ABSTRACT
COVID-19 caused by the virus SARS-CoV-2 has gripped essentially all countries in the world, and has infected millions and killed hundreds of thousands of people. Several innovative approaches are in development to restrain the spread of SARS-CoV-2. In particular, BCG, a vaccine against tuberculosis (TB), is being considered as an alternative therapeutic modality. BCG vaccine is known to induce both humoral and adaptive immunities, thereby activating both nonspecific and cross-reactive immune responses in the host, which combined could effectively resist other pathogens including SARS-CoV-2. Notably, some studies have revealed that SARS-CoV-2 infectivity, case positivity, and mortality rate have been higher in countries that have not adopted BCG vaccination than in countries that have done so. This review presents an overview of the concepts underlying BCG vaccination and its nonspecific immuological effects and protection, resulting in ‘trained immunity’ and potential utility for resisting COVID-19.
Introduction
Coronaviruses (CoVs) are generally regarded as nonlethal human pathogens, mostly causing common cold.Citation1 However, during the last 18 years, two human pathogenic CoVs, namely severe acute respiratory syndrome Coronavirus (SARS-CoV) and Middle-East respiratory syndrome Coronavirus (MERS-CoV), have caused epidemics that originated in China (2002) and Saudi Arabia (2012), respectively, and spread over to several countries with high morbidity and mortality.Citation2 Thus, in the recorded history of humans, the existing SARS-CoV-2 outbreak is the third human CoV outbreak. SARS-CoV-2 has higher genetic relatedness with SARS-CoV (nearly 80%) than with MERS-CoV (nearly 50%).Citation3
COVID-19, a Corona Virus Disease, emerged in early December 2019 and is caused by a novel CoV named SARS-CoV-2 due to the association of COVID-19 symptoms with those of severe acute respiratory distress (SARD) and the resemblance of COVID-19 with the previous human CoV disease, SARS, that originated in Guangdong region of China in 2002 and spread over 27 countries before its termination in mid-2003.Citation4 Since the first COVID-19 case was confirmed in Wuhan city of Hubei province in China on December 7, 2019, COVID-19 has gripped essentially all countries in the world, infected over 25 million confirmed people and killed almost one million people (WHO COVID-19 Situation report-201, August 8, 2020).Citation5 Consequent to its panic spread, morbidity, mortality, economic significances, COVID-19 was declared by the World Health Organization (WHO) as a Public Health Emergency of International Concern (Pandemic) on February 1, 2020 and on March 11, 2020 as pandemic.Citation6 Though the origin source of SARS-CoV-2 has not been traced to date, the virus is assumed to be of zoonotic origin (from animals), primarily bats, followed by maintenance of virus in the ecosystem through human-human transmission cycle. A few of the ongoing studies have further expanded COVID-19 hosts, including dogs, cat, and few wild animals.Citation4,Citation7
COVID-19, in comparison to SARS, has different epidemiological features as reflected in demographic studies. SARS-CoV-2 can replicate in both upper and lower respiratory tracts, causing mild or no symptoms in the early stages of infection.Citation8–10 Thus, a person infected with SARS-CoV-2 can spread the infection in early-stages or during the incubation period, causing difficulty in controlling the pandemic. In contrast, SARS-CoV is transmitted when the patients are severely ill, and almost no transmission occurs in the early stage.Citation11,Citation12
Therefore, the current COVID-19 syndemic is extreme and harder to control than the previous SARS outbreak. In the absence of any suitable treatment for SARS-CoV-2 infection, only supportive care is being provided to the patients of COVID-19.Citation13 Different types of therapies that have been used before for the treatment of MERS-CoV and SARS-CoV infections or other viral diseases are being considered for treatment of SARS-CoV-2 infection, as it is a new disease and lack any standard therapeutic option. These therapies include immunosuppressants, steroids, anti-viral drugs, plasma from recuperated patients, Chinese medications, and repurposed drugs. Pharmaceutical companies are in a race to produce antibodies and vaccines against SARS-CoV-2.Citation12,Citation14
Bacillus Calmette–Guérin (BCG) vaccine is widely used in infancy as prevention against tuberculosis (TB). Epidemiological and randomized studies suggest that BCG vaccine provides a protective effect against infant mortality through nonspecific heterologous protection to other infectionsCitation15 maybe through innate immune epigenetic mechanisms.Citation15–17 Exposure to BCG vaccine can reduce the severity of COVID-19 and may lower mortality, and thus, it may be helpful in development of therapeutic or preventive strategies that can impact SARS-CoV-2 infections.
