ABSTRACT
Objective
We introduced a multi-component cancer prevention awareness program to primary care practices across New York State to evaluate its impact on adolescent human papillomavirus (HPV) vaccination rates.
Methods
Eight pediatric and three family medicine practices were recruited to participate in this program. On-site training sessions were provided for all practice providers and staff to discuss the importance of HPV vaccine and cancer prevention and teach strategies for delivering a strong vaccine recommendation. Each practice received a study-specific booklet that included HPV vaccine information and other commonly provided cancer prevention guidance. These booklets were distributed to all adolescents and their parents during well visits over a one-year period. Practice specific and county-wide HPV vaccination rates were assessed before and 12 months after the program training session.
Results
One year after program initiation, aggregate data show statistically higher vaccine series initiation rates among 11–12 and 13–18-year-olds and higher vaccine series completion rates among 13–18-year-olds. The greatest and most consistent improvements were seen in vaccine initiation rates for the 11–12-year-old cohort. Disparities in vaccine uptake were observed by gender and medical specialty.
Conclusion
Cancer prevention education targeting providers, office staff, patients, and parents, improved adolescent HPV vaccine series initiation rates.
Introduction
Human papillomavirus (HPV) causes 14 million new infections and ~45,000 new cancer diagnoses among the United States (US) women and men each year. The HPV vaccine, recommended to be administered to adolescents starting at age 11 or 12 years, is safe and effective in the prevention of HPV infection and its complications, yet adolescent HPV vaccine uptake remains low. According to the 2018 National Immunization Survey-Teen data, 51% of adolescents aged 13–17 years had completed the HPV vaccine series, leaving almost half of the nation’s adolescents vulnerable to the future development of HPV-associated cancers.Citation1
The most common reasons reported for vaccine delay or non-vaccination include lack of provider vaccine recommendation and the perceived association of vaccine with sexual activity.Citation2,Citation3 HPV vaccine acceptance, on the other hand, is associated with the understanding that the vaccine prevents HPV-associated cancers. Of concern, a study from 2019 found that up to two-thirds of surveyed women did not know if the HPV vaccine was successful in the prevention of cervical cancer, highlighting a gap in community awareness that needs to be addressed.Citation4 Another factor consistently associated with vaccination is the receipt of a strong provider recommendation to be immunized.Citation5 Recent studies, however, found that many primary providers report that they have limited knowledge regarding HPV and are uncomfortable delivering a strong recommendation.Citation6,Citation7
Clinicians have offered suggestions as to what they need to improve HPV vaccine uptake, including instructions on how to deliver a strong vaccine recommendation, education of office staff, and written materials explaining vaccine benefits that can be provided to families.Citation7,Citation8 We developed a multi-component cancer education program designed to teach providers strategies for delivering a strong vaccine recommendation, educate practice staff on the importance of HPV vaccine in disease prevention, and provide parents and adolescents with written guidance about HPV vaccination, as part of an overall emphasis on adolescent-based cancer prevention practices. In our pilot program, we reported that implementation of these strategies resulted in an increase in HPV vaccination rates in six suburban upstate New York pediatric practices, where the practice lead was an active member of the American Academy of Pediatrics (AAP), New York State (NYS) Chapter 1.Citation9 Here, we aim to show that the benefits of the program can be successfully generalized to pediatric and family medicine practices with diverse patient populations.
Methods
Practice recruitment
Between 2017 and 2018, we recruited pediatric and family medicine practices across upstate and western New York State to participate in a cancer prevention education program to improve their adolescent HPV vaccination rates. The initiative included practice-wide HPV vaccine education sessions and the subsequent year-long distribution of cancer prevention booklets to adolescents receiving care at recruited practices. As part of a quality improvement initiative, participating providers from these practices were eligible for Maintenance of Certification credit.
Cancer prevention booklets
The cancer prevention booklets were developed to combine HPV vaccine information with other routinely provided cancer prevention guidance, in an effort to de-emphasize the association of HPV vaccine and sex, while re-framing the message of HPV vaccine and cancer prevention, with a goal of increasing adolescent HPV vaccine uptake. The booklets used for this project are as previously describedCitation9 with modifications made in collaboration with Roswell Park Comprehensive Cancer Institute. The full-color 8-page monograph includes basic, easy-to-read educational material regarding cancer risks associated with tobacco use and exposure to ultraviolet light from tanning beds and sunbathing. Content included in the booklet also emphasizes the important role of regular physical activity, good nutritional habits, and vaccination against HPV in reducing lifelong risks for developing cancer.
