ABSTRACT
Brazil currently has a 10-valent pneumococcal conjugate vaccine (PCV10) pediatric national immunization program (NIP). However, in recent years, there has been significant progressive increases in pneumococcal disease attributed to serotypes 3, 6A, and 19A, which are covered by the 13-valent PCV (PCV13). We sought to evaluate the cost-effectiveness and budget impact of switching from PCV10 to PCV13 for Brazilian infants from a payer perspective. A decision-analytic model was adapted to evaluate the clinical and economic outcomes of continuing PCV10 or switching to PCV13. The analysis estimated future costs ($BRL), quality-adjusted life-years (QALYs), and health outcomes for PCV10 and PCV13 over 5 y. Input parameters were from published sources. Future serotype dynamics were predicted using Brazilian and global historical trends. Over 5 y, PCV13 could prevent 12,342 bacteremia, 15,330 meningitis, 170,191 hospitalized pneumonia, and 25,872 otitis media cases, avert 13,709 pneumococcal disease deaths, gain 20,317 QALYs, and save 172 million direct costs compared with PCV10. The use of PCV13 in the Brazilian NIP could reduce pneumococcal disease, improve population health, and save substantial health-care costs. Results are reliable even when considering uncertainty for possible serotype dynamics with different underlying assumptions.
Introduction
Pneumococcal diseases are caused by the bacterium Streptococcus pneumoniae, which has more than 90 immunologically distinct serotypes and is one of the most significant causes of morbidity and mortality worldwide.Citation1 Noninvasive pneumococcal diseases include otitis media (OM), sinusitis, and community-acquired pneumonia (CAP) and are highly prevalent illnesses in children under 5, adults over 65, as well as immunocompromised individuals. Invasive pneumococcal disease (IPD), such as meningitis, bacteremia, and sepsis, is the most severe presentations and can lead to death.Citation2
Vaccination is the most effective strategy for the prevention of pneumococcal diseases. Pneumococcal conjugate vaccines (PCVs) are consistently managed by pediatric National Immunization Programs (NIPs) and have reduced the pneumococcal disease burden significantly since their introduction.Citation2-4 In Brazil’s private sector, the 7-valent (PCV7) became available to children under 5 y of age at high-risk of pneumococcal diseases during 2002–2010. The 10-valent (PCV10) was introduced universally for all children 0–2 y of age as a 3 + 1 schedule in 2010 and later changed to 2 + 1 schedule in 2016.Citation5 In 2019, PCV13 was approved in Brazil’s NIP exclusively for patients over 5 y of age with high-risk conditions.
Recently in Brazil, there have been significant progressive increases in 3 and 19A serotype IPD.Citation2,Citation6 In 2018, approximately 40% of all registered IPD cases in children under 5 y were caused by serotype 19A and 52.3% were attributed to serotype 3, 6A, and 19A combined.Citation5 The editorial report from the Board of Directors of the Latin American Pediatric Association (ALAPE) revealed Latin American countries incorporating PCV10 in pediatric NIPs (Brazil, Chile, Paraguay, Peru, and Colombia) have subsequently experienced increases in serotype 19A.Citation7 Moreover, a recent World Health Organization (WHO) position regarding PCVs in 2019 advises that the choice of the appropriate vaccine in a country should be based on, among other factors, local and regional prevalence of serotypes and antimicrobial resistance patterns.Citation8
Although several studies have reported efficacy and immunogenicity of PCV10 to serotype 19A,Citation1,Citation9,Citation10 epidemiologic surveillance from countries that use PCV10 universally shows increases of 3 and 19A serotype IPD, including cases in the vaccinated population.Citation11-15 In contrast, real-world effectiveness data have shown that PCV13 NIPs have reduced and stabilized the incidence of serotype 3, 6A, and 19A serotype disease cases.Citation16-21
PCV NIPs require a significant amount of government resources to vaccinate large birth cohorts. Nevertheless, population disease trends, serotype replacement, herd effects, antimicrobial resistance, health-care system budget impact, and cost-effectiveness are all important factors that decision-makers need to factor in when choosing a PCV for pediatric NIPs. A cost-effectiveness analysis (CEA) quantifies the costs and health gains for a given population and compares alternatives. These analyses are often used as tools for prioritizing the allocation of resources to interventions which have the greatest gain in health for the least amount of resources.
There are several published studies evaluating the CEA of universal pediatric PCV NIPs in Brazil. One CEA study from 2006 found that a hypothetical universal PCV7 NIP for children aged <1 y compared with no vaccination program was cost-effective.Citation22 Another study assessed the historical cost-effectiveness of pre-vaccine (2006–2009) versus post-vaccine (2001–2014) era in one Brazilian state, Santa Catarina, and concluded that inclusion of PCV10 in the NIP had been cost-effective from the Brazilian federal government perspective.Citation23 Santori et al.Citation24 also concluded that PCV10 was cost-effective compared with no program; however, there was uncertainty in the estimated result due to quality and availability of data and long-term vaccine effects at the time of analysis.