This review provides a comprehensive summary on the beneficial effects and protection offered by the BCG vaccine against COVID-19. As of now, only a limited approved therapeutics and/or vaccines are available/or are in final clinical trials for disease control, and as a result, the COVID-19 pandemic has become a huge threat/crisis to global public health.Citation12,Citation18
SARS-CoV-2/COVID-19 treatment alternatives
In the absence of an approved, safe and effective vaccine or a standard therapeutic option for mitigating the COVID-19 pandemic, scientists and public health experts have suggested the use of currently existing licensed vaccines/drugs, i.e., called repurposing of vaccines/drugs (used against other bacterial/viral infections) such as BCG vaccine,Citation19 antimicrobials, didanosineCitation20 and hydroxychloroquine,Citation21,Citation22 antiparasitics, ivermectinCitation23 or combination of hydroxychloroquine and ivermectin,Citation24 antibiotics, azithromycinCitation25 or antiviral drugs ARTCitation26 like lopinavir/ritonavir, favipiravir, and remdesivirCitation26–28 for the treatment of COVID-19.Citation29 Similarly, many advanced and novel therapeutics are also being tested for the treatment of COVID-19, including CAR T-cell,Citation30 stem-cell,Citation31 RNA Synthesis inhibitors,Citation32 Protease Inhibitors,Citation33 monoclonal antibodies,Citation34,Citation35 and immunoglobulins-based therapy.Citation36
COVID-19 vaccines-update
A new mRNA vaccine (mRNA-1273) was developed by a clinical stage biotechnology company, named Moderna, Inc., USA, which is a pioneering messenger RNA (mRNA) therapeutics and vaccines firm, has initiated Phase-I clinical trials on March 16, 2020 against SARS-CoV-2. This mRNA vaccine encoding for a prefusion stabilized form of the Spike (S) protein of SARS-CoV-2.Citation37 The Phase-I study evaluated the safety and immunogenicity of three dose levels of mRNA-1273 (25, 100, and 250 μg) administered on a two-dose vaccination schedule, given 28 days apart in 45 healthy adults enrolled in the study. The phase 3 clinical trial of mRNA-1273 has started in the U.S. Clinical research site enrolling approximately 30,000 adult individuals.
Ad5-nCoV is the first COVID-19 vaccine in a clinical trial in China.Citation38 The vaccine developed by CanSinoBIO’s adenovirus-based viral vector vaccine technology platform. In the human trial of the vaccine, it is found safe and induces a rapid immune response. The AZD1222 CoV vaccine, formerly known as ChAdOx1 nCoV-19, developed from ChAdOx1 virus. The ChAdOx1 vaccine is in clinical trials to prevent infection of SARS-CoV-2. AstraZeneca Plc announced it selected Emergent BioSolutions to produce the 300 million doses of the COVID-19 vaccine candidate. The oxford- AstraZeneca in the phase I/II trial tolerated and generated strong immune responses in all the participants in the United Kingdom. The vaccine has entred in its final trial in Brazil along with studies going on in the United Kingdom and South Africa. Similarly Pfizer also trigerred strong T-cell response in phase I/II and has entered the pase III trial. The Sinovac Biotech Ltd. developed the CoronaVac SARS-CoV-2 vaccine based on an inactivated virus. Sinovac Biotech got approval from Chinese authorities to conduct both the phase-I and phase-II human clinical trials for CoronaVac in China. DNA vaccine INO-4800 designed to prevent SARS-CoV-2 from infecting humans is under phase-I/II clinical trial by INOVIO and Seoul National University Hospital and is expected to be available by November 2020.Citation39
The Johnson and Johnson and Janssen Pharmaceutical research teams, in collaboration with Beth Israel Deaconess Medical Center, part of Harvard Medical School, have selected its lead vaccine candidate Ad26-COV2-S, recombinant for prevention of coronavirus infection. They have started the Phase-I/II a first-in-human clinical trial of its investigational SARS-CoV-2 vaccine, Ad26.COV2-S, recombinant. The NVX-CoV2373 is a prefusion protein CoV vaccine candidate made using Novavax’s proprietary nanoparticle technology, Matrix-M, which is an adjuvant to enhance immune responses and stimulate high levels of neutralizing antibodies. In preclinical trials, NVX-CoV2373 showed efficient binding with receptors of the SARS-CoV-2. Sanofi-GSK using a combination of innovative technologies developed an adjuvanted SARS-CoV-2 vaccine to prevent COVID-19 disease. The companies may start phase-I clinical trials in the second half of 2020 and, aim to complete the development of the vaccine by the second half of 2021. Vaxart develop an oral H1 influenza vaccine targeting the SARS-CoV-2. The phase-II clinical study showed that oral H1 flu tablet vaccine protects against influenza infection after just one dose. Based on these results, it is considered to protect against mucosal respiratory viruses such as SARS-CoV-2.
