 months of life (β coefficient = −5.71, p = .018), with an average hospitalization rate equal to 118 x 100000 infants compared to an average of 112 x 100000 infants for BP, recalling that these two etiologies accounted for 91.2% of hospitalizations for whooping cough, as shown in .
Figure 2. A) Pertussis hospitalization rates for all etiologies stratified by age classes. Trend test. <3 months: β = 9.12, p = .002; 3–4 months: β = 0.96, p = .419; 5–10 months: β = 1.30, p = .053; >10 months: β = 0.52, p = .117. B) Bordetella pertussis-related hospitalization rates stratified by age classes. Trend test for B. pertussis hospitalization rates. <3 months: β = 18.11, p < .001; 3–4 months: β = 3.71, p = .002; 5–10 months: β = 1.54, p = .001; >10 months: β = 0.57, p = .011. dtp3 and dt_dtp3: diphtheria-tetanus-pertussis vaccines. Trend test for vaccination coverage (VC). VC(dtp3): β = −0.23, p=.003; VC(dt_dtp3): β=-0.27, p=.001
Discussion
The present study demonstrated that pertussis represents a reemergent public health issue in Italy, especially for infants too young to be vaccinated, being the hospitalization rates for BP increased for infants aged <3 months. Our results are consistent with trends previously reported: incidence was highest among younger infants (2–3 months of age) and decreased steadily from the 4th through the 12th months of age.Citation4
A decrease in vaccine coverage rate in children was also noticed and this might be a contributing factor to pertussis resurgence, although it was estimated to be >95% in the study period.Citation10,Citation11
The highest incidence of pertussis hospitalizations was observed between spring and summer, in line with previous epidemiological analysis.Citation11 During the winter season, when other respiratory viruses circulate and the minimum incidence of pertussis is usually observed, cases with atypical clinical presentations could occur and might be often unrecognized.Citation12 Clinical suspicion has, in fact, a low sensitivity in infants since clinical manifestations can overlap with several other respiratory infections: there is therefore the need for systematic use of laboratory tests for the diagnosis of pertussis in this age group, also to avoid the risk for hospital outbreaks.Citation3,Citation12 Moreover, the molecular characterization of the circulating strains may contribute to identify variant strains possibly due to vaccine pressure.Citation3
The percentage of cases bearing the code 033.9, related to pertussis due to unspecified pathogens, was 48.6% indicating that more efforts should be made to implement the etiological diagnosis in order to reduce the under-notification of the disease, whereas in Italy pertussis notification is made on a clinical diagnosis.Citation8,Citation11
Pertussis epidemiology is changing in Italy, with a lower incidence in children and an increased incidence in adolescents and adults: comparing period with a low (1971–1989), intermediate (1990–1996) and high (1998–2002) vaccination coverage, the percent distribution of cases has appeared markedly changed, declining by half in the 0–4 y age group, while increasing by 1.5-fold in the 5–9 y age group and three-fold in the 10–14 y age group.Citation13 Thus, adolescents and adults represent a significant source of infection for unvaccinated or incompletely immunized newborns/infants in whom the severity of the disease can be serious.Citation13
The current Italian immunization schedule suggests the first dose to be given at the age of 3 months.Citation7 The first dose produces partial protection mainly against severe disease, but higher rates of immunity (80–90%) do not occur until after administration of the third dose.Citation14 Hence, some infants remain unimmunized or incompletely immunized during their first months of life.Citation15 Pediatric populations, too young to be protected by vaccination, not only have a greater risk of contracting the infection but also bear the greatest disease burden.Citation14 In California, during the 2010 outbreak, the highest rates of disease and hospitalization occurred in infants <6 months old.Citation16 In the UK, during the 2011–2012 outbreak the highest incidence occurred in infants <3 months old.Citation17 In addition, during those outbreaks, the majority of deaths occurred in infants <3 months old. Thus, in line with our results, deaths occurred in infants who were age-ineligible to commence the routine childhood immunization series at 3 months of age with pertussis vaccine. Therefore, when considering prevention strategies against pertussis, it is critical to include approaches that prevent pertussis transmission to young infants.