Education session
The lead investigator (MS) facilitated a one-hour, on-site education session for all office staff members and medical providers working at each of the recruited practices. First, health-care providers attending the education session completed an anonymous, self-administered survey assessing baseline knowledge and attitudes about HPV vaccine and cancer prevention and their perceived frequency with which adolescent patients receive cancer prevention counseling during their office visits. The investigator then provided education regarding HPV infection-associated cancer epidemiology, current HPV vaccine recommendations, factors associated with HPV vaccine uptake and refusal, and interventions known to improve adolescent HPV vaccine uptake at both the provider and the practice level. Strategies for delivering a strong provider vaccine recommendation were modeled, and the importance of reviewing the vaccine status of adolescents during every encounter was emphasized. An open discussion with ample time to address specific questions from attendees ensued. After all questions were addressed, the content of the cancer prevention booklet was reviewed with the group.
The study team provided each practice with a sufficient number of cancer prevention booklets to distribute to their adolescent patients and their families for 1 year. The logistics for booklet distribution (during check-in, upon patient rooming, or during an encounter with the provider) and decisions regarding the use of the booklet during the visit were left to the discretion of the providers and staff at each practice.
Measures and analysis
Practice-specific HPV vaccination rates were determined immediately before the practice-wide education session and again 12 months later. In an effort to control for external factors that could be associated with community-wide changes in HPV vaccination rates, we collected and recorded overall county-wide HPV vaccination rates for the same start and end dates. Practice- and county-specific HPV vaccination rates were obtained from New York State’s Immunization Information System (NYSIIS). New York State law mandates that all vaccines administered to individuals younger than 19 years of age be entered into this vaccine registry. HPV vaccination rates at the desired time points were collected and recorded for each practice and for the county where each practice is located. Changes in vaccination rates were calculated for each practice, and for each county, descriptive statistics are used to report our findings including estimates for 95% CI around practice and county vaccination rates.
Results
Eight general pediatric practices (A through H) and three family medicine practices (I through K), serving more than 31,000 total adolescents participated in this program. All practices accepted patients with either public or private insurance coverage. Three practices served primarily rural populations, three practices served primarily suburban populations, and the remaining five practices served a combination of suburban, urban, and/or rural communities ().
Table 1. Characteristics of the participating pediatric practices
Fifty-eight health-care providers from the 11 practices completed the anonymous survey. While 95% strongly agreed that cancer prevention education is within the scope of their practice, only 88–91% of the providers strongly agreed that HPV infection is associated with cancer development (n = 53), that their adolescent patients are at risk for acquiring HPV infection (n = 51), or that HPV vaccine is an important tool for cancer prevention (n = 52). Of the 50 participants who listed perceived provider and/or parent barriers to adolescent HPV vaccination, 29 (58%) and 17 (34%) specifically listed the association of the vaccine with sex and the effects of media (including social media), respectively.
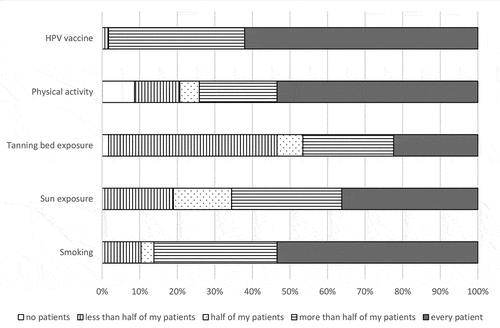
Self-reported delivery of cancer prevention guidance to adolescent patients varied by behavioral risk factor, with more than half of respondents reporting that they counsel every adolescent patient regarding avoidance of tobacco use and the importance of HPV vaccine and physical activity in cancer prevention. On the other hand, less than half of providers counseled every adolescent patient regarding sun and tanning bed exposure as cancer risks (). Of note, close to 40% of surveyed providers reported that they do not provide guidance regarding the importance of HPV vaccine for cancer prevention to all eligible patients.
