ABSTRACT
Objectives
To evaluate cost-utility of universal Hepatitis B vaccination program in the Beijing city (Beijing).
Methods
A decision-Markov model was constructed to determine the cost-utility of the universal immunization program for infants (universal vaccination program) by comparing with a hypothetic nonvaccination strategy in Beijing. Parameters in models were extracted from Beijing Center for Disease Control and Prevention (CDC) annual work report, Beijing health statistical yearbook, National Health Survey report, Beijing 1% population sample survey report, Beijing Health and Medical Price Monitoring Data Platform, and public literatures. The incremental cost‑utility ratio (ICUR) was used to compare alternative scenarios. One-way sensitivity analysis and probabilistic sensitivity analysis were used to assess parameter uncertainties.
Results
The universal vaccination program had increased the utility and reduced cost among infants born in 2016 in Beijing. The ICUR was CNY −24,576.61 (US$ −3779.16) per QALY for universal vaccination program comparing with non-vaccination scenario from healthcare perspective. It was estimated that the universal vaccination would save direct medical treatment cost of CNY 2,262,869,173.50 (US$ 347,962,414.43) and prevent loss of 18322.25 QALYs within lifetime of target cohort. Discount rate accounted for the most remarkable influence on ICUR in one-way sensitivity analysis. The result of probabilistic sensitivity analysis illustrated that all of the ICURs were located in the fourth quadrant of the cost-utility incremental plot undergone 5000 times of Monte Carlo simulation.
Conclusions
Current universal hepatitis B vaccination program in Beijing was highly cost utility. The investment was reasonable for current universal vaccination program in Beijing.
Introduction
Hepatitis B virus (HBV) is one of the most serious infectious diseases, approximately infected 257 million people worldwide, leading to numerous serious public health issues all over the worldCitation1. It was estimated that at least 90 million people were suffering from diseases related to HBV in China, which imposed heavy economic burden to the government.Citation2–4 In 2014, the overall HBsAg positive rate of pregnant women was 6.09% in China, meanwhile it’s noted that perinatal transmission of HBV was still the dominant route of HBV infection.Citation5,Citation6 HBV infection that occurred during infanthood and earlier childhood was more likely developed into chronic hepatitis B infection, and further developed into cirrhosis or hepatocellular carcinoma (HCC).Citation7,Citation8
The Global Health Sector Strategy on Viral Hepatitis dedicated to eliminate incidence of chronic HBV infection by 2030.Citation9,Citation10 To achieve this goal, it was important to control HBV infection among children, especially in newborns. HBV vaccine immunization for newborns was the most effective approach to control perinatal HBV infection, which was recommended for all newborns in China since 1992. The first dose was vaccinated within 24 hours of birth, which was extremely vital to prevent perinatal HBV infection, and the following 2nd and 3rd vaccination could boost the protective effect of immunization.Citation11 However, the expense of vaccine was entirely payed by their parents, which could influence the coverage rate of HBV vaccination.Citation12 Then, Chinese government integrated HBV vaccine immunization into Expanded Program of Immunization (EPI) in 2002, which reduced the financial burden of parents. To expand the coverage of HBV vaccine in less-developed areas, Ministry of Health/Global Alliance for Vaccine and Immunization GAVI Cooperation Project (MOH/GAVI Cooperation Project) was also implemented in 2002.Citation13 Since June of 2005, the government decided to bear all the expenditure generated from universal vaccination program, which also meant HBV vaccine was free for all newborns.Citation14
Beijing, as the capital of China, was the first few cities that implemented HB vaccine immunization for newborns since 1992. Current HB-vaccination included three 10 μg doses of recombinant HB-vaccine for infants regardless their mothers’ HBV status since 2006.Citation15,Citation16 An official epidemic report revealed that the HBsAg prevalence rate of children under 5 years old declined from 0.59% to below 0.1% in Beijing from 1992 to 2013.Citation17,Citation18 HBV vaccination program in Beijing has made great progress in controlling HBV infection among children.