Few studies report on the cost-effectiveness directly comparing PCV10 versus PCV13. One study assessed PCV10 compared with PCV13 over a 1-y time horizon and concluded that switching PCVs in the Brazilian infant NIP would not be cost saving.Citation25 However, the analysis did not consider life-years or quality-adjusted life-years (QALYs); assumed cross-protection against 19A for PCV10, although in recent years this has not been observed in Brazil; excluded serotype replacement in the analysis, which is regularly observed with PCV use; and did not include indirect effects in non-vaccinated individuals and the adult population. Outside the context of the Brazilian health-care system, there are several analyses from other countries that consider these factors when assessing the cost-effectiveness of PCV10 versus PCV13. Pugh et al. (2020) evaluated the clinical and economic benefit of replacing PCV10 with PCV13 in Colombia, Finland, and the Netherlands and determined that a PCV13 program would save costs and prevent more disease in Colombia and Finland, as well as be cost-effective in the Netherlands at 1 x GDP per capita (€34,054/QALY), compared with PCV10.Citation26 Another publication using a similar modeling technique estimated that PCV7 and PCV13 are estimated to have saved 1,840 lives due to pneumococcal disease in Mexico over an 8-y period.Citation27 Switching to PCV10 was estimated to result in 311,259 more cases of pneumococcal disease, 410 associated deaths, and cost over 6.7 USD billion MXN over a 10-y period compared to maintaining PCV13. In Malaysia, a country that has yet to adopt a PCV into its NIP, PCV13 compared with PCV10 was projected to avert an additional 190,628 cases of pneumococcal disease and 1126 deaths, gain 2,280 QALYs, with higher cost-saving potential.Citation28 Other examples are reported for Canada, demonstrating that switching to PCV10 from PCV13 was estimated to result in higher net costs due to increases in disease caused by uncovered serotypes;Citation29 as well as Italy, which concluded that Switching from PCV13 to PCV10 would increase the incidence of pneumococcal disease primarily linked to reemergence of serotypes 3 and 19A.Citation30 Therefore, the aim of this study was to build upon previous cost-effectiveness analyses conducted in Brazil and to incorporate other critical evaluation factors, such as life-year and QALY outcomes, historical local serotype behavior, current epidemiology, serotype replacement, and herd effects. We evaluated the cost-effectiveness and budget impact of replacing PCV10 with PCV13 over a 5-y period in Brazil for vaccination of children up to 2 y of age after the implementation of a universal PCV10 program.
Methods
Model structure and assumptions
The incremental cost per QALY gained was calculated using a decision-analytic model to compare the cost-effectiveness of switching from a PCV10 NIP to a PCV13 NIP from a federal government perspective. The model structure has been described in detail elsewhere.Citation26,Citation27,Citation30 Briefly, the model uses retrospective country-level observed surveillance data on serotype-specific pneumococcal disease to estimate prospective serotype disease behavior. Therefore, for vaccine serotypes contained in both PCV10 and PCV13, as well as non-vaccine serotypes, the model predicts future disease behavior from retrospective real-world data in the population dependent on which vaccine is being evaluated. Given the observed historical serotype dynamics, the model calculates future IPD, pneumococcal pneumonia, and pneumococcal OM cases over a 5-y time horizon for the following scenarios: (1) maintaining PCV10 in the Brazilian NIP and (2) switching to PCV13 in the Brazilian NIP. We estimated the number of cases of IPD (meningitis and bacteremia), pneumococcal hospitalized pneumonia, pneumococcal OM, meningitis disease sequelae, deaths, direct health-care costs related to vaccine acquisition and disease burden, life-years gained, and QALYs gained, for both PCV13 and PCV10 over 5 y.
Epidemiology data
Population statistics were taken from the Instituto Brasileiro de Geografia e Estatística (IBGE) for years 2009 to 2018 and were stratified into 7 age groups, 0–<2 y, 2–4 y, 5–17 y, 18–34 y, 35–49 y, 50–64 y, and 65+ y (). Vaccine coverage was assumed to be 95% of infants using a 3-dose (2 + 1) vaccination schedule. The percent of infants vaccinated was tested in sensitivity analyses.
Table 1. Population, clinical, and economic parameters
Invasive pneumococcal disease
To estimate historical trends, serotype‐specific IPD distribution data and the proportion of IPD causing meningitis for all age groups were based on Sistema Regional de Vacunas (SIREVA) II, which is an international prospective surveillance program organized by the WHO, with the purpose of monitoring the epidemiology of bacterial pneumonia and meningitis.Citation31 The reference center in Brazil is the Núcleo de Meningites, Pneumonias e Infecções (NMPI) of the Bacteriology Center of Instituto Adolfo Lutz (IAL). It is worth noting that this is a passive, laboratory-based surveillance system, which captures the IPD serotype distribution in Brazil but contributes to a limited estimation of IPD incidence rates. Therefore, incidence data were obtained from the Colombian Individual Registration of Health Services (RIPS) database, a health benefit information system from all health maintenance organizations, which provides a good estimation of overall IPD rates,Citation26 and the data have been published elsewhere.Citation26 Colombia data were chosen since the countries share population and health-care assistance similarities and had first introduced a PCV10 program in 2010. Moreover, the Colombia surveillance system also showed an increase in non-PCV10 serotypes in recent years, mainly due to serotype 19A, which is mirrored in Brazil; therefore, we assume the incidence trends should be somewhat comparable between the two settings.Citation15 For the base case, the age group-specific serotype distributions in Brazil were applied to the overall incidence rates in Colombia by age-group.
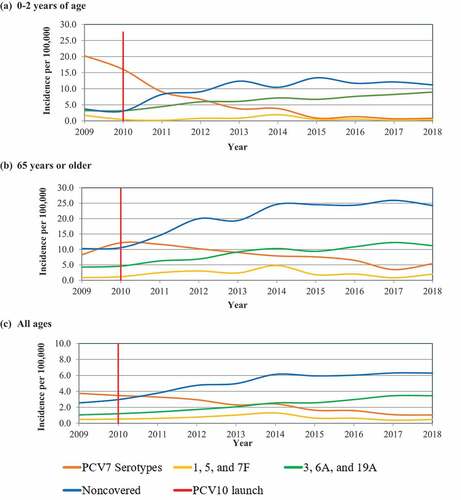
The modeled historical estimates for incidence of IPD among children 0–2 y, 65 y and older, and for all ages combined are shown in . Epidemiologic data are presented specifically for children 0–2 y and those 65 or older, since these populations are the most susceptible to pneumococcal disease and form a large proportion of the total disease burden. In the base case and start of the model (2018), 42% of disease in children 0–2 y of age and 26% for 65 y or older was caused by serotypes 3, 6A, and 19A, respectively. Given the uncertainty in the true incidence of IPD in Brazil, these data were tested in scenario analyses.