Figure 1. Percentage of family medicine and pediatric providers (n = 58) who deliver verbal counseling regarding cancer risk reduction to their adolescent patients
Aggregate practice data show that 1 year after program start, HPV vaccine series initiation rates in both the 11–12 year (33% versus 40%) and 13–18 year (70% versus 73%) cohort and HPV vaccine completion rates in the 13–18 year cohort (56% versus 59%) were significantly higher than baseline (). When stratified by practice specialty, pediatric practices had statistically higher HPV vaccination rates than family medicine practices for vaccine series completion rates (26% versus 18%) among 11–12 year olds and series initiation (73% versus 69%) and completion (66% versus 58%) rates among 13–18 year olds (). When the aggregate data were further analyzed by gender, 1 year after study start, HPV vaccine initiation and completion rates were found to be consistently higher among females in both age cohorts ().
Table 2. Aggregate HPV vaccination rates at baseline and 1 year following the start of the education program
Table 3. Aggregate HPV vaccination rates by practice type 1 year following the start of the education program
Table 4. Aggregate data on HPV vaccination rates by gender at baseline and 1 year following the start of the education program
Baseline practice-specific HPV vaccination rates for 11–12 year old adolescents ranged from 24% to 57% for series initiation and from 4% to 29% for series completion (). Six and seven of the eleven practices had baseline rates at or above countywide rates for HPV vaccine initiation and completion, respectively (). Over the one-year study period, all of the recruited practices demonstrated increases in HPV vaccine series initiation rates. Six of the eleven practices increased their vaccine initiation rates by more than 5%, with the largest practice-specific increase of 25%. On the other hand, only 7/11 practices increased their HPV vaccine series completion rates. Only one of these practices had an increase in vaccine completion rates that exceeded 5%.
Figure 2. Rates of practice specific and county-wide (a) HPV vaccine series initiation; 11–12 years; (b) HPV vaccine series completion; 11–12 years; (c) HPV vaccine series initiation; 13–18 years; (d) HPV vaccine series completion; 13–18 years before and 12 months after the initiation of the cancer prevention education program
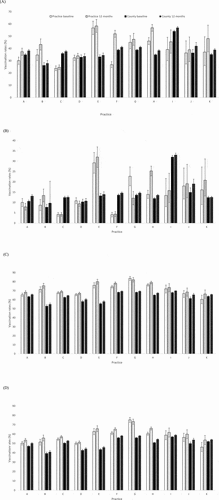
Baseline practice-specific HPV vaccination rates for 13–18 year old adolescents ranged from 60% to 84% for series initiation and from 46% to 75% for series completion (). Ten of the eleven practices had baseline rates at or above county-wide rates for HPV vaccine initiation and completion at the same time point (). Over the 1-year study period, 10 of the 11 recruited practices demonstrated increases in HPV vaccine series initiation and completion rates, with highest practice-specific improvements of 6% and 8% for initiation and completion, respectively.
Discussion
Here, we describe an expansion of our previously piloted HPV quality improvement program, to include eleven new pediatric and family medicine practices across New York State.Citation9 While overall vaccination rates appear to be low, aggregate data show that the vaccination rates among recruited practices are higher than national rates for vaccine initiation (73% versus 68%) and completion (59% versus 57%) among 13–17 year old adolescents. We present a low-cost intervention that offers the potential to incrementally increase practice-wide vaccine uptake. One year after the initiation of this multi-component education program, aggregate data showed statistically higher HPV vaccine series initiation rates among 11–12 and 13–18-year-old adolescents and higher HPV vaccine completion rates among 13–18 year old adolescents when compared to baseline.
The largest and most consistent practice-specific changes were seen for vaccine series initiation rates among 11–12-year-olds, with improvements as high as 25%. There are several reasons why this may be the case. Many parents of young adolescents, despite understanding that the HPV vaccine is recommended at age 11 or 12 years, often cite the association of vaccine with sex as the reason for their decision to delay the vaccine.Citation10 It is possible that framing the HPV vaccine as a method of cancer prevention, through provider recommendation and use of the cancer prevention booklets, may have influenced parents of 11- and 12-year-old adolescents to accept rather than delay vaccine until their child is older. It is also not surprising that increasing vaccine awareness at the time of a well-child visit would result in the administration of the first dose of vaccine, thus improving vaccine initiation, whereas systematic changes, such as reminder/recall systems, may be more likely to bring adolescents back to the office for vaccine series completion.
