ABSTRACT
This study explored the association between provider recommendation and adolescent vaccine coverage. We analyzed data from the 2008 to 2018 National Immunization Survey-Teen including coverage with one dose of quadrivalent meningococcal conjugate vaccine (MenACWY), Tetanus-diphtheria-acellular pertussis vaccine (Tdap), and one and three doses of Human papillomavirus (HPV) vaccine. We compared vaccine coverage between those who recalled a provider recommendation and those who did not. Among those who received a provider recommendation for MenACWY, coverage trended from 67.8% (2008) to 88.1% (2013), contrasted to those who did not, trending from 30.9% to 73.1%. Among those with a provider recommendation for Tdap, coverage trended from 47.6% to 89.7%, contrasted to those who did not, trending from 35.6% to 82.2%. Among females with a provider recommendation for HPV vaccine, receipt of initial dose of HPV vaccine trended from 57.5% (2008) to 74.3% (2018), contrasted to those who did not, trending from 18.1% to 49.8%, and among males, trended from 17.2% (2010) to 75.1% (2018) for those with a provider recommendation, compared to 0.5% to 44.7% for those without. In 2013, coverage difference by provider recommendation was 26.0% among females for one dose of HPV vaccine and 21.9% for three doses, and among males was 44.8% and 20.8%, respectively, while it was lower at 15% for MenACWY and 7.6% for Tdap. For each vaccine, coverage was higher with a provider recommendation; the largest difference was noted for HPV vaccine. This finding verifies for providers the importance of their recommendation, especially for the HPV vaccine.
Introduction
Since their introduction, vaccine coverage with the quadrivalent meningococcal conjugate vaccine (MenACWY) and the Tetanus-diphtheria-acellular pertussis vaccine (Tdap) has steadily increased and has now reached the Healthy People 2020 target of at least 80% among 13- to 15- year-old adolescents.Citation1,Citation2 In contrast, human papillomavirus (HPV) vaccine coverage for both initiation and series completion has increased slowly and remains far below the Healthy People 2020 goal.Citation1,Citation2 Vaccine coverage, and the factors which contribute to the acceptance of vaccines, is a multifaceted topic that requires investigation.Citation3,Citation4 Investigating the factors that contribute to the acceptance of HPV vaccine is of particular importance, since HPV vaccine coverage remains lower than that of MenACWY and Tdap.Citation1,Citation2 Darden and colleagues found that one of the reasons parents stated most frequently for their child not receiving the HPV vaccine was that it was “not recommended.”Citation5 In general, there is evidence that for individual vaccines, provider recommendations matter.Citation6–11
The degree to which provider recommendation influences coverage with adolescent vaccines across time, vaccine type, and between sexes, warrants further exploration. A recent study found an association between a provider recommendation and HPV vaccine uptake among males.Citation12 However, to our knowledge, no published studies compare the association of a provider recommendation on vaccine coverage between MenACWY, Tdap, and HPV vaccine. The association between a provider recommendation and vaccine coverage may differ between each of the adolescent vaccines and could contribute to the existing differences in adolescent vaccine coverage. The purpose of this study was to compare the associations over time of provider recommendation and coverage for each of the adolescent vaccines: MenACWY, Tdap, and HPV vaccine.
Methods
Data for this study were from the 2008 to 2018 public use files of the National Immunization Survey-Teen (NIS-Teen). This study compared the association between provider recommendation and vaccine coverage across all three adolescent vaccines only for the years in which the NIS-Teen asked respondents about provider recommendation for all three adolescent vaccines (2008–2013). Beginning in 2010, parents of males were surveyed about HPV vaccine and recommendation.Citation13 After 2013, the NIS-Teen reports provider recommendation only for HPV vaccine but not for Tdap or MenACWY.Citation14 Therefore, reports of associations between provider recommendation and vaccine coverage for the years 2014–2018 are only for the HPV vaccine.
The NIS-Teen is sponsored by the National Center for Immunization and Respiratory Diseases.Citation15 It is performed annually and targets families with adolescents between 13 and 17 years of age living in non-institutionalized households in the United States.Citation15 The survey is conducted in two parts. The first part uses random digit dialing to select parents for a telephone interview. The interview includes questions pertaining to parental recall of provider recommendation of vaccines. The second part surveys the providers of those who responded to the first part to verify their child’s vaccination history.Citation14,Citation15 The NIS-Teen calculates and provides design variables including sampling weights so that analyses can generate accurate population estimates that account for non-response and other sources of bias.Citation14
This study included all males and females age 13–17 years with provider verified vaccine status. Responses of “don’t know” or “refused” were treated as missing data for all variables. Data from residents of the US Virgin Islands and Guam were not included.