Tuberculosis and bacillus calmette–guérin (BCG) vaccine
An important potential approach to COVID-19 control is the use of BCG vaccineCitation40 for COVID-19, which was developed by Jean-Marie Canille Guerin and Albert Calmette around 100 years back for immunization against Tuberculosis (TB), one of the most dreaded human diseases caused by M. tuberculosis (MTB). TB is predominant in developing countries and connected with poverty status, and control of TB is at least an issue of equity and human rights.Citation41 In areas with high burden of TB, control strategies for TB are overpowered by the rising cases of TB, occurring in parallel with HIV/AIDS pandemic.Citation42
Further, the situation is being complicated due to the emergence of drug resistance among Mycobacterium tuberculosis, i.e., multidrug resistant tuberculosis (MDR-TB) or extensively drug resistant tuberculosis (XDR-TB). TB is endemic, and no country is TB-free in the world. It is assessed that 33% of the world population is infected with M. tuberculosis but shows no symptoms, and only 5–10% develop TB during their lifetime.Citation43 In high burden countries, the annual risk of TB infection in children is estimated to be 0.5–2%. Childhood deaths from TB are commonly caused by meningitis or disseminated disease. BCG vaccine is a live, debilitated strain of M. bovis, which is closely related to M. tuberculosis, but the bacterium is commonly responsible for causing TB in cattle, known as bovine-TB. The BCG vaccine has existed for more than 100 years and is one of the most commonly used vaccines even today, worldwide.Citation44
BCG vaccine coverage
BCG vaccine was included into the Expanded Programme on Immunization (EPI) (WHO) in 1974, and has been used as the primary TB vaccine since then. Every year, around 100 million children are given BCG vaccine worldwide.Citation45,Citation46 Among children, BCG prevents infection by most of the severe forms of TB, and reduces the incidence of miliary and meningeal TB by around 85% and TB-related deaths by 66%.Citation47,Citation48 BCG has been prescribed during childhood in nations having a high burden of TB for decades. Currently, 152 low- and middle-income countries (LMIC) have adopted universal child vaccination policy at birth or in the first week of birth.Citation49
The data on BCG vaccination is available from 180 countries, and among them currently, 157 countries recommend a policy of universal BCG vaccination. The remaining countries, either due to questionable efficacy of the BCG vaccine in protection against TB infection or due to reduction in incidence of TB, have either stopped BCG vaccination or do not suggest mass BCG immunization and rather provide vaccination only to “at-risk” groups. Some countries, like the United Kingdom, run programs for universal BCG vaccination. In contrast, others, like the United States and Canada, either recommend BCG for high-risk groups or do not support nationwide BCG vaccination (https://en.wikipedia.org/wiki/BCG_vaccine 2020).
