ABSTRACT
A safe and effective vaccine candidate is urgently needed for the ongoing COVID-19 pandemic, caused by SARS-CoV-2. Here we report that recombinant SARS-CoV-2 RBD protein immunization in mice is able to elicit a strong antibody response and potent neutralizing capability as measured using live or pseudotyped SARS-CoV-2 neutralization assays.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the etiologic agent for the Coronavirus Disease 2019 (COVID-19). After the outbreak of COVID-19 in late 2019, a safe, effective and scalable vaccine against the SARS-CoV-2 is in urgent need. Like other coronaviruses, the SARS-CoV-2 spike (S) protein contains two subunits, S1 and S2, and the receptor-binding domain (RBD) is embedded in the S1 subunit.Citation1 Previous studies on SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) vaccine development indicate that the spike RBD predominantly elicits neutralizing antibodies in animals,Citation2,Citation3 while antibodies directed outside of the RBD may have no neutralizing capabilities.Citation2,Citation4 SARS-CoV RBD, but not the entire S protein, induces higher level of neutralizing antibodies to prevent the disease.Citation2 Similarly, SARS-CoV RBD fused with IgG constant region (RBD-Fc) also induces a protective humoral response against SARS-CoV.Citation2 Furthermore, recombinant MERS-CoV RBD immunization protects the rhesus macaques challenged with live MERS-CoV.Citation5 On the other hand, the non-neutralizing antibodies were shown to induce antibody-dependent enhancement (ADE) which can enhance infections caused by heterologous SARS-CoV strains or mediate harmful immune responses.Citation2 Considerable evidence has proved that the recombinant RBD-based subunit vaccine is a promising candidate vaccine against the SARS-CoV-2 infection.
To examine the immunogenicity of SARS-CoV-2 RBD, his-tagged recombinant RBD covering the a.a. 333–516 of the S protein was cloned into pSecTag2A expression vector and expressed in-house using the Expi293 mammalian cell expression system (ThermoFisher). Since RBD contains a complex tertiary structure with many intramolecular disulfide bonds, the use of mammalian cells can help express proteins with proper folding and post-transcriptional modifications. In addition, through structural modeling, the selected RBD fragment maintains a complete structure without residues that may cause undesirable immunogenicity. RBD-Fc fusion protein was acquired from Sino Biological (#40592-V05H). Both proteins were confirmed of their purity (>95%) and angiotensin-converting enzyme 2 receptor binding specificity, and they were formulated with alum for vaccination. Groups of C57BL/6 mice were inoculated subcutaneously with 5 μg of RBD-Fc (two doses: day 0 and 21) or RBD (three doses: day 0, 21, 49), and mice receiving PBS were used as controls. To assess the humoral immunity in response to RBD-based antigen immunization, RBD-specific IgG antibody titer was determined by ELISA. The results () showed that, after the first dose, RBD-Fc vaccination immediately induced a strong RBD-specific IgG titer on day 21, and the second dose slightly boosted the RBD-specific IgG response on day 35; the titer level remained stable until day 63. In contrast, RBD initially elicited subdued RBD-specific humoral response on day 21 as no detectable RBD-specific humoral response was observed in 4 out of the 5 vaccinated mice. The second vaccination of RBD resulted in a significant increase in anti-RBD-specific IgG titer on day 35, and additional immunization on day 49 further boosted the anti-RBD IgG titer on day 63. On day 63, similar levels of RBD-specific IgG titers were observed in the RBD-Fc and RBD groups. In addition, neutralizing antibodies (100% neutralizing titer, NT100) were further monitored using the SARS-CoV-2 neutralizing assays in Vero E6 cells and a lentiviral-based SARS-CoV-2 pseudovirus neutralization assay based on luminescence readout. As shown in , after the first immunization, RBD-Fc induced neutralizing titers of 1:40 to 1:160 on day 21. Subsequent booster increased the neutralizing titers to 1:320 and 1:1280 on day 35 and day 63, respectively. For the RBD immunization, the neutralizing responses in mice sera were barely detected on day 21 and 35, however, after the third dose on day 49, significant neutralizing titers were detected, and the titers were ranged between 1:40 and 1:2560 on day 63. PBS-immunized group did not demonstrate any neutralizing titers. The data obtained from the pseudovirus neutralization assays were similar to that performed using SARS-CoV-2 virus, hence validating the vaccine effectiveness.