This study’s primary outcome measure was coverage difference for each of the three vaccines examined. Coverage differences, which quantify the association between provider recommendation and vaccine coverage, are defined as the difference between the percentages vaccinated of those who recall a provider recommendation and those who do not. We estimated coverage differences, and their respective 95% confidence intervals, in separate log-binomial regression models for each vaccine. Similar models estimated coverage differences in groups defined by sex, provider recommendation, and study year.
Initial models included interaction terms to assess if the association between provider recommendation and vaccine coverage differed by sex or by year (treating year as a continuous variable). Significant interaction was found between sex and provider recommendation for the HPV vaccine (both one dose and three doses), as well as MenACWY in 2010 and 2011 and for Tdap in 2009. However, the interaction between sex and provider recommendation for the MenACWY vaccine and Tdap vaccine were deemed not clinically significant (supplementary Table 1), so measures of association were stratified by sex only for the HPV vaccine. Year was determined to be a significant effect modifier for the relationship between provider recommendation and vaccine coverage among all vaccines investigated, and thus, estimates of coverage differences were stratified by year.
We explored coverage with one or more doses of MenACWY, one or more doses of Tdap, and one or more and three or more doses of HPV vaccine. The main predictor variable was recall of a provider recommendation of each vaccine. Parents were asked to recall separately whether a provider recommended each vaccine. Parents were asked if a doctor or other health-care professional had ever recommended that their teen receives “meningitis shots,” “tetanus booster shots,” or “hpv shots.”Citation16
As secondary analyses, provider-verified vaccine coverage was compared, independent of recommendation, between groups defined by sex and the year studied. Sex was reported as either “male” or “female.” The NIS-Teen did not offer respondents the option of reporting sex as “non-binary” or “other.” Other demographic variables used to describe the study population included race/ethnicity, reported by the NIS-Teen as either “Hispanic,” “Non-Hispanic White only,” “Non-Hispanic Black only,” or “Non-Hispanic other + multiple race.” Language was defined as the language in which the interview was conducted and was reported as “English,” “Spanish,” or “Other.” Poverty status was obtained from the NIS-Teen and was based on the US Census poverty thresholds, and reported as “above poverty >$75 K,” “above poverty ≤$75 K,” or “below poverty.” Census region was based on the state of residence and reported as “Northeast,” “Midwest,” “South,” or “West.” The number of children in the household was defined as the number of children under 18 y of age in the household and classified in the NIS-Teen as “one,” “two or three,” or “four or more.”
Percentages of the population reporting a provider recommendation were estimated for each vaccine and were calculated separately for males and females. Construction of separate statistical models was especially relevant for the HPV vaccine, since the ACIP initiated its recommendation for HPV vaccination in different years for males and females.Citation17,Citation18
Data from years 2008–2018 were combined into one data set for analysis based on recommendations in the 2018 User’s Guide.Citation16 SAS version 9.4 was used for producing basic frequencies, while R version 3.5.0 was used for log-binomial regression modeling, using the “survey” package version 3.33–2. All analyses used the design variables including sampling weight for the provider-verified data provided in the NIS-Teen data in order to account for the survey’s complex sampling design and to obtain accurate estimates of provider recommendation and vaccine coverage. Comparisons between estimates were regarded as significant if the associated p value was less than 0.05, or if the estimates’ 95% confidence intervals (CI) failed to overlap.
Because NIS-Teen data are de-identified and publicly available online, the University of Oklahoma Health Sciences Center Institutional Review Board (IRB) for Human Research determined that this study did not meet the criteria for human subjects research.
