?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Prevalence of hepatitis B (HB) remains high among adults in insular regions of southeast China. To address this issue, large-scale HB vaccination was implemented in Putuo County of Zhoushan City in 2013. To examine the effects of this large-scale HB vaccination, a cross-sectional serological survey was conducted on two isolated islands, Putuo (intervention group) and Dinghai (control group) Counties of Zhoushan City in southeastern China five years later. The data showed lower prevalence of HBsAg and negative results for all HBV markers in the intervention group compared to the control group (4.2% and 6.0%, 20.8% and 33.0%), while the positive rate for anti-HBs and only anti-HBs were higher (68.4% and 53.4%, 47.9% and 34.3%). Therefore, large-scale adult HB vaccination could lower the HB epidemic level. However, the proportion of susceptible people who were negative for all HBV markers remained high. Hence, several additional measures to limit the spread of HBV should be taken by the government.
Hepatitis B (HB) virus (HBV) infection and its associated complications remain serious public health concerns worldwide. China is a highly endemic area for HBV infection.Citation1 Hepatitis B vaccination (HepB) is the most economical and effective method to prevent and control HB. In 1992, HepB was first recommended for routine immunization of infants, which was paid for by the parents. Subsequently, it was integrated into the National Expanded Program of Immunization (EPI) of China in 2002, and provided free of charge.Citation2 Numerous surveys revealed that HepB was highly successful and increasingly effective. The most recent large, nationwide survey in China conducted in 2006, showed that the weighted positive rate of hepatitis B surface antigen (HBsAg) was 7.2% among those aged 1–59 years, and the rate among children aged <5 years was only 1.0%.Citation3 A national seroepidemiological study in 2014 showed that the HBsAg prevalence had significantly decreased (2.6%) in the population aged 1–29 years in China.Citation4 However, vaccination efforts were not perfect because many adults were born before prevention of HBV was possible.Citation4
Zhoushan City, which located in southeast China, is an isolated group of islands connected to the mainland by a bridge. A survey conducted by Chen et al. revealed that the prevalence of HBsAg in Zhoushan City was 10.4% in 2012, and the infection rate under 20 years was 1.7%.Citation5 Though it significantly declined compared to 19.33% in 1997Citation6 and 15.5% in 2007, Citation7 the rate was still high among adults and the effect would be limited if the vaccination program is restricted to newborns in these islands.Citation5 Some experts proposed that adult HB immunization should be included into EPI. To address this issue, with the support of Mega-Project for National Science and Technology Development for the “12th Five-Year Plan of China’, the Putuo County in Zhoushan City, provided the HepB (dosage 20 mg, Tianyi Biotech, Beijing, China) free of charge to adults aged 20–59 years who had not completed three doses of HepB (20 ug) from June to Nov. 2013.Citation8 In this program, 199,319 injections were administered to 80857 persons (36.93% of all the people who reside in Putuo County) and 53,172 people completed three doses (65.76%).
Epidemiological assessment is necessary to accurately determine the current burden of disease and the role of existing interventions, and to provide insights for future priority actions. Hence, the purpose of this study was to investigate the effects of large-scale HepB vaccination five years later by comparing the data from Putuo County with another similar county (Dinghai County) that did not receive this vaccination.
We conducted a cross-sectional serological survey in Putuo and Dinghai Counties of Zhoushan City in southeastern China from Jan. to Mar. 2019. The target population included local residents and migrants who resided in these counties for ≥6 months and were aged 1–94 years. The results of Putuo County represent the intervention group and Dinghai County represent the control group. A randomized controlled study design was used, with a 1:1 allocation ratio of two groups (Nt:Nc = 1:1). According to past literatureCitation1 and pre-investigation results, the estimated HBsAg positive rate Pt in the intervention group was 3%, and the control group Pc was 6%. We defined the α value as 0.05 (two-sided) and β as 0.1 (one-sided). Based on the sample size calculation formula:
Where represents the average of Pt and Pc,
represents the average of 1-Pt and 1-Pc, N = 1002. There should be 1200 participants in each group if the shedding rate is 20%, and the total number is 2400. Participants in this serological survey were selected by multi-stage random sampling. According to the survey plan, we used cluster sampling in the first stage and simple random sampling in the second stage.