Figure 1. Base case historical invasive pneumococcal disease incidence (IPD) per 100,000 in infants 0–2 y of age in (a) 0–2 y of age, (b) 65 y or older, and (c) all ages. Data presented represent the launch of 10-valent pneumococcal conjugate vaccine (PCV10) and the historical serotype trends contained in 7-valent pneumococcal conjugate vaccine (PCV7); 1,5, and 7 F serotypes; 3, 6A, and 19A serotypes; and non-covered vaccine serotypes. Real-world data are based on the serotypes causing IPD reported annually in Brazil and incidence reported in Colombia
Noninvasive pneumococcal disease
The historical retrospective incidence of general OM from 2009 to 2014 was obtained from Santori et al.Citation24 for children 0–2 y of age. Santori et al.Citation24 included medical outpatient visits in children aged 2 to 23 months, from August 2008 through July 2015 in Goiania municipality, Brazil. The model and analysis used a conservative approach, and only included children in this age group for OM incidence rates. The incidence from 2014 to 2018 was assumed to be stable in Brazil in OM disease using data from 2014, with an annual incidence of 1,573 per 100,000 for all-cause OM. It was assumed that 20% of all-cause outpatient OM was caused by Streptococcus pneumoniae and was assumed to change proportionally to the changes in serotypes causing IPD. The percent of all-cause outpatient OM due to Streptococcus pneumoniae was tested in sensitivity analyses.
For all-cause hospitalized pneumonia incidence, data were obtained from Andrade et al.Citation32 from the years 2005 to 2015. Data reported by Andrade et al.Citation32 were representative of the full Brazilian population taken from the Unified Health System (SUS) in the National Hospitalization Information Database (SIH) stratified by age groups. This cost-effective analysis only included inpatient pneumonia cases in the calculations. Hospitalized incidence data were assumed constant from 2016 to 2018, using 2015 annual all-cause hospitalized pneumonia incidence. The same assumption was taken for inpatient pneumonia comparable to OM, that approximately 20% of all-cause inpatient pneumonia cases were caused by Streptococcus pneumoniae and assumed to change proportionally to IPD. Given the uncertainty in this percentage, it was varied in the sensitivity analyses.
Mortality and sequelae
Age-specific all-cause mortality rates per 100,000 for each age group were taken from IBGE 2018 data.Citation30 Age-specific case-fatality rates (CFR) for bacteremia, meningitis, and hospitalized pneumonia were taken from the most recent year of Novaes et al.Citation33 which calculated CFR from the number of deaths/confirmed cases for each clinical syndrome registered in the SIH-SUS. The analysis used CFR from SIH-SUS for hospitalized pneumonia and meningitis (). It was assumed that bacteremia had the same CFR as meningitis. Two long-term sequelae associated with meningitis were included in the model, which were neuromotor disorders and hearing loss, and probabilities for both were taken from a cost-effectiveness analysis of PCVs in Brazil, 0.17 and 0.13, respectively.Citation22 Base case CFRs and long-term sequelae assumptions were tested in scenario analyses.
Costs data
Direct costs were taken from publicly available sources and were inflated to 2019 Brazilian Reals (BRL). The direct costs for bacteremia, meningitis, OM, and hospitalized pneumonia were taken from SIH/SUS, calculated by taken the total cost value to the health-care system and dividing it by the number of hospitalizations recorded in 2017.Citation34 The calculation was performed for each disease state and age group (). The cost of OM was calculated using the same method as IPD and pneumonia and taken from SIH/SUH, but only for the 0–2 y age group, which was estimated to be 312 USD BRL per episode.Citation34 Cost for the two-long term sequelae due to meningitis was taken from a cost-effectiveness analysis of PCVs in Brazil, 16,200 USD for a lifetime neurologic disorder and 14,464 USD for lifetime hearing loss.Citation22
The last vaccine prices practiced by the Ministry of Health and published on the Federal Official Gazette were considered, 57.22 USD and 58.94 USD per dose for PCV10 and PCV13, respectively.Citation35,Citation36 The cost of immunization per child was based on the price per dose in a 2 + 1 schedule, as per guidance on the respective vaccine labels. All administration costs were assumed equal across vaccines.
Utility and decrements data
To calculate QALYs, individuals who did not experience a disease event had a baseline utility that was age-specific taken from a healthy population, shown in .Citation37 A utility decrement was applied for each case of disease experienced annually. Decrements of 0.0070 and 0.0232 were assumed for bacteremia and meningitis, respectively.Citation38 Decrements of 0.0050 and 0.0060 were assumed for OM and hospitalized pneumonia, respectively.Citation39 Long-term sequelae involving neurologic impairment and hearing loss following a case meningitis carried a lifetime utility decrement of 0.40 and 0.20.Citation40,Citation41 Long-term sequelae input parameters were tested by excluding them in a scenario analysis, thereby omitting their associated negative utility decrements.
Analysis
Base case analysis
Clinical outcomes, direct costs, and QALYs were estimated assuming PCV10 was maintained in the NIP or where PCV10 was replaced with PCV13. In a switch from PCV10 to PCV13, disease trends began to change upon introduction of the PCV13 program. Vaccine and non-vaccine serotypes were separately modeled by age group and based on historical surveillance data. The PCV13 switch scenario was predicted using the United States (US) historical data in the base case, and additional scenarios were tested using surveillance from other countries. For PCV10, the base case analysis used the serotype distribution from Brazil and applied this to the incidence of IPD in Colombia. Sensitivity analyses were run using PCV10 NIP country case counts of IPD from Brazil and Chile.