A major source of pertussis infection during the first year of life, when infants are not completely immunized, is household contact. Although the vaccination of persons in households with a young infant might substantially reduce the disease burden of pertussis by limiting its transmission (cocoon strategy), it was demonstrated that individuals vaccinated with components of aP were protected against the disease, but not against bacterial colonization:Citation18 these individuals can become asymptomatically infected, and can then transmit infection to susceptible individuals.Citation19–21 In addition, because vaccinated individuals develop maximal antibody titers within 2 weeks, if vaccination occurs solely during the postpartum period, there will be a short time (≤2 weeks post-vaccination) when the infant could be at risk for transmission.Citation14 Parental “cocoon” strategy is not favorable in a context of low pertussis incidence and would be more expensive to implement and less cost-effective than other strategies designed to protect infants from pertussis, as the maternal immunization.Citation14–22
Vaccination of women with aP during pregnancy, stimulating the development of maternal anti-pertussis antibodies, which pass through the placenta, is expected to provide protection to infants from pertussis until they are old enough to be vaccinated themselves.Citation12
Several studies revealed an effectiveness of over 90%: more than 9 out of 10 cases of pertussis in infants in the first months of life could be avoided with vaccination of pregnant women in the third trimester.Citation23–26
Vaccination during pregnancy is now recommended by the national health organizations of several countries.Citation25,Citation27,Citation28
Defining the optimal timing for maternal immunization is critical, as the half-life of anti-pertussis antibodies is short and seroprotection should be extended as long as possible.Citation29 In Italy, anti-pertussis vaccination was recommended for each pregnancy, during the third trimester, ideally at the 28th week.Citation30
In addition, as deaths occurred in the first 3 months after birth, a pertussis booster in pregnancy may certainly be the key to address strategies to prevent infant death.Citation31
Recommendations of health-care providers have a crucial role in vaccine uptake, as they can educate pregnant women on the benefits of immunization for the young infant.Citation14
Limitations. Of particular relevance for this study, a pertussis diagnosis was based on the presence of a pertussis diagnosis code in the hospital discharge records. Miscoding as well as under- and missed-diagnoses, especially among infants with milder or atypical illness, are possible. Both the failure to code and the failure to diagnose pertussis would result in underestimation of the true rate of disease in this population.Citation3,Citation4,Citation32 Nevertheless, as this observational study was conducted on a national scale, it was able to give a good picture of pertussis burden in Italian infants.
In conclusion, pertussis-related hospitalizations and deaths among infants less than 12 months of age in Italy are higher than expected given the overall vaccination rate, especially among infants below 3 months of age, and it is still dramatically increasing. The present investigation underlines the need to implement urgently the public health efforts to maintain a high vaccination coverage and to promote additional immunization strategies besides one booster dose in adolescents, including vaccination during pregnancy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Frassanito A, Nenna R, Nicolai A, Pierangeli A, Tozzi AE, Stefanelli P, Carsetti R, Concato C, Schiavoni I, Midulla F, et al. Pertussis study group. Infants hospitalized for Bordetella pertussis infection commonly have respiratory viral coinfections. BMC Infect Dis. 2017;17:492. doi:10.1186/s12879-017-2567-6.
- Tozzi AE, Pandolfi E, Celentano LP, Massari M, Salmaso S. Ciofi degli Atti ML, EUVAC-NET study group. Comparison of pertussis surveillance systems in Europe. Vaccine. 2007;25:291–97. doi:10.1016/j.vaccine.2006.07.023.
- Fedele G, Stefanelli P. Pertussis in infants and the resurgence of a vaccine preventable disease: what to do? Comment Ann Ist Super Sanita. 2017;53:100–03. doi:10.4415/ANN_17_02_04.
- Masseria C, Martin CK, Krishnarajah G, Becker LK, Buikema A, Tan TQ. Incidence and burden of pertussis among infants less than 1 year of age. Pediatr Infect Dis J. 2017 Mar;36:e54–e61. doi:10.1097/INF.0000000000001440.
- Carloni I, Ricci S, Azzari C, Galletti S, Faldella G, de Benedictis FM. Fatal pertussis in infancy, Italy. J Infect. 2017;75(2):186–89. doi:10.1016/j.jinf.2017.04.005.
- Cherry JD. Pertussis in Young Infants Throughout the World. Clin Infect Dis. 2016;63:S119–S122. doi:10.1093/cid/ciw550.
- Ministry of Health. Vaccine scheduler. [accessed 2019 Nov 9]. http://www.salute.gov.it/imgs/C_17_pagineAree_4829_listaFile_itemName_0_file.pdf
- Fiasca F, Gabutti G. Mattei a trends in hospital admissions for pertussis infection: a nationwide retrospective observational study in Italy, 2002-2016. Int J Environ Res Public Health. 2019;16:E4531. doi:10.3390/ijerph16224531.
- Italian Institute of Statistics. [accessed 2019 Dec 2]. http://demo.istat.it/MinistryofHealth. Vaccination coverage. [accessed 2019 Dec10]. http://www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=20
- Gonfiantini MV, Carloni E, Gesualdo F, Pandolfi E, Agricola E, Rizzuto E, Iannazzo S, Ciofi Degli Atti ML, Villani A, Tozzi AE. Epidemiology of pertussis in Italy: disease trends over the last century. Euro Surveill. 2014;19:20921. doi:10.2807/1560-7917.es2014.19.40.20921.
- Vittucci AC, Spuri Vennarucci V, Grandin A, Russo C, Lancella L, Tozzi AE, Bartuli A, Villani A. Pertussis in infants: an underestimated disease. BMC Infect Dis. 2016;16:414. doi:10.1186/s12879-016-1710-0.
- Gabutti G, Azzari C, Bonanni P, Prato R, Tozzi AE, Zanetti A, Zuccotti G. Pertussis. Hum Vaccin Immunother. 2015;11(1):108–17. doi:10.4161/hv.34364.
- Forsyth K, Plotkin S, Tan T, Wirsing von König CH. Strategies to decrease pertussis transmission to infants. Pediatrics. 2015;135:e1475–1482. doi:10.1542/peds.2014-3925.