Blood samples (2 ml per person) were collected from each participant in the population. Demographic information was collected by a questionnaire. Serum was separated, stored at −20°C, and sent to KingMed Diagnostics Inc. in Hangzhou for HBsAg, HB surface antibody (anti-HBs), and HB core antigen antibody (anti-HBc) quantification. An Architect-i2000 (Abbott, USA) was used for the chemiluminescence immunoassay (CLIA). HBsAg, anti-HBs, and anti-HBc above 0.05 IU/ml, 10 mIU/ml, and 1 S/CO were considered positive, respectively. Detailed quality control measures were implemented at all stages of this serological survey. All researchers received appropriate training before the survey. The survey was approved by the Ethics Committee of Zhejiang Provincial Center for Disease Control and Prevention (No.T-043-R).
Data were independently entered by two investigators and checked for accuracy using Epidata software, Version 3.02. All statistical analyzes were conducted using SPSS for Windows, version 20.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were expressed as frequencies (percentages), and Pearson’s chi-square test and continuity-corrected χ2 test were used to determine group differences. A p-value ≤0.05 (2-sided) was considered to be statistically significant.
A total of 3000 serum samples were tested. Information on age or gender was missing for 73 participants (2.4%). Thus, a total of 2937 samples were analyzed. Demographic characteristics of the study population are shown in . The age groups and their proportions in the intervention group and the control group individually were as follows: aged 1–19 years (45.7% and 47.9%), aged 20–59 years (43.7% and 41.6%), and aged >60 years (10.7% and 10.4%). In terms of gender, 46.2% of the participants were male in the intervention group and 49.1% were male in the control group. The number of participants distributed between the two districts was similar in proportion to the population of each group. There were no statistically significant differences in age or gender among the two groups (p > .05). In terms of HepB history, 68.4% of the respondents in the intervention group and 58.9% in the control group responded that they had been vaccinated (p < .001).
Table 1. Demographic data of the study population
The prevalence of HBV markers was compared between the two groups (). Among the eligible study population, 63 persons (4.2%) were positive for HBsAg in the intervention group and 86 (6.0%) were positive in the control group (p < .05); the ratio was significantly lower in the intervention group than in the control group among males, aged 1–19 years, who had been vaccinated (p < .05). A total of 1024 persons (68.4%) were positive for anti-HBs in the intervention group and 769 (53.4%) were positive in the control group (p < .001); 718 persons (47.9%) were positive for only anti-HBs (isolated anti-HBs) in the intervention group and 494 (34.3%) were positive in the control group, which provided evidence of HepB-induced immunity (p < .001), however, there were no statistically significant differences among the two groups in people aged >60 years. A total of 311 persons (20.8%) in the intervention group and 475 persons (33.0%) in the control group were negative for all HBV markers (p < .001), indicating that they were susceptible to HBV. Moreover, the ratio of the intervention group was lower than the control group among those aged 20–59 years (p < .001). There was no statistically significant differences in anti-HBc among the two groups, which is a marker of HBV exposure indicative of past or present infection (p > .05); however, it was lower in the intervention group than in the control group among males aged 20–59 years (p < .05).
Table 2. Distribution of HBV markers in two counties
Table 3. Distribution of HBV markers in different regions, stratified by gender or age
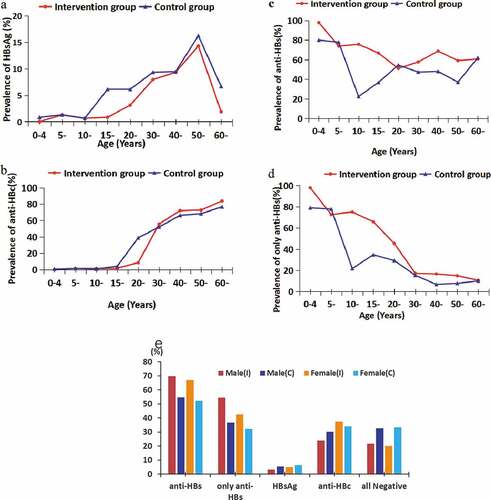
The rate of HBsAg positivity, a marker of current infection, increased gradually from <1% in those aged 1–4 years to 14.29% (intervention group) or 16.23% (control group) in those aged 50–59 years, reaching a peak in this age group and declining sharply in the older age groups (). The prevalence of anti-HBc increased with age in both the groups and reached a peak in the >60 years age group (). The prevalence of anti-HBs was >50% in all age groups of the intervention group, but was <50% in most age groups of the control group (). The isolated anti-HBs prevalence decreased with age in both groups (). The prevalence of HBsAg and anti-HBc among female participants was higher than males, while anti-HBs and only anti-HBs was lower than males ().