Cost-utility analysis serves as a powerful measure in evaluating the economic and health benefit of HBV vaccination program. Studies had been carried out in regions with different HBV epidemic levels. A research proved that universal vaccination program was cost saving and well controlled for long-term prognosis of HBV diseases in Taiwan.Citation19 A previous study in Gambia provided evidence that HB-vaccine for neonates was cost saving in low-income country.Citation20 With monitored data, Boccalini et al. evaluated the effectiveness of HBV immunization during 3 periods in 20 years and found that the universal vaccination strategy was clinically and economically favorable during the first 20 year in Italy.Citation21
Studies in Chinese mainland also illustrated the cost effectiveness of universal HB vaccination. Process of 3 dose of 5 μg HB vaccination was proved to be cost saving for newborns,Citation22 while the implementation cost of government was not included in this study. After that, it was reported that the process of 3 dose of 5 μg HB vaccination could less cost effective than the series of 10 μg HB vaccination due to lower vaccine efficacy.Citation23 However, in which the parameters of parameters of 10 μg HB-vaccine strategy were inferred based on the 5 μg HB vaccine strategy. In this research, we aimed to evaluate the cost utility of 10 μg HB-vaccine program, which is different from previous studies. Besides, it’s a long-lasting task to conduct economical evaluation on HBV immunization program, due to considerable changes occurred in related epidemic parameters, such as prevalence of HBV infection, vaccination coverage, vaccination cost, and medical cost. Finally, Beijing is one of the largest cities characterized with large population amount, complex population structure and well-designed HBV immunization policy. However, there is no research related to cost-utility of universal HB vaccination which could contribute to explain the cost effective of HB-vaccine strategy in special large city like Beijing. Therefore, we chose Beijing as a representative to conduct economic evaluation of HBV immunization program.
Materials and methods
Decision-Markov model
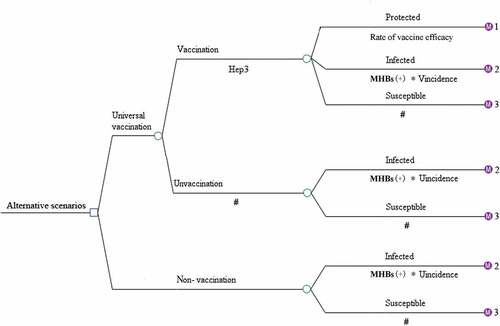
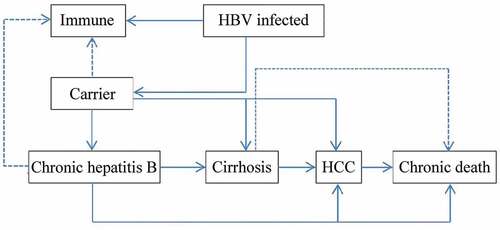
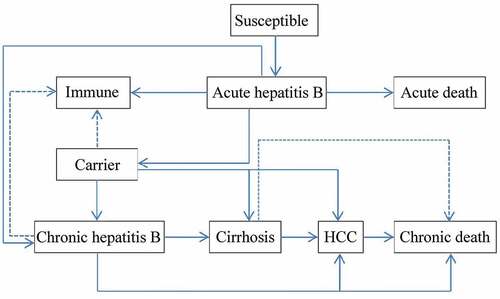
A decision tree was constructed to represent two alternative scenarios: with universal HB-vaccination program and without vaccination program and followed with Markov model to represent the process of vaccine-protected, HBV infection, and susceptible of HBV infection (). In universal vaccination scenario, newborn cohort received three doses of 10 μg HB-vaccine regardless the mothers’ HBV status. Since few infants would quit the full process of immunization, those who didn’t complete the full process of immunization, receiving only one or two doses of HB vaccine were ignored. There were six health status included in HBV infection Markov process: immunity, HBV carriers, chronic hepatitis (CHB), cirrhosis, hepatocellular carcinoma (HCC), and death (). Beyond that, infants who were neither protected by vaccine nor undergone perinatal infection would enter the susceptible Markov process, including three more health status than the HBV infection Markov process, which were: susceptible, asymptomatic acute hepatitis and systematic acute hepatitis (). The Decision-Markov model was simulated yearly with a cohort of 202349 born in 2016 within a maximum 82 cycles according to their life expectancyCitation24,Citation25 ().