Results
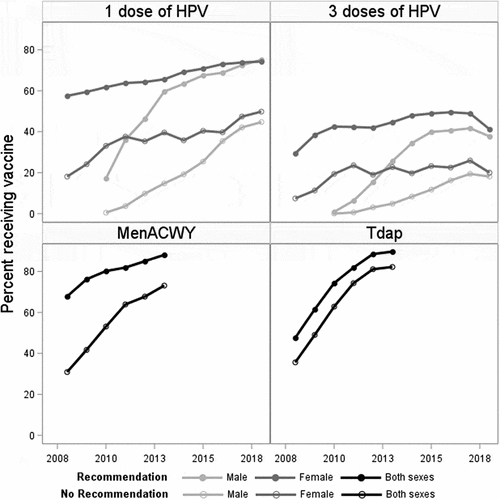
Patient demographics are outlined in . Information pertaining to the number of adolescents, number of telephone surveys, and response rate are included in the Appendix. shows the population estimates for the percent of adolescents who were covered with each vaccine, estimated using log-binomial regression, categorized by provider recommendation and by sex (for HPV vaccine) from 2008 to 2018. The figure depicts that vaccine coverage is higher across all vaccines among those with a provider recommendation compared to those without a recommendation. These coverage differences are demonstrated by the width of the gap between the lines shown in . The association between provider recommendation and vaccine coverage is quantified in terms of coverage differences (). Coverage differences are defined for each vaccine as the difference between the percentages vaccinated of those who recall a provider recommendation and those who do not. In 2013, the coverage difference for the MenACWY vaccine was 15% compared to 7.6% for Tdap. Among females in 2013, the difference in the coverage with one dose of HPV was 26.0% and 21.9% for three doses, and for males, it was 44.8% for one dose and 20.8% for three doses.
Table 1. Patient demographics and percentage of respondents who recalled a provider recommendation for MenACWY, Tdap, and HPV vaccines.a,b
Table 2. Coverage differencesa [%(95%CI)] for each vaccine
Log-binomial modeling, which included assessment of recommendation-time interactions, revealed that the association between provider recommendation and vaccine coverage changed across time for MenACWY, Tdap, and HPV vaccine. The recommendation-time interactions detected in the log-binomial models were also seen in the coverage differences calculated using those models’ estimates. For MenACWY, the coverage difference decreased from 36.9% in 2008 to 15.0% in 2013. For Tdap, the difference decreased from 12.5% in 2009 to 7.4% in 2012 and saw no significant change before 2009 or after 2012 (). Among females, for one dose of HPV vaccine, coverage difference was 39.4% in 2008 and 24.4% in 2018 with year-to-year variability noted (). For three doses of HPV vaccine, among females, the coverage difference was 22.0% in 2008 and 21.2% in 2018. Among males, the difference in the coverage for HPV vaccine increased from 16.7% for one dose and 0.8% for three doses in 2010, to 44.8% and 20.8%, respectively, in 2013. After 2013, coverage difference for HPV vaccine among males decreased to 30.4% in 2018. For three doses among males after 2013, the coverage difference increased to 28.2% in 2015 before decreasing to 19.6% in 2018 ().
Population estimates for vaccine coverage, the percentage of adolescents who received each vaccine, are outlined in . The vaccine coverage for MenACWY and Tdap did not differ between males and females. Among those who received a provider recommendation for MenACWY, coverage trended from 67.8% (2008) to 88.1% (2013), contrasted to those who did not, trending from 30.9% to 73.1%. Among those with a provider recommendation for Tdap, coverage trended from 47.6% to 89.7%, contrasted to those who did not, trending from 35.6% to 82.2%. HPV vaccine coverage differed by sex. Among females with a provider recommendation for HPV vaccine, receipt of one dose of the vaccine trended from 57.5% (2008) to 74.3% (2018), compared to 18.1% to 49.8% among those without a recommendation. Receipt of three doses of the HPV vaccine among females with a provider recommendation trended from 29.4% to 49.5% in 2016 and then decreased to 41.2%, contrasted to those who did not, trending from 7.4% to 25.9% in 2017 and then decreased to 20% in 2018. Among males with a provider recommendation, receipt of one dose of HPV vaccine rose from 17.2% (2010, first-year males were surveyed about provider recommendation) to 75.1% (2018) compared to those without a recommendation from 0.5% to 44.7%. Receipt of three doses of HPV vaccine among males with a provider recommendation trended from 0.9% to 41.7% in 2017 and then decreased to 37.7% in 2018, compared to 0.03% to 19.4% in 2017 and then decreased to 18.1% in 2018 for those without.
Table 3. Estimated coverage [%(95%CI)]a of adolescents for MenACWY, Tdap, and HPV vaccines, 2008–2018
Discussion
This novel study comparing the association between provider recommendation and vaccine coverage for three adolescent vaccines found that recalling a provider recommendation was associated with a higher probability of vaccination. This difference was greater for HPV vaccine than for the MenACWY or Tdap vaccines (). The association between provider recommendation and vaccine coverage, calculated as a difference in percentages, coverage difference, or risk difference, was higher for the HPV vaccine than for the MenACWY or Tdap vaccines. Therefore, a provider recommendation may hold more importance for the HPV vaccine compared to MenACWY and Tdap.