An annual 5% discount rate was chosen based on Brazil’s National Committee for Health Technology Incorporation (CONITEC) guidelines for economic evaluationCitation42 and was applied to costs and effectiveness outcomes beyond the first year. The analysis was from the Brazilian federal government perspective over a 5-y time horizon.
Scenario analyses
Scenario analyses were performed to assess uncertainty in Brazil IPD incidence. For historical data and subsequent projections, the Colombia IPD data were tested in sensitivity analysis using Chilean IPD case data given that PCV10 was implemented in the NIP over multiple years. The incidence data for Chile were taken from Chilean laboratory surveillance system for IPD from 2011 to 2016.Citation14 This scenario analysis assumed a stable incidence rate from 2016 to 2018, using the 2016 IPD incidence rate. Moreover, a scenario analysis was run using Brazilian IPD case data reported by SIREVA II from 2009 to 2018.Citation2
Separate scenario analyses were run for PCV13 future serotype dynamic projections. We conducted scenario analyses where serotype behavior was predicted using the United Kingdom (UK), Canada, and Quebec historical trends, rather than US trend data. In order to test the influence of including sequelae due to meningitis in the model, we conducted a scenario omitting the probability of incurring lifetime hearing loss and neurologic disorders, and thus omitting their associated incurred costs and negative utility weights. Vaccination coverage was tested by assuming only 89% of infants in Brazil were vaccinated starting in 2019 and projecting forward. The percentage of all-cause pneumonia and all-cause OM due to Streptococcus pneumoniae was conservatively assumed to be 10% for both parameters in scenario analyses. Finally, case-fatality rates were changed to assess the impact on results using case-fatality rate data from a cost-effectiveness analysis of PCV10 compared with PCV13 in Mexico.Citation27
To assess the cost-effectiveness over a different time horizon, the model was run using a 10-y time horizon. Moreover, a scenario analysis was run to assess the cost-effectiveness using a 1% discount rate applied to future costs and outcomes.
Results
The public health and economic impact of PCV13 use in the Brazilian NIP for all children was predicted over 5 y from a direct health-care government perspective.
Base case results
Epidemiologic results
Base case results for IPD are presented in . Considering that 43% of IPD was caused by 19A, 6A, and 3 serotypes in children 0–2 y of age in 2018, we estimated that this absolute percentage could increase to 48% by 2023 if maintaining a PCV10 NIP, whereas by implementing PCV13 this percentage could decrease the absolute percentage to 24% of IPD ()). Based on epidemiologic assumptions, IPD incidence could decrease from 21 per 100,000 to 15 per 100,000, a 26% relative decrease, if PCV13 is implemented. However, if PCV10 is continued universally, IPD incidence could have a relative increase by 19% with an overall incidence of 25 per 100,000 in children 0–2 y of age.
Figure 2. Base case invasive pneumococcal disease (IPD) serotype distribution at time of decision to switch and forecasted at 5 y with implementing either PCV10 or PCV13 on the Brazilian NIP in (a) 0 to 2 y of age, (b) 65 y of age or older, and (c) all ages. “At time of decision” indicates the “current state of pneumococcal disease considering the switch to the PCV13 strategy”
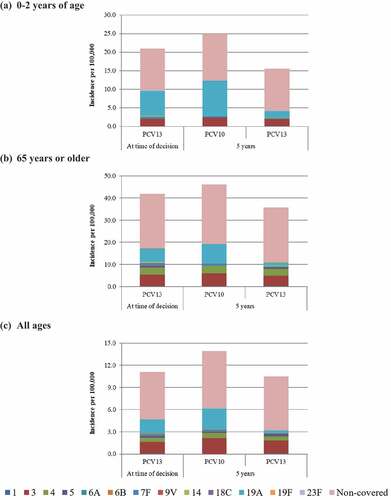
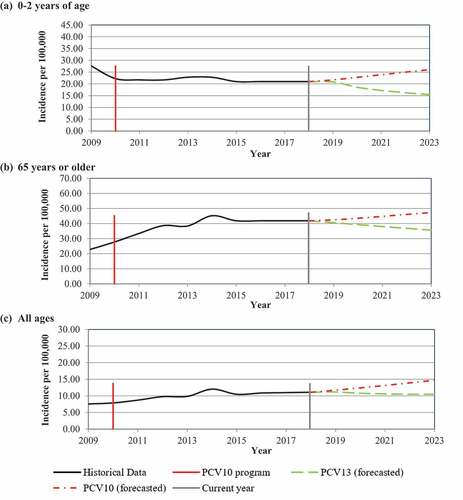
Figure 3. Base case predicted invasive pneumococcal disease incidence (IPD) based on observed real-world data per 100,000 in (a) 0–2 y of age, (b) 65 y or older, and (c) all ages. Data presented represent Historical Data for overall IPD incidence, the year 10-valent pneumococcal conjugate vaccine (PCV10) program was implement in Brazil, the current year reflecting the year the choice was made between maintaining PCV10 on the NIP or switching to PCV13, and predicted IPD for PCV10 (forecasted) and PCV13 (forecasted) depending on the choice made in the current year
For adults aged 65 or older, approximately 27% of all IPD was caused by the three additional serotypes in PCV13 in 2018 at the start of the analysis. The use of PCV13 was estimated to decrease the contribution of these serotypes to 17% of all IPD after 5 y ()). However, continuing PCV10 use universally in infants for 5 y was predicted to increase 19A, 6A, and 3 IPD in adults 65 or older, with these serotypes causing 31% of all IPD. Thus, adults over 65 could see a relative decrease in IPD by 15% with a corresponding incidence of 36 per 100,000 if PCV13 replaced PCV10 in the pediatric NIP. However, this population could also see a relative increase in IPD by 10% after 5 y if PCV10 is maintained.