Table 1. Parameters of decision-Markov models
Parameters
Epidemical data
The risk of perinatal infection depends on mothers’ HBsAg status, and research shows that if maternal HBsAg is positive, the possibility of perinatal infection of vaccinated and unvaccinated infants could be 5.90% and 40.34% respectively.Citation28–31 Prevalence of HBsAg among pregnant women was reported to be 3.86% in Beijing in 2005, and a seroepidemic investigation in Beijing showed that the HBsAg positive rate was decline by years in every age groups from 2006 to 2014.Citation15,Citation18 It could predict that HBsAg positive rate also showed a downward trend. We therefore adopted a base value of mothers’ HBsAg positive rate of 3.10% in 2014 in eastern regions of China in this study, and identified a range of 1.00%~3.86% for this parameter.Citation5,Citation22,Citation27As for infants who did not infected during perinatal period or were not protected by vaccine, the incidence of acute HBV infection among them could be 3.00% after their 1 year-old.Citation32 This parameter was derived from existed study in Beijing and tested at the range of ±50% in sensitivity analysis since the potential underestimation. ().
Vaccination coverage and efficacy
Beijing was one of the first cities implementing the process of 3 doses of 10 μg HB vaccine.Citation16 Newborns were required to receive 3 doses of 10 μg HB vaccine in 0-1-6 months (a timely birth-dose followed with two booster doses) in Beijing. Vaccination rate was estimated at the range of 99.90%~100.00%, and the base value for analysis was 99.95% as the vaccination rate was over 99.90% since 2004 according to data from annual work report on Beijing CDC.Citation43 For the infants who completely finished three-dose immunization process, the vaccine efficacy could range from 93.20% to 99.50%.Citation26It was assumed that the protective effect of HBV vaccine would be the same regardless of the maternal HBV infection status and this effect would last for lifelong.
Transmitted probabilities
Parameters of transmitted probabilities in Markov model were acquired from published literatures (). Perinatal infected infants would start with HBV carrier and immunity status at the rate of 11.00% and 89.00% respectively.Citation33–39 Unprotected and uninfected infants would start with a susceptible status, and further develop into acute hepatitis state at the rate of 3.00%.Citation32 Acute hepatitis (AHB) would last for maximum 6 months, which was a transient status maintaining for max of one circle.Citation40,Citation41 People who infected HBV might die from AHB or chronic HBV infection, cirrhosis, and HCC.Citation32,Citation48 Age-specific mortality rate was derived from Beijing 1% population sample survey report in 2015.Citation42 (Appendix)
Parameters of cost
Cost includes vaccination cost and direct treatment cost of HBV-related diseases from a healthcare perspective. Cost of per dose vaccine was derived from public literatures, including cost of vaccine, labor resource, public office expenses and medical consumables generated from Beijing CDC, hospitals and communities.Citation43 Cost of vaccination was adjusted to price level in 2016 using consumer price index (CPI). Direct treatment cost of HB-related diseases was deduced by multiplying average treatment charge for per visit and average visit times per year.Citation47,Citation48 Average treatment cost per visit was calculated according to treatment record of 124553 patients that extracted from Beijing Health and Medical Price Monitoring Data Platform (BHMP), which was from HIS system of 39 secondary and tertiary-level hospitals in 2016 in BeijingCitation47 (). Average annual visit times was estimated by employing data from the fifth national health service survey in Beijing. Average annual visit times was estimated at 1.38 and 2.39 times for outpatients and inpatients of HCC, and 1.52 and 1.08 times for outpatients and inpatients of other four HB-related diseases (AHB, CHB, cirrhosis, and HBV carriers), respectively.Citation48 AHB can be divided into asymptomatic acute hepatitis and symptomatic acute hepatitis, and here we only included the cost caused by symptomatic acute hepatitis. Cost during future years was discounted at the rate of 3.00%.Citation44–46 Details of direct medical cost are provided in Appendix .
Parameters of utility
Utility scores were derived from literatures, which were assessed by conducting household investigation with Europe quality-5 dimensions (EQ-5D) in Beijing community.Citation32 Utility score of AHB, CHB, cirrhosis, HCC and HBV carriers were 0.892, 0.912, 0.755, 0.712, and 0.968 respectively. QALY loss scores were obtained by subtracting utility score of each HBV state from the health utility score (health utility score = 1). We assumed that the utility score of asymptomatic acute hepatitis status was the same as general population (health utility score = 1). Utility during future years was discounted at the rate of 3.00%.Citation44
Cost-Utility analysis
Cost utility analysis was conducted to analyze and compare the incremental cost-utility ratio (ICUR) of HBV immunization program and nonvaccination program, and then calculated the prevented losses attribute to immunization program. The universal vaccination program was considered to be cost-utility when ICUR ≤ 3 times of the annual per capita GDP (CNY 115,000.00, US$ 17,683.60 in Beijing in 2016) and it was highly cost utility when ICUR does not exceed per capita GDP according to WHO recommendation.Citation49,Citation50 We further estimated the QALY loss and direct treatment cost of each HB-related disease in different scenarios, to evaluate the health and economic burden due to HBV infection during cohort’s lifetime.