Our findings highlight an important question: Why is HPV vaccine coverage low despite the positive effect of provider recommendation? One explanation is that the type of provider recommendation, including its strength or content, may differentially influence vaccine coverage. Moss and colleagues examined communication styles and adolescent vaccine coverage using the NIS-Teen 2010 dataset, and found that the communication style used influences vaccine coverage.Citation7 Our study builds on this, analyzing data from 2008 to 2018 and examining the association between recommendation and vaccine coverage over time and among adolescent vaccines. However, our study did not address the type of recommendation provided. There is evidence that providers use weaker recommendations for HPV vaccine than for the other adolescent vaccines.Citation19 Further study should be performed to better understand differences in the type of provider recommendation between the adolescent vaccines, and how this impacts vaccine coverage.
Another explanation for low HPV vaccine coverage despite evidence that provider recommendation appears to be effective may be related to low rates of HPV vaccination in the population without a provider recommendation, which could be related to societal factors and misconceptions outside the practice setting. There is evidence that there are misconceptions regarding the HPV vaccine.Citation5,Citation20 However, Nyhan and colleagues found an intervention that corrected misperceptions about the influenza vaccine actually decreased intent to vaccinate among those inclined not to vaccinate.Citation3 While provider recommendation influences HPV vaccine coverage, we may not yet understand how to maximize recommendation effectiveness.
Other societal factors may contribute to the high coverage of MenACWY and Tdap vaccines, despite the lower coverage differences that we observed for these vaccines. Such factors include social norms and greater parental awareness of outbreaks of diseases such as pertussis and meningitis. State vaccine mandates may contribute to some of the differences observed, as 36 states mandate MenACWY receipt, as contrasted to 50 for Tdap, and four for HPV.Citation21 The variation in vaccine mandates further highlights the importance of a provider’s recommendation in favor of the HPV vaccine, as very few states mandate receipt of the HPV vaccine.
In addition, Lindley and colleagues examined differences in HPV vaccine receipt between males and females and found that among parents who intended not to vaccinate their child with the HPV vaccine, the most common reason cited by parents of males was lack of provider recommendation, whereas for parents of females, it was lack of knowledge about the vaccine.Citation22 This highlights the need for increased provider recommendation of the HPV vaccine, especially for males.
Among males, this study shows that the effect of a provider recommendation increased over time for HPV vaccine, but in more recent years has decreased. This could have been due to the low initial coverage of HPV vaccine among males. Moreover, parents of males may be more accepting than parents of females of a provider’s recommendation for a vaccine that prevents a sexually transmitted disease. In a 2010 review of the literature on the acceptability of HPV vaccine among males, Liddon et al. found that among mothers of males, support of HPV vaccination was higher with knowledge that the vaccine protected against genital warts and cervical cancer compared to knowledge that the vaccine protected against cervical cancer alone.Citation23 Other factors, including slow acceptance of a new vaccine, may have contributed to this increase in effect of provider recommendation on HPV vaccine coverage for males over time. The decrease in association between provider recommendation and HPV vaccine coverage for males in recent years requires further exploration. This decrease, particularly for three doses, may be partially explained by the fact that in 2016, the Advisory Committee on Immunization Practices changed their recommendation, including a two-dose schedule for those who initiate the HPV vaccine series at ages 9 through 14 y.Citation24 Thus, assessing receipt of three doses of vaccine may underestimate series completion for years 2016–2018.
Limitations
This study has several limitations. An observational study cannot establish causality between provider recommendation and vaccine coverage. In addition, parental report of provider recommendation occurred after receipt of vaccine and could be subject to recall bias. Respondents might be more likely to recall receiving a provider recommendation that is aligned with their ultimate decision to receive a vaccine. Those who receive a vaccine may be more likely to recall a recommendation, and those who elect not to receive a vaccine may be more likely to fail to recall a recommendation. In this worst-case scenario, the study would overestimate the link between recommendation and vaccination. However, vaccine coverage was highest for MenACWY and Tdap, yet recall of a provider recommendation for these vaccines was lower than for HPV vaccine. These findings are evidence that recall bias did not influence parental responses.
Another limitation is that some adolescents classified as having adequate provider data may have incomplete provider data. Also, the phrasing used to ask parents if they received a provider recommendation for the Tdap vaccine did not include a description of the components of the vaccine other than tetanus, and thus, some parents may not have recalled a recommendation for the Tdap vaccine if they identified it as a pertussis or diphtheria vaccine.