In general, serotypes not covered by PCV13 were predicted to cause substantial IPD burden for all age groups and this estimated burden was similar across vaccines. In the full population comprising all ages, the burden of 19A was a key driver of IPD ()). The use of PCV13 was estimated to reduce 19A disease to 3% of all IPD after 5 y, whereas maintaining PCV10, 19A disease could increase to 20% of all IPD in the full population.
Cost-effectiveness results
Results for the base case are shown in . Using PCV13, we estimated 24,934 life-years gained and 20,317 QALYs gained over 5 y compared with PCV10. PCV13 was estimated to prevent an additional 12,342 bacteremia cases, 15,330 meningitis cases, 25,872 otitis media cases, and 170,191 hospitalized pneumonia cases over a 5-y period compared with PCV10. It was estimated that 6,979 IPD and 6,730 hospitalized pneumonia deaths could be avoided by a switch from PCV10 to PCV13. Moreover, the use of PCV13 in Brazil was estimated to be a cost-saving strategy compared with maintaining PCV10. Despite higher vaccine-related costs associated with PCV13, switching to PCV13 was estimated to save the Brazilian government approximately 172 USD million BRL over 5 y due to direct costs avoided by prevention of substantial pneumococcal disease burden.
Table 2. Base case results on incremental cost, outcomes, and cost-effectiveness of PCV13 compared with PCV10 Brazil national immunization program over a 5‐year time horizon
Scenario analyses results
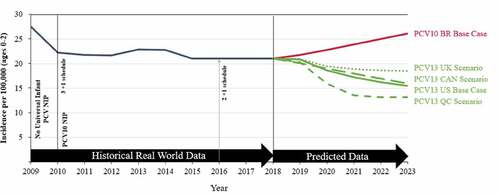
Three alternative scenarios were used to predict PCV13 IPD serotype dynamics based on the UK, Canada, and Quebec historical trends (). Each scenario’s trendline predictions mirrored the US base case, showing a range of predictions, all with decreasing IPD incidence, mainly due to decreases in 19A, 6A, and 3 serotype IPD.
Figure 4. Scenario analyses showing the predicted invasive pneumococcal disease incidence based on observed real-world data per 100,000 in children 0–2 y of age in Brazil. Scenario analyses were run where 13-valent pneumococcal conjugate vaccine (PCV13) serotype dynamics were predicted using the United Kingdom (UK), Canada (CAN), and Quebec (QC) historical trends. For reference, the base case serotype dynamics are presented for PCV13 using historical United States (US) trends and 10-valent pneumococcal conjugate vaccine (PCV10) using historical Brazil (BR) trends. Implementation of PCV10 on the Brazilian national immunization program (NIP) and schedule changes are shown
Overall cost-effective results and cost savings for the use of PCV13 compared with PCV10 did not change when altering major assumptions in the base case. PCV13 remained cost saving compared with PCV10 across several scenario analyses ().
Table 3. Scenario analyses results for total direct costs, total quality-adjusted life-years gained, incremental difference between PCV13 and PCV10, and the cost-effectiveness of PCV13 compared with PCV10 Brazil national immunization program
The scenarios included varying the trend line estimates used to forecast vaccine impact given experiences in different countries, changing the incidence of IPD using both Brazil and Chile case data from passive surveillance reports, altering the time horizon to 10 y, lowering the percentage of all-cause pneumonia and all-cause OM due to Streptococcus pneumoniae to 10%, omitting long-term sequelae from the analysis, changing the vaccination coverage to 85%, using Wasserman et al.Citation27 CFRs instead of Novaes et al.Citation33 and changing the discount rate to 1% for all future costs and outcomes.
Discussion
The goal of this study was to conduct a cost-effectiveness analysis using a decision‐analytic model and real‐world data to predict the potential public health and economic impact of switching from PCV10 to PCV13 in the Brazilian NIP. This study will help enrich the cost-effectiveness literature regarding PCVs in Brazil and is the first to estimate the cost-effectiveness of switching vaccination programs from PCV10 to PCV13 for the full population over 5 y. The analysis showed that even with its higher acquisition cost, PCV13 could ultimately have a greater public health impact by preventing more pneumococcal disease.
We demonstrated that the use of PCV13 could decrease pneumococcal disease primarily due to serotypes 3, 6A, and 19A in those aged 0–2 and those 65 y or older, given that a large burden of the remaining disease in Brazil are caused by these serotypes. Moreover, serotype 19A is associated with antimicrobial resistance, the need for longer treatment, and thus the use of more health-care resources.Citation43 This expansion of multidrug resistance among invasive 19A strains after PCV10 introduction has been observed in Brazil.Citation6 While this cost-effectiveness analysis did not incorporate data on vaccine prevention for antimicrobial resistance, antimicrobial resistance is another important aspect to consider when choosing a PCV for a NIP locally, according to a recent WHO publication.Citation8
This analysis was tailored to the Brazilian population by using data on the complex nature of real-world historical PCV use (serotype distribution, disease trends, vaccine type, herd effects, uptake, etc.) to predict future disease behavior. A strength of this study was testing the serotype dynamics by using trendlines from multiple countries to predict how PCV13 could impact pneumococcal disease. Under these various scenarios using the US, UK, Canada, and Quebec serotype dynamics, the use of PCV13 followed similar serotype trends and was shown to be cost saving compared with maintaining PCV10. Additionally, by using real-world effectiveness data to predict future IPD, the framework inherently incorporates IPD indirect herd effects in projections, whereas most other cost-effectiveness studies may miscalculate these effects and/or serotype replacement by only using point estimates of vaccine efficacy.Citation44
Vaccine serotype coverage was the main driver of the clinical and economic results in this analysis. The analysis demonstrated that estimated rates of total IPD, pneumococcal pneumonia, and pneumococcal OM could be further reduced in children and adults if PCV13 is included in the NIP, shedding light on the added public health impact that higher-valent vaccines provide to the population directly and indirectly. Higher valent-vaccines may soon be available, a 15-valent (PCV15, PCV13 serotypes + 22 F and 33 F) and a 20-valent (PCV20, PCV15 serotypes + 15B/C, 12 F, 11A, 10A, and 8),Citation45,Citation46 which contain several of the new emerging serotypes causing IPD globally.Citation47-49 These higher-valent PCVs that cover additional serotypes in their formulations may add even more clinical and economic benefit by preventing more pneumococcal disease burden compared with lower-valent PCV alternatives. Thus, considerable increases in serotype protection may be obtained with the highest-valent PCV, which can ultimately drive cost-effectiveness analysis results as shown in this analysis.