Sensitivity analysis
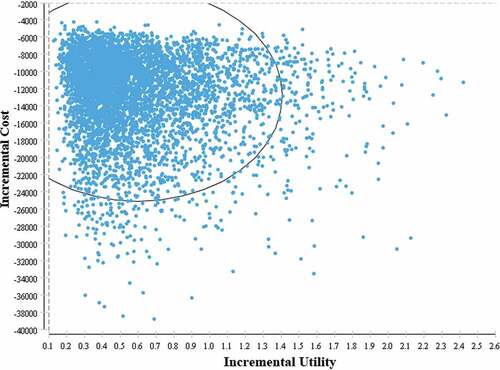
One-way and a probabilistic sensitivity analysis were conducted to assess the parameter uncertainties. Direct treatment cost, discount rate and some of transmitted probabilities rate varied in one-way sensitivity analysis to find out influence of single parameter uncertainties (). Probabilistic sensitivity analysis used second-order Monte Carlo simulation with 5000 iterations by setting parameters in different distributions (). Universal vaccination program was cost utility when the ICURs were located in the fourth quadrant of the ICE scatter plot. Cost data was processed and calculated with EXCEL 2010. Decision-Markov model was constructed and analyzed in TreeAgePro2011 (Tree Age Software, Inc, Williamstown, MA).
Results
Cost utility of universal HBV vaccination
Universal vaccination program could improve total QALY gains, as well as save medical cost. The average cost and QALY gains were estimated within the lifetime of cohort and were presented in . Compared with nonvaccination strategy, universal vaccination program would save an average cost of CNY 11,072.47(US$ −1702.62) and further lengthen an average of 0.45 QALY gains among the cohort of 202349 newborns. The ICUR was CNY −24,576.61 (US$ −3779.16) for per QALY gained. This result could be considered cost saving according to WHO recommendation.
Table 2. Average cost and QALYs gains and ICUR of two scenarios
Model-predicted disease burden and QALY loss
Direct medical cost and QALY loss due to HB-related diseases under different scenarios within 82 cycles were estimated by Markov model (). Results showed that universal vaccination program would save direct medical cost of CNY2,262,869,173.50 (US$ 347,962,414.43) and prevent a total loss of 18322.25 QALYs due to HBV-related diseases, among which CHB accounted for 54.17% and 38.86% of medical cost saving and loss of QALY respectively. medical treatment cost-savings due to universal vaccination program has considerably exceeded the government input on HBV vaccination program (CNY 22,364,564.54, US$ 3,439,009.19).
Table 3. Direct treatment cost and QALY loss of HB-related patients under different scenarios
Sensitivity analysis
According to the result of one-way sensitivity analysis, parameters that following parameters have a significant impact on ICUR, including discount rate, mortality of HCC, mortality of cirrhosis, direct medical cost of CHB, and mortality of CHB. While none of single parameter could change the conclusion that universal vaccination program was highly cost utility. Result of probabilistic sensitivity analysis indicated that all of the ICUR scatter points (5000 Monte Carlo simulations) were located in the fourth quadrant of the cost-utility incremental plot (), which identified that the model was robust and reliable even taking uncertainties of each parameter into account.
Discussion
Universal vaccination program was free for all the newborns in Beijing since 2005, which greatly expanded the coverage of HBV vaccination. Based on economic evaluation, universal vaccination program in Beijing could save an average of CNY 24,576.61 (US$ 3779.16) when one more QALY gained. A similar study conducted in China reported that within 100 circles, the ICER was US$ 1708.06 for per QALY gained for universal vaccination program.Citation22 The average cost and utility of universal vaccination were estimated at CNY 2225.74 and 26.88 QALYs in Zhejiang province.Citation31 Average QALY gained in these studies were lower than that in our study, the reason might lie in the lower utility scores in our study especially for general population. A previous study, conducted in a high epidemic area (HBsAg positive rate of pregnant women was 9.50%) of Taiwan, presented that universal vaccination would save US$ 5715 when acquiring one more QALY from perspective of healthcareCitation19. But it was not directly comparable because the parameter of utility was not discounted to the birth year.