Practice-specific HPV vaccination rates remain low across all participating practices. Just under 40% of our surveyed providers reported that they do not routinely provide guidance regarding HPV vaccine as cancer prevention to all their eligible patients, revealing provider-level vaccine hesitancy. Some providers reported that they do not strongly agree that their patients are at risk for acquiring HPV infection (12%) or that the HPV vaccine is important for cancer prevention (10%). These beliefs are contrary to existing data that describe the high lifetime probability of HPV acquisition, the known risk of cancer development following persistent HPV infection, and vaccine efficacy of close to 100% for the prevention of HPV infections caused by vaccine types.Citation11,Citation12 Future interventions to increase adolescent HPV vaccine uptake might also focus on improving provider vaccine confidence, which could ultimately lead to more routine delivery of strong provider vaccine recommendations.
The perception that HPV vaccine is associated with sex was the most commonly reported parental barrier to vaccine uptake. While this has been a stated concern since the vaccine became available, available data show that advocating for and administering HPV vaccine does not influence adolescent sexual behavior.Citation13 Yet, parents continue to cite concerns regarding sexual activity as reasons for declining or delaying vaccine.Citation10,Citation14 In order to optimally protect adolescents from HPV-associated complications, the HPV vaccine message should be re-framed from one of the preventions of a sexually transmitted infection to one of the cancer preventions. Toward this goal, the educational booklets used in this program combined HPV vaccine information with other guidance that parents already associate with cancer risk reduction. Underwood, et al. found the use of educational booklets about adolescent vaccines (as part of multi-component educational interventions) to be associated with an increase in HPV vaccine uptake.Citation15 Furthermore, bringing this information to the adolescents directly includes them in their healthcare decision making. Health promotion interventions geared to children and adolescents have been successful in changing health behaviors and influencing future outcomes.Citation16
Pediatric practices that were recruited for our study had statistically higher HPV vaccination rates than family medicine practices for both vaccine series completion among 11–12 and 13–18-year-olds and vaccine series completion among 13–18-year-olds. This finding is consistent with a long-standing observation that HPV vaccine uptake tends to be higher across pediatric practices compared with family medicine practices.Citation17 More recently, pediatric providers reported higher levels of engagement with HPV vaccine recommendation practices, defined by frequency, strength, and style of vaccine recommendations, when compared with their family medicine colleagues.Citation7 This may be explained, at least in part, by the high emphasis of disease prevention in pediatric medicine, with chronic disease management being reported as a higher priority by family medicine providers.Citation8
More than a decade has passed since the HPV vaccine was first routinely recommended for all males starting at age 11 or 12 years, yet we still observed significant gender disparities in HPV vaccine initiation and completion rates. This is particularly worrisome given the epidemiology of HPV-associated diseases among males. Each year, between 2012 and 2016, in the U.S. alone, estimated 19,000 men were diagnosed with an HPV-associated malignancy, most frequently manifesting as oropharyngeal cancer.Citation18 HPV vaccination has already been shown to be associated with a reduction in vaccine-type oral HPV prevalence among young adults.Citation19,Citation20 These findings highlight the need for cancer prevention awareness programs to be directed toward both males and females alike.
Multi-component interventions that engage providers, patients, and families, including in-office patient education and the use of information sheets, have been found to be effective strategies to improve HPV vaccine acceptance.Citation21-23 Key aspects of our cancer prevention program included (a) teaching providers strategies to deliver a strong vaccine recommendation, (b) education of all office staff to increase practice-wide vaccine confidence, and (c) distribution of booklets highlighting strategies to reduce lifetime risks of developing cancer, including HPV vaccination. First, the delivery of a strong vaccine recommendation is the most commonly cited reason for vaccination, yet providers lack confidence in their ability to communicate with families about this vaccine.Citation5,Citation6,Citation23 On-site training for providers included didactic education and allowed for individual questions and concerns regarding their vaccine recommendation practices to be addressed. Second, involving the entire practice in the education process is important to ensure that the office philosophy conveys a consistent message to patients and families. Previously published studies show that support staff, including nurses and medical assistants, have limited HPV knowledge, mixed attitudes toward the vaccine, and were not confident in their ability to communicate with families about the HPV vaccine.Citation8 Higher vaccination rates were seen in clinics that described vaccination as a team effort rather than a sole responsibility of the providers.Citation8 Lastly, understanding the role that HPV vaccine plays in cancer prevention is consistently associated with vaccine acceptance.