Like all cost-effectiveness analyses, there are some limitations mainly related to issues with the quality and underreporting of incidence rates, as many countries in Latin America have passive laboratory-based surveillance systems. For example, we applied the serotype distribution derived from Brazil IPD isolates of Streptococcus pneumoniae serotypes reported to Colombia’s incidence rate. However, we tested the incidence data in scenario analyses using both Brazil and Chile IPD isolate case data. Results showed that the use of PCV13 would remain cost saving compared with maintaining PCV10 in the Brazilian NIP, even when using other assumptions for IPD incidence rates. For all-cause OM, data were taken from one municipality, Goiania. However, Santori et al. (2017) used the electronic Outpatient Visit Information System (OVIS), in which all outpatient visits occurring in SUS facilities in Goiania are recorded in real time, and likely reflect an accurate capture of all-cause OM reflective of the Brazilian population.Citation24 This study did not examine schedule adherence (two primary infant doses and one booster), something that is variable across Brazil. Without strong adherence, there can be resurgent outbreaks of the disease which subsequently weakens herd immunity. Therefore, in this analysis, uncertainty remains on how much pneumococcal burden could be influenced by adherence to the schedule and how adherence to the priming doses compared with the booster dose influences resurgence.
In conclusion, this cost-effectiveness analysis showed the use of PCV13 in the Brazilian NIP could reduce pneumococcal disease rates and improve population health. Additionally, the Brazilian government could save substantial health-care costs due to greater disease cases averted. We demonstrated that the results are consistent even when considering uncertainty for other possible serotype dynamics with different underlying assumptions.
Disclosure of potential conflicts of interest
This work was supported by Pfizer Inc. and participated in model development, study design, data collection, analysis, interpretation of results, writing the manuscript, and in the decision to submit the article for publication. All authors are employees of Pfizer Inc. and may hold stock or stock options and contributed to the interpretation of the results, writing of the manuscript, and approved the final version of the manuscript.
Acknowledgments
RTI Health Solutions and employees, Michele Wilson, Cheryl McDade, and Aaron Lucas, are acknowledged for their role in the model development.
References
- Brandileone M-C-C, Almeida SCG, Minamisava R, Andrade A-L. Distribution of invasive Streptococcus pneumoniae serotypes before and 5 years after the introduction of 10-valent pneumococcal conjugate vaccine in Brazil. Vaccine. 2018;36:2559–66. doi:10.1016/j.vaccine.2018.04.010.
- Centers for Disease Control and Prevention. Pneumococcal disease. In: Hamborsky J, Kroger A, Wolfe S, editors. Epidemiology and prevention of vaccine-preventable diseases. 13th ed. Washington (DC): Public Health Foundation; 2015. p. 279–96.
- Avila-Aguero ML, Ulloa-Gutierrez R, Falleiros-Arlant LH, Porras O. Pneumococcal conjugate vaccines in Latin America: are PCV10 and PCV13 similar in terms of protection against serotype 19A? Expert Rev Vaccines. 2017;16:1–4. doi:10.1080/14760584.2017.1334555.
- Global Burden of Disease (GBD). 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1191–210. doi:10.1016/S1473-3099(18)30310-4.
- Mott MP, Caierão J, Cunha GR, Del Maschi MM, Pizzutti K, d’Azevedo P, Dias CAG. Emergence of serotype 19A Streptococcus pneumoniae after PCV10 associated with a ST320 in adult population, in Porto Alegre, Brazil. Epidemiol Infect. 2019;147:e93–e93. doi:10.1017/S0950268819000013.
- Cassiolato AP, Almeida SCG, Andrade AL, Minamisava R, Brandileone M. Expansion of the multidrug-resistant clonal complex 320 among invasive Streptococcus pneumoniae serotype 19A after the introduction of a ten-valent pneumococcal conjugate vaccine in Brazil. Plos One. 2018;13:e0208211. doi:10.1371/journal.pone.0208211.
- The Latin American Pediatric Association (ALAPE). ALAPE Board of Directors opinion on the pneumococcal vaccine. 2017.
- World Health Organization (WHO). Summary of WHO Position Paper on Pneumococcal conjugate vaccines in infants and children under 5 years of age. 2019.
- Temple B, Toan NT, Dai VTT, Bright K, Licciardi PV, Marimla RA, Nguyen CD, Uyen DY, Balloch A, Huu TN, et al. Immunogenicity and reactogenicity of ten-valent versus 13-valent pneumococcal conjugate vaccines among infants in Ho Chi Minh City, Vietnam: a randomised controlled trial. Lancet Infect Dis. 2019;19:497–509. doi:10.1016/S1473-3099(18)30734-5.
- Wijmenga-Monsuur AJ, van Westen E, Knol MJ, Jongerius RMC, Zancolli M, Goldblatt D, van Gageldonk PGM, Tcherniaeva I, Berbers GAM, Rots NY, et al. Direct comparison of immunogenicity induced by 10- or 13-valent pneumococcal conjugate vaccine around the 11-month booster in dutch infants. PLoS One. 2015;10(12):e0144739. doi:10.1371/journal.pone.0144739.