Prevalence of all HBV markers were compared in the two groups by age and gender (). For the 1–19 years age group, anti-HBs and isolated anti-HBs prevalence was significantly higher in the intervention group than in the control group irrespective of gender (p < .001), and there were no statistically significant differences for HBsAg and anti-HBc (p > .05). For the 20–59 years age group, anti-HBs and isolated anti-HBs prevalence was significantly higher in the intervention group (57.4%, 31.9%) than in the control group (47.0%, 13.0%) among males (p < .05, p < .001), while anti-HBc in the intervention group (37.5%) was lower than in the control group (60.1%, p < .001). The prevalence of anti-HBs was significantly higher in the intervention group (59.1%) than in the control group (46.2%) among females (p < .001). However, there were no statistically significant differences for the other HBV markers. There was no statistically significant differences for all HBV markers in the >60 years age group (p > .05).
Table 4. Distribution of HBV markers in different regions, stratified by gender and age
HBV infection is highly endemic in China. It was estimated that 93 million people harbor HBsAg, leading to high mortality and societal burden.Citation9,Citation10 The annual cost of HB and its related diseases is about ¥900 billion (US 145 USD billion).Citation9,Citation11 Previous studies revealed that HepB is the most effective method to prevent HB and it achieved great success in pediatric immunity.Citation4,Citation12 However, HBsAg prevalence remains high in Chinese adults (8.5–10.5%), Citation3,Citation13 and hence cannot be effectively controlled only through newborn immunization. Adult HB immunity is a research hotspot. Recently, many scholars have explored adult HB immune procedures, strategies, and persistence.Citation14–16 However, research on the entire population, procedures to conduct large-scale immunization, whether such immunization is effective, and the immune effect of the population in the region are limited. This study obtained evidence of recent community-based status of HBV epidemiology and evaluated the effects of large-scale HepB in an insular region.
The prevalence of HBsAg, as a single indicator of infection with HBV, varies widely in different regions and populations. Regions are classified according to the prevalence of HBsAg into high (≥8%), higher moderate (5–7.99%), lower moderate (2–4.99%), and low (<2%) endemic areas.Citation17,Citation18 Compared with the prevalence of HBsAg (10.4%) in Zhoushan City in 2012, two counties (4.2% and 6.0%) have become moderately endemic areas in this study and the intervention group (Putuo County, 4.2%) has become a lower moderate endemic area through large-scale adult HepB. Meanwhile, the ratio of HepB history, the prevalence of anti-HBs and isolated anti-HBs in the intervention group were much higher than in the control group, and negative results for all HBV markers was lower. These results suggested that the implementation of large-scale adult HepB is effective for HBV control, and regular immunization should be performed in susceptible adult populations.
The prevalence of HBsAg varied among the different age and gender populations in both groups in this study. In this study, females showed a slightly higher prevalence of HBsAg than males, which was similar to previous studies, Citation17 while there was no gender-specific difference in HBsAg-positive rate in the 1–59 years age group. The HBsAg-positive rate in females aged 60 years was higher than in males, although the reason for this difference is unclear due to the small sample size, and further research is needed. The peak age of acquisition of the HBsAg positivity rate observed in this study was in the 50–59 years age group, which was different from the previous findings in 2009 in the 40–49 years age group.Citation13 It demonstrated the effectiveness of EPI. Meanwhile, the percentage of all HB marker negative remained high and >20% of people were susceptible to HBV. More vaccination policy should be adopted to protect these people.
Anti-HBc can be detected in all individuals who have previously been infected with HBV, irrespective of whether they have recovered or are actively infected, and the isolated anti-HBc can be found in high-risk groups as the “window” stage, followed by acute HBV infection.Citation15 In our study, the prevalence of anti-HBc increased significantly with age, which was similar to previous studies.Citation5,Citation13 It indicated that horizontal transmission played an important role in China. According to the 2006 National Serological Survey, the prevalence of anti-HBc in adults aged 20–59 years was 47%.Citation3 In our study, it was higher than the national data in both groups. However, the prevalence of HBc in the 20–29 years age group in the intervention group was 8.33% and this ratio was >50% in both groups among people older than 30 years. This may be due to low HepB coverage in the ≤30 years age group, indicating poor implementation of the universal newborn vaccination policy from 1992.
This study had some limitations. First, the study was based on a seroepidemiological survey and compared two similar regions, but lacked data before the large-scale vaccination intervention. Second, because HBeAg and anti-HBeAg were not tested in this study, we could not categorize the adults with isolated anti-HBc. Third, this survey did not obtain the three-dose HepB records.
In summary, large-scale adult HepB vaccination could lower the HB epidemic level. Although the prevalence of HBsAg had significantly dropped in the intervention region, the prevalence of HBV in adults and negative for all HBV markers in all age groups remained high. The government should undertake several additional measures to limit the spread of HBV, especially among susceptible people.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank the Putuo and Dinghai Centers for Disease Control and Prevention (CDC), and other relevant personnel for their contributions to this study.