Additionally, there are limitations related to this telephone survey with concern for non-response and a shift to cell phone use by respondents. While there is potential for under- or over-representation of certain populations, the NIS-Teen’s complex survey design accounts for this possibility. Despite the limitations of the NIS-Teen data set, it is a large national survey that is strengthened through the provider report of vaccine coverage and provides the ability to make national inferences.
Further research is needed on provider recommendation and vaccine coverage, including analysis of the impact of recommending all three adolescent vaccines concurrently compared with a provider recommendation emphasizing HPV vaccine, comparisons of transcripts of provider recommendations with parent perceptions, and assessing the reasons for not vaccinating males with HPV vaccine. Further research is also needed on the various types of recommendations, as it is important to identify the impact of each on vaccine delivery. Also, this study compared data for all three adolescent vaccines from 2008 to 2013 because NIS-Teen asked respondents about provider recommendation for all three adolescent vaccines during these years, and in subsequent years provider recommendation is only reported for HPV vaccine. Thus, comparisons were not made or analyzed after 2013. However, this study was able to show the association between provider recommendation and HPV vaccine in more recent years. This is a potential area of research for practice-based research networks, who could involve their participant clinics in studies designed to address changes in provider recommendation, or other factors, that might contribute to adolescent vaccine coverage. In addition, the societal perceptions of the MenACWY and Tdap vaccines, and public health factors that contribute to their acceptability should be studied.
Conclusion
For each vaccine studied, vaccine coverage was higher among those with a provider recommendation compared to those without a recommendation, with the largest coverage difference noted for HPV vaccine. Provider recommendation is an essential factor associated with coverage with the HPV vaccine, especially for males. HPV vaccine coverage is much lower in those whose parents do not report a provider recommendation. The results of this study should emphasize to providers the vital nature of their recommendation to parents in favor of adolescent vaccine receipt, particularly for HPV vaccine.
Disclosure of Potential Conflicts of Interest
Alexandria C. Caldwell has no financial disclosures. Christi A. Madden has no financial disclosures. David M. Thompson has no financial disclosures. Michael C. Garbe has no financial disclosures. James R. Roberts has no financial disclosures. Robert M. Jacobson serves on external safety review committees for two post-licensure safety studies of HPV vaccine both funded by Merck & Co. and on an external data monitoring committee for a series of pre-licensure trials of a pneumococcal vaccine also funded by Merck & Co. Paul M. Darden has no financial disclosures.
CASRO Response Rate
“The NIS-Teen CASRO response rate equals the product of the resolution rate (83.5%, Row E), the screening completion rate (86.1%, Row G), and the interview completion rate among eligible households (71.1%, Row I). The resolution rate is the percentage of the total telephone numbers selected that are classifiable as non-working, nonresidential, or residential. The screening completion rate is the percentage of known households that are successfully screened for the presence of age-eligible teens. The interview completion rate is the percentage of households with one or more age-eligible teen that complete the household interview.”
-Data User’s Guide for the 2013 NIS-Teen Public-Use Data File p.10
Supplemental Material
Download MS Word (25 KB)Acknowledgments
Research reported in this publication was supported by the National Institutes of Health under Award Numbers UG1OD024950, U54GM104938, and Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant number D55HP23210. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsoring agency was not involved in study design, collection, analysis or interpretation of data, in the writing of the report or in the decision to submit this manuscript for publication.
Supplementary materials
Supplemental data for this article can be accessed online at http://dx.doi.org/10.1080/21645515.2020.1817713.
Additional information
Funding
References
- U.S. Department of Health and Human Services. Healthy People 2020. Washington (DC); 2010. [accessed 2018 August 8]. www.healthypeople.gov/2020
- Walker TY, Elam-Evans LD, Yankey D, Markowitz LE, Williams CL, Fredua B, Singleton JA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(33):718–23. doi:10.15585/mmwr.mm6833a2.
- Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33(3):459–64. doi:10.1016/j.vaccine.2014.11.017.
- Nyhan B, Reifler J, Richey S, Freed GL. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133(4):e835–42. doi:10.1542/peds.2013-2365.
- Darden PM, Thompson DM, Roberts JR, Hale JJ, Pope C, Naifeh M, Jacobson RM. Reasons for not vaccinating adolescents: national Immunization Survey of Teens, 2008-2010. Pediatrics. 2013;131(4):645–51. doi:10.1542/peds.2012-2384.