- Desmet S, Peetermans W, Lagrou K. Switch in childhood pneumococcal vaccine in Belgium. Lancet Infect Dis. 2018;18:945–46. doi:10.1016/S1473-3099(18)30484-5.
- Rinta-Kokko H, Palmu AA, Auranen K, Nuorti JP, Toropainen M, Siira L, Virtanen MJ, Nohynek H, Jokinen J. Long-term impact of 10-valent pneumococcal conjugate vaccination on invasive pneumococcal disease among children in Finland. Vaccine. 2018;36(15):1934–40. doi:10.1016/j.vaccine.2018.03.001.
- Naucler P, Galanis I, Morfeldt E, Darenberg J, Ortqvist A, Henriques-Normark B. Comparison of the impact of pneumococcal conjugate vaccine 10 or pneumococcal conjugate vaccine 13 on invasive pneumococcal disease in equivalent populations. Clin Infect Dis. 2017;65:1780–89. doi:10.1093/cid/cix685.
- Ministry of Health Chile, Institute of Public Health Chile. Behavior of Streptococcus pneumoniae serotypes 3 and 19A in Chile. Lab Surveillance Bull Inst Public Health Chile. 2016;6:21.
- Ministry of Health Columbia, National Institute of Health Columbia. SIREVA II (Surveillance Network System for Agents Responsible for Pneumonia and Bacterial Meningitis) Laboratory Surveillance of invasive isolates of Streptococcus pneumoniae Colombia 2006-2018. 2019.
- Hanquet G, Krizova P, Valentiner-Branth P, Ladhani SN, Nuorti JP, Lepoutre A, Mereckiene J, Knol M, Winje BA, Ciruela P. Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: implications for adult vaccination. Thorax. 2019;74(5):473. doi:10.1136/thoraxjnl-2018-211767.
- Griffin MR, Mitchel E, Moore MR, Whitney CG, Grijalva CG. Declines in pneumonia hospitalizations of children aged <2 years associated with the use of pneumococcal conjugate vaccines - Tennessee, 1998-2012. MMWR Morb Mortal Wkly Rep. 2014;63:995–98.
- Harboe ZB, Dalby T, Weinberger DM, Benfield T, Mølbak K, Slotved HC, Suppli CH, Konradsen HB, Valentiner-Branth P. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis. 2014;59(8):1066–73. doi:10.1093/cid/ciu524.
- Janoir C, Lepoutre A, Gutmann L, Varon E. Insight into resistance phenotypes of emergent non 13-valent pneumococcal conjugate vaccine type pneumococci isolated from invasive disease after 13-valent pneumococcal conjugate vaccine implementation in France. Open Forum Infect Dis. 2016;3(1):ofw020. doi:10.1093/ofid/ofw020.
- Kawai K, Adil EA, Barrett D, Manganella J, Kenna MA. Ambulatory visits for Otitis media before and after the introduction of pneumococcal conjugate vaccination. J Pediatr. 2018;201:122–127.e121. doi:10.1016/j.jpeds.2018.05.047.
- Steens A, Bergsaker MA, Aaberge IS, Ronning K, Vestrheim DF. Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine. 2013;31:6232–38. doi:10.1016/j.vaccine.2013.10.032.
- Vespa G, Constenla DO, Pepe C, Safadi MA, Berezin E, Moraes JCD, Campos CAHD, Araujo DV, Andrade ALSSD. Estimating the cost-effectiveness of pneumococcal conjugate vaccination in Brazil. Revista panamericana de salud publica. 2009;26:518–28. doi:10.1590/S1020-49892009001200007.
- Kupek E, Viertel I. Postintroduction study of cost-effectiveness of pneumococcal vaccine PCV10 from public sector payer’s perspective in the State of Santa Catarina, Brazil. Value Health Reg Issues. 2018;17:109–14. doi:10.1016/j.vhri.2017.12.008.
- Sartori AL, Minamisava R, Bierrenbach AL, Toscano CM, Afonso ET, Morais-Neto OL, Antunes JLF, Cristo EB, Andrade AL. Reduction in all-cause otitis media-related outpatient visits in children after PCV10 introduction in Brazil. PloS One. 2017;12(6):e0179222–e0179222. doi:10.1371/journal.pone.0179222.
- Gomez JA, Lopes de Abreu AJ, Caceres DC, Nieto J, Ortega-Barria E. Estimated annual health and cost impact of PHiD-CV immunization program in Brazil. Pediatr Infect Dis J. 2019;38:e260–e265. doi:10.1097/INF.0000000000002436.
- Pugh S, Wasserman M, Moffatt M, Marques S, Reyes JM, Prieto VA, Reijnders D, Rozenbaum MH, Laine J, Åhman H, et al. Estimating the impact of switching from a lower to higher valent pneumococcal conjugate vaccine in Colombia, Finland, and The Netherlands: a cost-effectiveness analysis. Infect Dis Ther. 2020;9(2):305–24. doi:10.1007/s40121-020-00287-5.
- Wasserman M, Palacios MG, Grajales AG, BaezRevueltas FB, Wilson M, McDade C, Farkouh R. Modeling the sustained use of the 13-valent pneumococcal conjugate vaccine compared to switching to the 10-valent vaccine in Mexico. Hum Vaccin Immunother. 2019;15(3):560–69. doi:10.1080/21645515.2018.1516491.
- Shafie AA, Ahmad N, Naidoo J, Foo CY, Wong C, Pugh S, Tan KK. Estimating the population health and economic impacts of introducing a pneumococcal conjugate vaccine in Malaysia- an economic evaluation. Hum Vaccin Immunother. 2020;16(7):1719–1727. doi:10.1080/21645515.2019.1701911.