Chronic hepatitis infection was still accounted for a largest proportion of HBV infection regardless of with or without HBV vaccination program. It was estimated that a sum of 81.23% of direct medical cost and 57.30% of QALY loss attribute to CHB, while cirrhosis and HCC would be prevented by implementing the universal vaccination program. According to one sero-epidemic investigation report, prevalence of HBsAg was 5.49% among Chinese population and 7400 million people were predicted to suffer from chronic hepatitis infection in 2014.Citation51 Therefore, it was necessary for the government to keep funding this program, for the sake of prevention of casualties and reduction of expenses due to HBV infection.
In this study, direct economic cost of HB-related diseases was calculated based on the medical information of 124553 patients from 39 secondary and tertiary-level hospitals in Beijing. A previous study estimated the medical cost of HBV-related patients in community and hospital in Beijing,Citation52 and reported that the direct medical cost of CHB was CNY 5059.90, which was remarkably lower than results in our study (CNY 14,743.24 for CHB). It could be explained that patients receive treatment at community experienced less severe illness than those at secondary and tertiary hospitals. Moreover, the direct medical cost of HBV carrier in Li’s research was higher than that in our study (CNY 2114.98 versus CNY 761.95), which could be caused by that the cost generated from pharmacy medicine and private clinic treatment was not included in our study.Citation53
There were several limitations in our study. Firstly, only universal vaccination program was considered in this study, which might overestimate the proportion of vertical transmission from mothers to children. However, it might not necessary to inject one more dose of HBIG for all of children that born to HBsAg positive mothers. A study conducted in China presented that 3 doses of 10 μg HB-vaccine could sufficient for children that born to HBsAg positive and HBeAg negative mothers. Moreover, anti-HBs GMC induced by HBIG might result in a rapid growth in short-term, and it could even lower than those who only received Hep3 in 7 and 12 months after vaccination.Citation26 But it was necessary to monitor the level of maternal HBV DNA, because intrauterine infection might occur when the level of maternal HBV DNA was higher than 10Citation7 IU/ml.Citation26,Citation54 Secondly, this study was based on the assuming that the protective effect of vaccination would last for lifelong, while the protection of HBV vaccine would weaken by age.Citation55 In addition the herd immunity was ignored in our study for using a Markov model rather than a dynamic model. But it was considered to be sufficient for a high vaccine coverage. Finally, medical cost generated from pharmacy and privacy clinics was not included in our study, which might underestimate the direct medical cost of HB-related diseases especially in CHB and HBV carriers.
In conclusion, the current universal vaccination program in Beijing was highly ‘cost utility.’ It could improve the QALY of the whole population and contribute to less cost. This strategy could significantly reduce the heavy economic burden caused by HBV-related diseases in Beijing, so it was necessary for government to keep continuous investment funding in the future. Results in this study were robust and could provide a reliable evidence for further vaccine policy making.
While conducting this study, Yiwei Guo played a role in putting forward the idea as well as analyzing these data and drafting the first edition of this paper. Yong yang, Qian Bai, Zhengwei Huang, Zongwu Wang, Dongxia Cai and Shuo Li played a role in editing and reviewing the manuscript. Xiaowei Man played a large role in collecting these data, developing the idea for manuscript. Xuefeng Shi played an important role in developing the idea and revising the manuscript.
Disclosure of potential conflicts of interest
None of the authors has any conflicts of interest.
Additional information
Funding
References
- World Health Organization. (2017). Global hepatitis report 2017. World Health Organization.
- World Health Organization. Hepatitis B[EB/OL]. 2019 July 18. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
- World Health Organization. Hepatitis B[EB/OL].2015 May 15. http://www.wpro.who.int/china/topics/hepatitis/qa_20150515/en/
- Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Forouzanfour MH, Assadi R, Bhala N, Cowie B. The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet. 2016;388(10049):1081–88. doi:10.1016/S0140-6736(16)30579-7.
- Xiao-Jin S, Fu-Zhen W, Hui Z. Hbsag screening among pregnant women and neonatal immunization in six provinces of china,2014. Chinese Journal of Vaccines and Immunization; 2016.
- Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373(9663):582–92. doi:10.1016/S0140-6736(09)60207-5.