Figure 1. SARS-CoV-2 RBD induces a strong humoral response in mice. Three groups of mice were vaccinated subcutaneously with PBS, 5 μg RBD-Fc/alum (two doses: day 0 and 21) or 5 μg RBD/alum (three doses: day 0, 21, 49), and serum was collected on day 21, 35 and 63. (A) RBD-specific serum IgG response was determined using ELISA on 100 ng RBD-coated microplates. Data represent means ± SEM (n = 3–5). (B) Neutralizing titers were determined by the live SARS-CoV-2 or pseudotyped SARS-CoV-2. Mouse serum was heat-inactivated (56°C, 30 min) prior to the neutralization assay. For live SARS-CoV-2 neutralizing assay, 2-fold serially diluted serum was mixed with 100 TCID50 of SARS-CoV-2 and then incubated with Vero E6 cells for three days. The serum dilution showing no cytopathic effect was recorded as the 100% neutralizing titer (NT100). For pseudotyped virus neutralization, lentivirus that carries the full SARS-CoV-2 spike gene and a defective HIV-1 genome encoding the reporter luciferase was used. Serially diluted serum was incubated with 1000 unit of pseudovirus, then added to hACE2-transfected 293 T cells, and incubated for three days. The luciferase activity was measured using the luminescence microplate reader and converted to neutralizing titers. Lines represent means (n = 3–5)
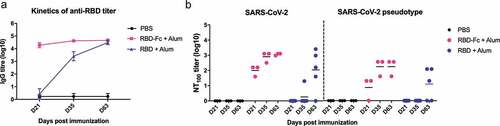
These results indicate that SARS-CoV-2 RBD is a promising candidate for vaccine development. Comparing with the slower and more variable responses observed in the RBD-immunized group, the fusion of the Fc region and RBD largely improves the immunogenicity. One dose of RBD-Fc protein in mice induced a robust antibody response with neutralizing capability against the SARS-CoV-2 (NT100: 1:40 to 1:160), the neutralizing titer is comparable with the recommended titer (1:80 to 1:160) of SARS-CoV-2 therapeutic plasma transfusion.Citation6,Citation7 Thus far, an mRNA-based vaccine that encodes the full-length S protein and adenovirus-based vaccine or DNA-based vaccine expressing full-length S protein are under clinical investigation, and they have reportedly induced moderate neutralizing antibodies against SARS-CoV-2.Citation8 However, these full S protein-based vaccine approaches possess risks of ADE manifestation when the SARS-CoV-2 establishes infections in the vaccine recipients.Citation4 On the other hand, scalability and stability of RBD subunit production are advantages of RBD-based vaccines.Citation9 Although there is no available Fc fusion protein-based vaccine, therapeutic Fc fusion protein drugs have been approved with tolerable safety profile.Citation10 In this study, we report that recombinant SARS-CoV-2 RBD is able to elicit potent antibody response with neutralizing capability, and its further development is warranted.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Alex Ma in Genomics Research Center, Academia Sinica for providing the cDNA.
Additional information
Funding
References
- Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi:10.1038/s41467-020-15562-9.
- Jiang S, He Y, Liu S. SARS vaccine development. Emerg Infect Dis. 2005;11:1016–20. doi:10.3201/1107.050219.
- Lin LCW, Huang CY, Yao BY, Lin JC, Agrawal A, Algaissi A, Peng BH, Liu YH, Huang PH, Juang RH, et al. Viromimetic STING agonist-loaded hollow polymeric nanoparticles for safe and effective vaccination against middle east respiratory syndrome coronavirus. Adv Funct Mater. 2019;29:1807616. doi:10.1002/adfm.201807616.
- Wang Q, Zhang L, Kuwahara K, Li L, Liu Z, Li T, Zhu H, Liu J, Xu Y, Xie J, et al. Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. ACS Infect Dis. 2016;2:361–76. doi:10.1021/acsinfecdis.6b00006.
- Lan J, Yao Y, Deng Y, Chen H, Lu G, Wang W, Bao L, Deng W, Wei Q, Gao GF, et al. Recombinant receptor binding domain protein induces partial protective immunity in rhesus macaques against middle east respiratory syndrome coronavirus challenge. EBioMedicine. 2015;2:1438–46. doi:10.1016/j.ebiom.2015.08.031.
- European Commission. An EU programme of COVID-19 convalescent plasma collec.tion and transfusion; 2020. Accessed 30 June 2020. https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/guidance_plasma_covid19_en.pdf.
- U.S. Food & Drug Administration. Recommendations for investigational COVID-19 convalescent plasma; 2020. Accessed 30 June 2020. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma.
- Diamond MS, Pierson TC. The challenges of vaccine development against a new virus during a pandemic. Cell Host Microbe. 2020;27:699–703. doi:10.1016/j.chom.2020.04.021.
- Chen WH, Strych U, Hotez PJ, Bottazzi ME. The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep. 2020;7:61–64. doi:10.1007/s40475-020-00201-6.
- Czajkowsky DM, Hu J, Shao Z, Pleass RJ. Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med. 2012;4:1015–28. doi:10.1002/emmm.201201379.