- Nicolai A, Nenna R, Stefanelli P, Carannante A, Schiavariello C, Pierangeli A, Scagnolari C, Moretti C, Papoff P, Bonci E, et al. Bordetella pertussis in infants hospitalized for acute respiratory symptoms remains a concern. BMC Infect Dis. 2013;13:526. doi:10.1186/1471-2334-13-526.
- Winter K, Harriman K, Zipprich J, Schechter R, Talarico J, Watt J, Chavez G. California pertussis epidemic, 2010. J Pediatr. 2012;161:1091–96. doi:10.1016/j.jpeds.2012.05.041.
- Public Health England (PHE). Public health England (PHE) enhanced pertussis surveillance. [accessed 2020 Jan 21]. https://webarchive.nationalarchives.gov.uk/20140722070137/http://www.hpa.org.uk/hpr/archives/2013/news0513.htm#prtsss
- Fedele G, Carollo M, Palazzo R, Stefanelli P, Pandolfi E, Gesualdo F, Tozzi AE, Carsetti R, Villani A, Nicolai A, et al. Parents as source of pertussis transmission in hospitalized young infants. Infection. 2017;45:171–78. doi:10.1007/s15010-016-0943-6.
- Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A. 2014;111:787–92. doi:10.1073/pnas.1314688110.
- Storsaeter J, Hallander H, Farrington CP, Olin P, Möllby R, Miller E. Secondary analyses of the efficacy of two acellular pertussis vaccines evaluated in a Swedish phase III trial. Vaccine. 1990;8:457–61. doi:10.1016/0264-410x(90)90246-i.
- von Linstow ML, Pontoppidan PL, von König CH, Cherry JD, Hogh B. Evidence of Bordetella pertussis infection in vaccinated 1-year-old Danish children. Eur J Pediatr. 2010;169:1119–22. doi:10.1007/s00431-010-1192-9.
- Meregaglia M, Ferrara L, Melegaro A, Demicheli V. Parent “cocoon” immunization to prevent pertussis-related hospitalization in infants: the case of Piemonte in Italy. Vaccine. 2013;31:1135–37. doi:10.1016/j.vaccine.2012.12.061.
- Skoff TH, Blain AE, Watt J, Scherzinger K, McMahon M, Zansky SM, Kudish K, Cieslak PR, Lewis M, Shang N, et al. Impact of the US maternal tetanus, diphtheria, and acellular pertussis vaccination program on preventing pertussis in infants <2 months of age: a case-control evaluation. Clin Infect Dis. 2017;65:1977–83. doi:10.1093/cid/cix724.
- Amirthalingam G, Campbell H, Ribeiro S, Fry NK, Ramsay M, Miller E, Andrews N. Sustained effectiveness of the maternal pertussis immunization program in england 3 years following introduction. Clin Infect Dis. 2016;63:S236–S243. doi:10.1093/cid/ciw559.
- Dabrera G, Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Fry NK, Ramsay M. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012-2013. Clin Infect Dis. 2015;60:333–37. doi:10.1093/cid/ciu821.
- van den Biggelaar AHJ, Poolman JT. Predicting future trends in the burden of pertussis in the 21st century: implications for infant pertussis and the success of maternal immunization. Expert Rev Vaccines. 2016;15:69–80. doi:10.1586/14760584.2016.1105136.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months. Advisory committee on immunization practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1424–26.
- Public Health England (PHE). Vaccination against pertussis (whooping cough) for pregnant women-2014. [accessed 2020 Jan 8] https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/529956/FV_JUNE_2016_PHE_pertussis_in_pregnancy_information_for_HP_.pdf
- Eberhardt CS, Blanchard-Rohner G, Lemaître B, Boukrid M, Combescure C, Othenin-Girard V, Chilin A, Petre J, de Tejada BM, Siegrist CA. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin Infect Dis. 2016;62:829–36. doi:10.1093/cid/ciw027.
- Ministry of Health. Circular of 21 november 2018. Recommended vaccinations for women of childbearing and pregnancy. [accessed 2020 Feb 2] http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2018&codLeg=66751&parte=1%20&serie=null
- Stefanelli P, Buttinelli G, Vacca P, Tozzi AE, Midulla F, Carsetti R, Fedele G, Villani A, Concato C, Pertussis Study Group. Severe pertussis infection in infants less than 6 months of age: clinical manifestations and molecular characterization. Hum Vaccin Immunother. 2017;13:1073–77. doi:10.1080/21645515.2016.1276139.
- Fiasca F, Necozione S, Fabiani L, Mastrodomenico M, Mattei A. Measles-related hospitalizations in Italy, 2004-2016: the importance of high vaccination coverage. Ann Glob Health. 2019;85:40. doi:10.5334/aogh.2455.
- Fiasca F, Mattei A, Vittorini P, Necozione S, Appetiti A, Angelone AM, Fabiani L. Bacterial meningitis hospitalizations after the 2009 L’Aquila earthquake: a retrospective observational study. Asian J Epidemiol. 2018;11:46–51. doi:10.3923/aje.2018.46.51.