- Merican I, Guan R, Amarapuka D, Alexander MJ, Chutaputti A, Chien RN, Sjalfoellah Noer HM, Leung N, Lesmana L, Phiet P. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15(12):1356–61. doi:10.1046/j.1440-1746.2000.0150121356.x.
- World Health Organization, W. H.. 2015. Guidelines for the prevention, care and treatment of persons with chronic hepatitis b infection. World Health Organization.
- Monitoring and evaluation for viral hepatitis B and C: recommended indicators and framework. Geneva: World Health Organization; 2016. accessed May 2017. Available at http://apps.who.int/iris/bitstream/10665/204790/1/9789241510288_eng.pdf?ua=1
- Global Health Sector Strategy on viral hepatitis 2016–2021, towards ending viral hepatitis. Geneva: World Health Organization; 2016. [accessed April 2016]. http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf
- World Health Organization. Hepatitis B vaccines: WHO position paper, July 2017–recommendations. Vaccine. 2019;37(2):223–25. doi:10.1016/j.vaccine.2017.07.046.
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y. Evaluation of the impact of hepatitis B vaccination among children born during 1992–2005 in China. J Infect Dis. 2009;200(1):39–47. doi:10.1086/599332.
- Cui FQ, Gong XH, Chen YS. Evaluation on impact of hepatitis B vaccine integrated into routine immunization in the areas of Ministry of Health/Global Alliance for Vaccine and Immunization (GAVI) Cooperation Project PR China. Zhong Guo Yi Miao He Mian Yi. 2009;15:289–93.
- Liao XY, Zhou ZZ, Wei FB, Qin HN, Ling Y, Li RC, Zhuang H, Nong Y, Sun K-X, Li J. Seroprevalence of hepatitis B and immune response to hepatitis B vaccination in Chinese college students mainly from the rural areas of western China and born before HBV vaccination integrated into expanded program of immunization. Hum Vaccin Immunother. 2014;10(1):224–31. doi:10.4161/hv.26311.
- Gao P, Wang H, Chen WX, Sun YN, Wu J, Pang XH, He X, Wu J. A sero-epidemiological study of hepatitis b among general population in beijing. Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi. 2016;37(5):658–62. doi:10.3760/cma.j..0254-6450.2016.05.014.
- Ji-ye FU, Qi DING, Wei ZHANG, Ju-guang WANG, Min LIU. Efficacy of 10 μg recombinant hepatitis b vaccine in 128 infants 5 years after primary vaccination series in haidian district of beijing. Occup Health. 2017;33(3):366–69. doi:10.13329/j.cnki.zyyjk.2017.0107.
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y. Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27(47):6550–57. doi:10.1016/j.vaccine.2009.08.048.
- Wu J, Zhang W, Han LL, Lin CY, Ma LX, Xing Y-L, Gao P, Gong X-H, Liu L-R, Huang F. [a sero-epidemiologiecal study on hepatitis b among general population in beijing]. Zhonghua Liu Xing Bing Xue Za Zhi. 2007;28:555–57.
- Hung HF, Chen THH. Probabilistic cost-effectiveness analysis of the long-term effect of universal hepatitis B vaccination: an experience from Taiwan with high hepatitis B virus infection and Hepatitis B e Antigen positive prevalence. Vaccine. 2009;27(48):6770–76. doi:10.1016/j.vaccine.2009.08.082.
- Kim SY, Salomon JA, Goldie SJ. Economic evaluation of hepatitis B vaccination in low-income countries: using cost-effectiveness affordability curves. Bull World Health Organ. 2007;85(11):833–42. doi:10.2471/BLT.06.038893.
- Boccalini S, Taddei C, Ceccherini V, Bechini A, Levi M, Bartolozzi D, Bonanni P. Economic analysis of the first 20 years of universal hepatitis B vaccination program in Italy: an a posteriori evaluation and forecast of future benefits. Hum Vaccin Immunother. 2013;9(5):1119–28. doi:10.4161/hv.23827.
- Lu SQ, McGhee SM, Xie X, Cheng J, Fielding R. Economic evaluation of universal newborn hepatitis B vaccination in China. Vaccine. 2013;31(14):1864–69. doi:10.1016/j.vaccine.2013.01.020.
- Yin J, Ji Z, Liang P, Wu Q, Cui F, Wang F, Liang X, Zhuang G. The doses of 10 μg should replace the doses of 5 μg in newborn hepatitis B vaccination in China: A cost-effectiveness analysis. Vaccine. 2015;33(31):3731–38. doi:10.1016/j.vaccine.2015.05.082.