- Wilson M, Wasserman M, Jadavi T, Postma M, Breton M-C, Peloquin F, Earnshaw S, McDade C, Sings H, Farkouh R. Clinical and economic impact of a potential switch from 13-Valent to 10-valent pneumococcal conjugate infant vaccination in Canada. Infect Dis Ther. 2018;7(3):353–71. doi:10.1007/s40121-018-0206-1.
- Ansaldi F, Pugh S, Amicizia D, Di Virgilio R, Trucchi C, Orsi A, Zollo A, Icardi G. Estimating the clinical and economic impact of switching from the 13-Valent pneumococcal conjugate vaccine (PCV13) to the 10-Valent pneumococcal conjugate vaccine (PCV10) in Italy. Pathogens (Basel, Switzerland). 2020;9:76.
- Pan American Health Organization (PAHO). Surveillance Network System for Agents Responsible for Pneumonia and Bacterial Meningitis [Internet]. 2019. https://www.paho.org/bra/index.php?option=com_content&view=article&id=1720:sistema-de-redes-de-vigilancia-dos-agentes-responsaveis-por-pneumonias-e-bacterial-meningitis&Itemid=463
- Andrade AL, Afonso ET, Minamisava R, Bierrenbach AL, Cristo EB, Morais-Neto OL, Policena GM, Domingues CMAS, Toscano CM. Direct and indirect impact of 10-valent pneumococcal conjugate vaccine introduction on pneumonia hospitalizations and economic burden in all age-groups in Brazil: A time-series analysis. PLoS One. 2017;12(9):e0184204. doi:10.1371/journal.pone.0184204.
- Novaes H, Sartori A, Soárez P. Hospitalization rates for pneumococcal disease in Brazil, 2004-2006. Revista De Saúde Pública. 2011;45:539–47. doi:10.1590/S0034-89102011005000028.
- Ministry of Health Brazil, Department of Health Care, Department of Regulation, Evaluation and Control. Hospital Information System (Sistema de Informações Hospitalares, SIH) of the National Unified Health System (Sistema Único de Saúde, SUS), SIH/SUS. Datasus; 2017.
- Ministry of Health Brazil. Executive Secretariat. Acquisition price of pneumococcal conjugate 10 valent vaccine by the Ministry of Health 2019.
- Ministry of Health Brazil. Executive Secretariat. Extract of bid number 14/2019. http://www.in.gov.br/web/dou/-/extrato-de-inexigibilidade-de-licitacao-n-14/2019-uasg-250005-162478890.
- Mittmann N, Trakas K, Risebrough N, Liu BA. Utility scores for chronic conditions in a community-dwelling population. PharmacoEconomics. 1999;15:369–76. doi:10.2165/00019053-199915040-00004.
- Bennett JE, Sumner W 2nd, Downs SM, Jaffe DM. Parents’ utilities for outcomes of occult bacteremia. Arch Pediatr Adolesc Med. 2000;154:43–48.
- Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22:4203–14. doi:10.1016/j.vaccine.2004.05.003.
- Cheng AK, Niparko JK. Cost-utility of the cochlear implant in adults: a meta-analysis. Arch Otolaryngology–head Neck Surg. 1999;125:1214–18. doi:10.1001/archotol.125.11.1214.
- Morrow A, De Wals P, Petit G, Guay M, Erickson LJ. The burden of pneumococcal disease in the Canadian population before routine use of the seven-valent pneumococcal conjugate vaccine. Can J Infect Dis Med Microbiol. 2007;18:121–27. doi:10.1155/2007/713576.
- Ministry of Health Brazil. Secretariat of Science, Technology and Strategic Inputs. Methodological guidelines: economic evaluation studies of health technologies. 2 ed. Department of Science and Technology, Brazil; 2014. p. 132.
- Song J-H. Advances in pneumococcal antibiotic resistance. Expert Rev Respir Med. 2013;7:491–98. doi:10.1586/17476348.2013.816572.
- Wasserman M, Sings HL, Jones D, Pugh S, Moffatt M, Farkouh R. Review of vaccine effectiveness assumptions used in economic evaluations of infant pneumococcal conjugate vaccine. Expert Rev Vaccines. 2018;17:71–78. doi:10.1080/14760584.2018.1409116.
- Trial to evaluate the safety and immunogenicity of a 20-valent pneumococcal conjugate vaccine in pneumococcal vaccine-naïve adult. ClinicalTrials.gov; 2018. https://clinicaltrials.gov/ct2/show/NCT03760146.
- Rupp R, Hurley D, Grayson S, Li J, Nolan K, McFetridge RD, Hartzel J, Abeygunawardana C, Winters M, Pujar H, et al. A dose ranging study of 2 different formulations of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Hum Vaccin Immunother. 2019;15:549–59. doi:10.1080/21645515.2019.1568159.
- Lo SW, Gladstone RA, van Tonder AJ, Lees JA, Du Plessis M, Benisty R, Givon-Lavi N, Hawkins PA, Cornick JE, Kwambana-Adams B, et al. Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: an international whole-genome sequencing study. Lancet Infect Dis. 2019;19(7):759–69. doi:10.1016/S1473-3099(19)30297-X.
- Levy C, Ouldali N, Caeymaex L, Angoulvant F, Varon E, Cohen R. Diversity of serotype replacement after pneumococcal conjugate vaccine implementation in Europe. J Pediatr. 2019;213:252–253.e253. doi:10.1016/j.jpeds.2019.07.057.
- Varghese J, Chochua S, Tran T, Walker H, Li Z, Snippes Vagnone PM, Lynfield R, McGee L, Li Y, Metcalf BJ, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect. 2020;26(4):512.e511-512.e510. doi:10.1016/j.cmi.2019.09.008.