- Darden PM, Jacobson RM. Impact of a physician recommendation. Hum Vaccin Immunother. 2014;10(9):2632–35. doi:10.4161/hv.29020.
- Moss JL, Reiter PL, Rimer BK, Brewer NT. Collaborative patient-provider communication and uptake of adolescent vaccines. Soc Sci Med. 2016;159:100–07. doi:10.1016/j.socscimed.2016.04.030.
- Rosenthal SL, Weiss TW, Zimet GD, Ma L, Good MB, Vichnin MD. Predictors of HPV vaccine uptake among women aged 19-26: importance of a physician’s recommendation. Vaccine. 2011;29(5):890–95. doi:10.1016/j.vaccine.2009.12.063.
- Staras SA, Vadaparampil ST, Patel RP, Shenkman EA. Parent perceptions important for HPV vaccine initiation among low income adolescent girls. Vaccine. 2014;32(46):6163–69. doi:10.1016/j.vaccine.2014.08.054.
- Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008-2009. Pediatrics. 2011;128(5):830–39. doi:10.1542/peds.2011-0950.
- Smith PJ, Stokley S, Bednarczyk RA, Orenstein WA, Omer SB. HPV vaccination coverage of teen girls: the influence of health care providers. Vaccine. 2016;34(13):1604–10. doi:10.1016/j.vaccine.2016.01.061.
- Lu PJ, Yankey D, Fredua B, O’Halloran AC, Williams C, Markowitz LE, Elam-Evans LD. Association of provider recommendation and human papillomavirus vaccination initiation among male adolescents aged 13-17 Years-United States. J Pediatr. 2019;206:33–41 e1. doi:10.1016/j.jpeds.2018.10.034.
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases, National Center for Health Statistics (U.S.). National Immunization Survey-Teen. A user’s guide for the 2014 public-use data file 2015. [accessed 2020 May 2] http://www.cdc.gov/nchs/nis/data_files_teen.htm
- Smith PJ, Hoaglin DC, Battaglia MP, Khare M, Barker LE. Statistical methodology of the National Immunization Survey, 1994-2002. Vital Health Stat. 2005;2(138):1–55.
- Jain N, Singleton JA, Montgomery M, Skalland B. Determining accurate vaccination coverage rates for adolescents: the National Immunization Survey-Teen 2006. Public Health Rep. 2009;124(5):642–51. doi:10.1177/003335490912400506.
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases, National Center for Health Statistics (U.S.). National Immunization Survey-Teen. A user’s guide for the 2013 public-use data file 2014. [accessed 2020 May 2] http://www.cdc.gov/nchs/nis/data_files_teen.htm
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2007;56(RR–2):1–24.
- Centers for Disease C, Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males–Advisory committee on immunization practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705–08.
- Shay LA, Street RL Jr, Baldwin AS, Marks EG, Lee SC, Higashi RT, Skinner CS, Fuller S, Persaud D, Tiro JA, et al. Characterizing safety-net providers’ HPV vaccine recommendations to undecided parents: A pilot study. Patient Educ Couns. 2016;99(9):1452–60. doi:10.1016/j.pec.2016.06.027.
- Roberts JR, Thompson D, Rogacki B, Hale JJ, Jacobson RM, Opel DJ, Darden PM. Vaccine hesitancy among parents of adolescents and its association with vaccine uptake. Vaccine. 2015;33(14):1748–55. doi:10.1016/j.vaccine.2015.01.068.
- State mandates on immunization and vaccine-preventable diseases [Internet]. 2019 [accessed 2019 August 11]. https://www.immunize.org/laws/
- Lindley MC, Jeyarajah J, Yankey D, Curtis CR, Markowitz LE, Stokley S. Comparing human papillomavirus vaccine knowledge and intentions among parents of boys and girls. Hum Vaccin Immunother. 2016;12(6):1519–27. doi:10.1080/21645515.2016.1157673.
- Liddon N, Hood J, Wynn BA, Markowitz LE. Acceptability of human papillomavirus vaccine for males: a review of the literature. J Adolescent Health. 2010;46(2):113–23. doi:10.1016/j.jadohealth.2009.11.199.
- Meites E, Kempe A, Markowitz L. Use of a 2-dose schedule for human papillomavirus vaccination – updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405–08.
Appendix
Number of completed telephone surveys, number of teens with adequate provider data, and final weighted frequency used in analysis (Excludes Virgin Islands and Guam):
From the 2018 NIS-Teen Data Users Guide (Landline)
From the 2018 NIS-Teen Data Users Guide (Cellphone)