- Beijing Municipal Bureau of Statistic NBS Survey Office in Beijing. Beijing statistical yearbook. [EB/OL]. [accessed 2019 July 16]. http://nj.tjj.beijing.gov.cn/nj/main/2017-tjnj/zk/indexeh.htm
- Beijing Municipal Health Commission Information Center. 1979-2018 average life expectancy of Beijing population [EB/OL]. [accessed 2019 April 23]. http://www.phic.org.cn/tjsj/wssjzy/jkzb/201304/t20130425_255009.html
- Lu Y, Liang XF, Wang FZ, Yan L, Li RC, Li YP, et al. Hepatitis b vaccine alone may be enough for preventing hepatitis b virus transmission in neonates of hbsag (+)/hbeag (?) mothers. Vaccine 2016; S0264410X16311239.
- Fu-Zhen W, Xiao-Hong G, Li-Rong L. Analysis on infection of hepatitis b virus among pregnant women in beijing. Chinese Journal of Vaccines & Immunization; 2007.
- Lei Z, Xi-en G, Caroline T, et al. Effects of hepatitis b immunization on prevention of mother-to-infant transmission of hepatitis b virus and on the immune response of infants towards hepatitis b vaccine. Vaccine. 2014.
- Wang F, Zhang G, Zheng H, Miao N, Shen L, Wang F, Dong P, Du F, Chen C, Zhang X, et al. Post-vaccination serologic testing of infants born to hepatitis b surface antigen positive mothers in 4 provinces of china. Vaccine. 2017;35(33):4229–35. doi:10.1016/j.vaccine.2017.06.019.
- Shu-ying FENG. Observation on immune effect of hepatitis b vaccine in infants born to hbsag positive mothers. Journal of Shanxi Medical University; 2011.
- Zeng Y, Luo M, Lin J, He H, Deng X, Xie S, Fang Y. Cost-effectiveness of augmenting universal hepatitis B vaccination with immunoglobulin treatment: a case study in Zhejiang Province, East China. Hum Vaccin Immunother. 2019;16(4):955–64. doi:10.1080/21645515.2019.1688031
- Xiao M, Wang H, Zhang W, Pang XH, Qiu Q, Wang YH. Cost-effectiveness analysis of hepatitis B vaccination strategy for high risk adults in Beijing. Chin J Dis Control Prev. 2015;19:730–34.
- Yang PC. Development of markov models for hepatitisB vaccination or treatment and cost-effectiveness analysis of pro-phylactic entecavir use for population based-on community in China. Guangdong Pharmaceutical University; 2016.
- Jing-Jing L. Economic evaluation of hepatitis B immunization strategy among newborns in Shandong. Shandong University; 2013.
- Ni YH. Natural history of hepatitis B virus infection: pediatric perspective. J Gastroenterol. 2011;46(1):1–8. doi:10.1007/s00535-010-0304-7.
- Tilson L, Thornton L, O’Flanagan D, Johnson H, Barry M. Cost effectiveness of hepatitis B vaccination strategies in Ireland: an economic evaluation. Eur J Public Health. 2007;18(3):275–82. doi:10.1093/eurpub/ckm123.
- Chu CM, Liaw YF, Yang CY, Sheen IS. Diagnosis of acute type B hepatitis by a solid phase u‐antibody capture radioimmunoassay for IgM class antibody to hepatitis B core antigen: a diagnostic proposal based on a prospective study. Liver. 1987;7(3):182–87. doi:10.1111/j.1600-0676.1987.tb00340.x.
- Liu J, Yang H, Lee M, Lu S, Jen C, Wang L, You S, Iloeje UH, Chen C. Incidence and determinants of spontaneous hepatitis b surface antigen seroclearance: a community-based follow-up study. Gastroenterology. 2010;139(2):474–82. doi:10.1053/j.gastro.2010.04.048.
- GONG X, WANG F, LI H. Investigation on transformation of 197 HBsAg carriers. Chin J Public Health. 2006;2006:58.
- Jia Y, Li L, Cui F, Zhang D, Zhang G, Wang F, Gong X, Zheng H, Wu Z, Miao N. Cost-effectiveness analysis of a hepatitis B vaccination catch-up program among children in Shandong Province, China. Hum Vaccin Immunother. 2014;10(10):2983–91. doi:10.4161/hv.29944.