Additional information
Funding
References
- Razavi-Shearer D, Gamkrelidze I, Nguyen MH, Chen D-S, Van Damme P, Abbas Z, Abdulla M, Abou Rached A, Adda D, Aho I, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383–403. PMID: 29599078. doi:10.1016/S2468-1253(18)30056-6.
- Zhou YH, Wu C, Zhuang H. Vaccination against hepatitis B: the Chinese experience. Chin Med J. 2009;122(1):98–102. PMID: 19187625.
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27(47):6550–57. PMID: 19729084. doi:10.1016/j.vaccine.2009.08.048.
- Cui F, Shen L, Li L, Wang H, Wang F, Bi S, Liu J, Zhang G, Wang F, Zheng H, et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China. Emerg Infect Dis. 2017;23(5):765–72. PMID: 28418296. doi:10.3201/eid2305.161477.
- Chen P, Yu C, Ruan B, Yang S, Ren J, Xu W, Luo Z, Li L. Prevalence of hepatitis B in insular regions of southeast China: a community-based study. PloS One. 2013;8(2):e56444. PMID: 23437134. doi:10.1371/journal.pone.0056444.
- Yu AJ, Yan HH. Seroepidemiological analysis of prevalence of hepatitis B by fishermen in Zhoushan, Zhejiang Province. Sci Travel Med. 1997;1:14–15.
- Tang A, Wang JY, Yan JB, Zhang QT. Multiple factors analysis of the hepatitis B virus infection of the fishermen. Strait Prevent Med. 2007;13(6):43–45.
- Zhang SH, Wang SZ, Zheng HY. Evaluation of comprehensive community intervention effect on hepatitis B in Putuo of Zhoushan in 2010–2014. Chin Prevent Medi. 2016;17(2):112–15. doi:10.16506/j.1009-6639.2016.02.008.
- Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol. 2013;28(Suppl 1):7–10. PMID: 23855289. doi:10.1111/jgh.12220.
- Hu M, Chen W. Assessment of total economic burden of chronic hepatitis B (CHB)-related diseases in Beijing and Guangzhou, China. Value Health. 2009;12(Suppl 3):S89–92. PMID: 20586991. doi:10.1111/j.1524-4733.2009.00636.x.
- Wang FS, Fan JG, Zhang Z, Gao B, Wang H-Y. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–108. PMID: 25164003. doi:10.1002/hep.27406.
- Zhou Y, He H, Deng X, Yan R, Tang X, Xie S, Yao J. Significant reduction in notification and seroprevalence rates of hepatitis B virus infection among the population of Zhejiang Province, China, aged between 1 and 29years from 2006 to 2014. Vaccine. 2017;35(34):4355–61. PMID: 28687404. doi:10.1016/j.vaccine.2017.06.078.
- Luo Z, Xie Y, Deng M, Zhou X, Ruan B. Prevalence of hepatitis B in the southeast of China: a population-based study with a large sample size. Eur J Gastroenterol Hepatol. 2011;23(8):695–700. PMID: 21617533. doi:10.1097/MEG.0b013e328347322b.
- Ren W, Ren J, Wu Z, Shen L, Shan H, Dai X, Li J, Liu Y, Qiu Y, Yao J, et al. Long-term persistence of anti-HBs after hepatitis B vaccination among adults: 8-year results. Hum Vaccin Immunother. 2020;16(3):687–92. PMID: 31526223. doi:10.1080/21645515.2019.1666612.
- Yao J, Ren W, Chen Y, Jiang Z, Shen L, Shan H, Dai X, Li J, Liu Y, Qiu Y, et al. Responses to hepatitis B vaccine in isolated anti-HBc positive adults. Hum Vaccin Immunother. 2016;12(7):1847–51. PMID: 27065099. doi:10.1080/21645515.2016.1139256.
- Qiu Y, Ren J, Yao J. Healthy adult vaccination: an urgent need to prevent hepatitis B in China. Hum Vaccin Immunother. 2016;12(3):773–78. PMID: 26337328. doi:10.1080/21645515.2015.1086519.
- Liu J, Lv J, Yan B, Feng Y, Song L, Xu A, Zhang L, Yan Y. Comparison between two population-based hepatitis B serosurveys with an 8-year interval in Shandong Province, China. Int J Infect Dis. 2017;61:13–19. PMID: 28577994. doi:10.1016/j.ijid.2017.05.015.
- Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–19. PMID: 22273662. doi:10.1016/j.vaccine.2011.12.116.