This study has several limitations. First, we included eleven practices from across the state, only three of which were family medicine offices, so data may not be generalizable to all primary care practices. These practices, however, did serve over 31,000 adolescents in a variety of community settings (rural, suburban, and/or urban) with a wide range of HPV vaccination rates (both below and above countywide rates). Secondly, while all the booklets were to be distributed to their adolescent patients during their well-child visits, there was no further standardization to their use. The flexibility of our study design to allow practices to use the educational materials in a manner they deemed best with their workflow was incorporated in an effort to optimize engagement and participation. We chose this less than rigorous study design, understanding the limitations of doing so. There was also no data collected regarding the delivery of strong provider recommendations. Lastly, our program was based primarily on didactic and written education targeting a variety, but a limited number of stakeholders in the vaccine delivery system. It is unclear whether the changes observed in the study are sustainable over a longer period of time.
We developed a multi-component education program designed to increase practice staff vaccine confidence, improve provider comfort with delivering strong vaccine recommendations, and spread awareness about the role of HPV vaccine as part of the overall approach to cancer prevention. The distribution of booklets laying out strategies for cancer prevention was readily incorporated into daily workflow. While our aggregate data showed statistical improvement in HPV vaccine series initiation and completion rates, practice-specific increases varied substantially by the medical office. In the future, the addition of systematic practice changes to education programs should be studied as they may result in larger, more sustainable increases in vaccination rates, particularly with regards to vaccine series completion. Further work on understanding and reducing the causes of provider vaccine hesitancy and the persistence in gender disparities in HPV vaccine uptake is clearly needed.
Abbreviations
| HPV | = | Human Papillomavirus |
| NYSIIS | = | New York State’s Immunization Information System |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Financial disclosures
The authors have no financial relationships relevant to this article to disclose.
Acknowledgments
We thank the New York State Immunization Information Systems team for their assistance in obtaining statewide and countywide vaccination rates.
Additional information
Funding
References
- Walker TY, Elam-Evans LD, Yankey D, Markowitza LE, Williams CL, Fredua B, Singleton JA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years – United States, 2018. MMWR. 2019;68:718–23. doi:10.15585/mmwr.mm6833a2.
- Gidengil C, Chen C, Parker AM, Nowak S, Matthews L. Beliefs around childhood vaccines in the United States: A systematic review. Vaccine. 2019;37:6793–802. doi:10.1016/j.vaccine.2019.08.068.
- Hirth JM, Fuchs EL, Chang M, Fernandez ME, Berenson AB. Variations in reason for intention not to vaccinate across time, region, and by race/ethnicity, NIS-Teen (2008-16). Vaccine. 2019;37:595–601. doi:10.1016/j.vaccine.2018.12.017.
- Domgue JF, Chido-Amajuoyi OG, Yu RK, Shete S. Beliefs about HPV vaccine’s success at cervical cancer prevention among US women. JNCI Cancer Spectrum. 2019;3:pkz064. doi:10.1093/jncics/pkz064.
- Dempsey AF, Pyrzanowski J, Campagna EJ, Lockhart S, O’Leary ST. Parent report of provider HPV vaccine communication used during a randomized, controlled trial of a provider communication intervention. Vaccine. 2019;37:1307–12. doi:10.1016/j.vaccine.2019.01.051.
- Leung SO, Akinwunmi B, Elias KM, Feldman S. Educating healthcare providers to increase human papillomavirus (HPV) vaccination rates: A qualitative systematic review. Vaccine X. 2019;3:100037. doi:10.1016/j.jvacx.2019.10037.
- Lake PW, Kasting ML, Christy SM, Vadaparampil ST. Provider perspectives on multilevel barriers to HPV vaccination. Hum Vaccin Immunother. 2019;15:1784–93. doi:10.1080/21645515.2019.1581554.