- Nguyen VTT, Law MG, Dore GJ. Hepatitis B‐related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat. 2009;16(7):453–63. doi:10.1111/j.1365-2893.2009.01117.x.
- Beijing 1% population sample survey in 2015. 2017. Office for Beijing 1% population sample survey, Beijing Municipal Bureau of Statistics. China Statistics Press.
- Dan W, Qian B, Shi-Wen S, Jiang WU, Min L, Yi-Wei G, et al. Survey on the overall cost of hepatitis b vaccination in beijing in 1992-2013. Chinese Preventive Medicine; 2019.
- Hutton DW, So SK, Brandeau ML. Cost‐effectiveness of nationwide hepatitis B catch‐up vaccination among children and adolescents in China. Hepatology. 2010;51(2):405–14. doi:10.1002/hep.23310.
- Jian Z, Economics SO, University NN. A study of the impacts of rmb exchange rate volatility adjustment on rmb exchange rate volatility mechanism. Review of Economy and Management; 2018.
- Hepatitis B vaccines: WHO position paper-recommendations. Vaccine. 2010;28(3):589–90. PMID: 19896455. doi:10.1111/j.1524-4733.2010.00733.x.
- Xuan Z, Yan J, Xiao-Wei M, Li-Ying Z, Qi-Xin X, Wei C. Recurrent health expenses in primary healthcare institutions in beijing. Chin Gen Pract. 2018;21:2209–15.
- Xu L, Wang Y, Collins CD, Tang S. Urban health insurance reform and coverage in China using data from national health services surveys in 1998 and 2003. BMC Health Serv Res. 2007;7(1):37. PMID:17335584. doi:10.1186/1472-6963-7-37.
- Statistical bulletin of Beijing’s national economic and social development in 2016. (EB/OL). [2017 Feb 25]. http://www.beijing.gov.cn/gongkai/shuju/tjgb/201706/t20170608_1838189.html
- Lee D, Park S M. Cost-Effectiveness Analysis of Hepatitis B Vaccination Strategies to Prevent Perinatal Transmission in North Korea: Selective Vaccination vs. Universal Vaccination[J]. Plos One, 2016, 11(11):e0165879.
- Chen C. Zhuang Hui: epidemiological status of chronic hepatitis B in China. Liver Doctor. 2018;000:8–9.
- Qiao FY, Wu M. A study on direct economic burden of diseases related to hepatitis B viral infection in Xicheng district of Beijing. Capital J Public Health. 2011;5:247–50.
- Yan LI, Huai W, Wei Z, Yan-Hong W, Zhong-Ping D, Hui LI, et al. Investigation on the cost of patients with different outcomes related to hepatitis b virus infection in Beijing. basic & clinical medicine; 2014.
- Wang Z, Zhang H, Yang H, Li X, Wen S, Guo Y, Sun J, Hou J. Quantitative analysis of HBV DNA level and HBeAg positive mothers and their babies: hBsAg passage through the placenta and rate of decay in babies. J Med Virol. 2003;71(3):360–66. doi:10.1002/jmv.10493.
- Guo Y, Gao P, Wang H, Wu J, Bai Q, Huang L, Shi X. Risk factors of hepatitis B virus infection between vaccinated and unvaccinated groups among spouses in 2006 and 2014: a cross-sectional study in Beijing. Hum Vaccin Immunother. 2019;1–10.
Appendix
Table A1. Characteristics of socio-demographic variables of HB-related patients in 2016
Figure A1. Selective standard of out/inpatients from beijing health and medical price monitoring data platform
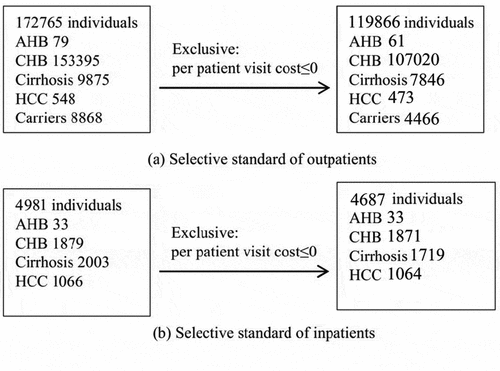
Table A2. Death rate data of 1% of population in 2015 in Beijing
Table A3. Direct treatment cost in 2016 in Beijing (CNY)