- Chuang E, Cabrera C, Mak S, Glenn B, Hochman M, Bastani R. Primary care team- and clinic level factors affecting HPV vaccine uptake. Vaccine. 2017;35:4540–47. doi:10.1016/j.vaccine.2017.07.028.
- Suryadevara M, Bonville CA, Cibula DA, Domachowske JB. Cancer prevention education for providers, staff, parents, and teens improves adolescent human papillomavirus immunization rates. J Pediatr. 2019;205:145–52. doi:10.1016/j.jpeds.2018.09.013.
- Hansen CE, Credle M, Shapiro ED, Niccolai LM. “It all depends”: A qualitative study of parents’ views of human papillomavirus vaccine for their adolescents at ages 11-12 years. J Cancer Educ. 2016;31:147–52. doi:10.1007/s13187-014-0788-6.
- Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660–64. doi:10.1097/OLQ.0000000000000193.
- About CDC HPV vaccines. Accessed May 7, 2020.https://www.cdc.gov/vaccines/vpd/hpv/hcp/vaccines.html
- Cook EE, Venkataramani AS, Kim JJ, Tamimi RM, Holmes MD. Legislation to increase uptake of HPV vaccination and adolescent sexual behaviors. Pediatrics. 2018;142:e20180458. doi:10.1542/peds.2018-0458.
- Dang JH, Stewart SL, Blumberg DA, Rodriguez HP, Chen MS. “There’s always next year”: primary care team and parent perspectives on the human papillomavirus vaccine. Hum Vaccin Immunother. 2020:1–10. Epub ahead of print. doi:10.1080/21645515.2019.1710410.
- Underwood NL, Gargano LM, Sales J, Vogt TM, Seib K, Hughes JM. Evaluation of educational interventions to enhance adolescent specific vaccination coverage. J Sch Health. 2019;89:603–11. doi:10.1111/josh.12786.
- Maisonneuve AR, Witteman HO, Brehaut J, Dube E, Wilson K. Educating children and adolescents about vaccines: A review of current literature. Expert Review of Vaccines. 2018;17:311–21. doi:10.1080/14760584.2018.1456921.
- Daley MF, Crane LA, Markowitz LE, Black SR, Beaty BL, Barrow J, Babbel C, Gottlieb SL, Liddon N, Stokley S, et al. Human papillomavirus vaccination practices: A survey of US physicians 18 months after licensure. Pediatrics. 2010;126:425–33. doi:10.1542/peds.2009-3500.
- Senkomago V, Henley SJ, Thomas CC, Mix JM, Markowitz LE, Saraiya M. Human papillomavirus – attributable cancers – United States, 2012-2016. MMWR. 2019;68:724–28. doi:10.15585/mmwr.mm6833a3.
- Chaturvedi AK, Graubard BI, Broutian T, Pickard RKL, Tong ZY, Xiao W, Kahle L, Gillison ML. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36:262–67. doi:10.1200/JCO.2017.75.0141.
- Schlecht NF, Masika M, Diaz A, Nucci-Sack A, Salandy A, Pickering S, Strickler HD, Shankar V, Burk RD. Risk of oral human papillomavirus infection among sexually active female adolescents receiving the quadrivalent vaccine. JAMA Netw Open. 2019;2:e1914031. doi:10.1001/jamanetworkopen.2019.14031.
- Rodriguez AM, Quynh T, Goodman M, Schmeler KM, Kaul S, Kuo YF. Human papillomavirus vaccine interventions in the US: A systematic review and meta-analysis. Am J Prev Med. 2020;56:591–602. doi:10.1016/j.amepre.2018.10.033.
- Dixon BE, Zimet GD, Xiao S, Tu W, Lindsay B, Church A, Downs SM. An educational intervention to improve HPV vaccination: A cluster randomized trial. Pediatrics. 2019;143:e20181457. doi:10.1542/peds.2018-1457.
- Reno JE, Thomas J, Pyrzanowski J, Lockhart S, O’Leary ST, Campagna EJ, Dempsey AF. Examining strategies for improving healthcare providers’ communication about adolescent HPV vaccination: evaluation of secondary outcomes in a randomized controlled trial. Hum Vaccine Immunother. 2019;15:1592–98. doi:10.1080/21645515.2018.1547607.