During 1940–1980, many nations started the program of BCG vaccination; however, a few nations, such as, Romania and Uzbekistan, had started vaccination much earlier in 1928 and 1937, respectively, while few African countries, for example, Nigeria and Sierra Leone, began to provide BCG vaccination as late as in 1991 and 1990, respectively. To date, nine countries have stopped the program of universal BCG vaccination. Spain and Denmark were among the first countries to stop in 1981–1986, while Austria and Germany halted the vaccination in 1990 and 1998, respectively. The remaining nations, including the Isle of Man, Slovenia, the United Kingdom, Finland, and France, stopped their respective BCG vaccination programs somewhere between 2005 and 2007. While some of these nations have stopped vaccination programs universally, many of them continue to provide BCG vaccination to high-risk groups or individuals, including those involved in occupations with high-risk of exposure to TB and children born in an environment with high burden of TB.Citation50
BCG vaccine immunomechanisms
The live attenuated BCG-TB vaccine proficiently induces nonspecific cross-protection against pathogens, which may be different from the cause of target disease.Citation51 BCG vaccination in children reduces mortality and elicits an improved innate immune response against different microorganisms in addition to M. tuberculosis, such as Staphylococcus aureus and Candida albicans. This nonspecific immune protection is provided by the innate immune cells, including monocytes and natural killer cells, and this development does not depend on T or memory B cells. The monocytes are epigenetically reprogrammed at the site of immunization such that there is histone modification in the promoter region of genes encoding proinflammatory cytokines and hence, there is a long-term change in their ability to respond to novel stimuli. These monocytes exhibit enhanced production of cytokines like IL1β, IFNγ and TNFα in response to infection with several different pathogens.Citation52,Citation53 The phenomenon of producing a memory-like response in innate immune cells is known as “trained immunity.”Citation54
BCG was first used as an immunotherapy for the treatment of bladder cancer around 40 years ago.Citation55 BCG being a liquid drug can be deposited directly into the bladder through a catheter. Immunotherapy is used to induce the immune system for attacking cancer cells. Several immune molecules and cell types were indetified involvedCitation56–58 in the action of BCG. It is apparent that the effects of BCG on the immune system are complex, multifactorial, and likely to evolve. The data suggest that the anticancer effects of BCG are due to the direct action on tumor cells by BCG and the host’s immune response.Citation58 The activation of the immune system led to recognition and subsequent destruction of tumor cells through nonspecific and specific cell-mediated mechanisms.Citation57The concept of trained immunity was first proposed by Netea et al.Citation59 and is defined as an improved nonspecific response by the innate immune cells to secondary infection caused by different or same microorganisms.Citation59 In adult humans, BCG vaccine induces trained phenotype in monocytes, leading to an increase in their capacity to secrete proinflammatory cytokines, which provide nonspecific protection against S. aureus and C. albicans.Citation15,Citation16,Citation60 BCG vaccination also induces the adaptive immune response, which leads to activation of both CD4+ and CD8 + T cellsCitation61 and increased production of interferon-gamma (IFN-γ), which increases the anti-mycobacterial activity of macrophages.Citation62 Further, cytokines help in B cell activation and successive antigen-specific antibody production by plasma cells. After vaccination, a pool of mycobacteria-specific CD8 + T cells multiplies, and remains in the peripheral blood for around 10 weeks. Beyond providing protection against TB, BCG vaccine also has some other clinical applications, particularly in immunotherapy, such as treatment of autoimmune diseases like type-1 diabetes and various cancers including melanoma.Citation54
BCG induced protection is also reported especially in case of respiratory virus infections. A study on BCG vaccination done in South Africa and in Guinea-Bissau reported up to 70% reduction in the incidence of respiratory tract infection including that of Respiratory Syncytial virus. A similar study showed the protective efficacy of BCG vaccination against influenza A virus and Herpes simplex virus (HSV) where it was shown that peritoneal macrophages are significantly activated by the vaccination to produce protective cytokines.Citation63
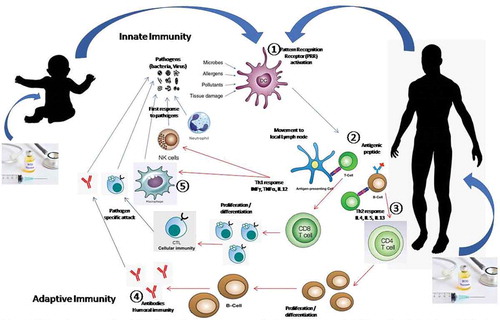
The two types of immune responses are innate and adaptive immune responses . In innate immune system, cells recognize and respond to pathogens in a nonspecific manner. Various types of cells, including B and T lymphocytes, dendritic cells, eosinophils, macrophages, mast cells, and neutrophils are involved in an innate immune response.Citation15,Citation64 Antigen presenting cells (APCs) kill the invading pathogens by engulfing and digesting them. Later, they move to the lymph node, where the antigens are presented to the naïve T cells, resulting in differentiation of T helper cells into different effector T cells (CD8 + T Cells/Th1; CD4 + T Cells/Th2).
The immediate defense against an infection is provided by the innate immune system and is vital for effective initiation of adaptive immunity.Citation65,Citation66 In contrast, adaptive immunity is mediated by T cells, B cells, and memory cells and it provides antigen-specific responses. T and B cells recognize a variety of antigens through unique T-cell receptors (TCRs) and B-cell receptors (BCRs), respectively. The activation of T and B cells lead to the development of killer cytotoxic T cells (cellular immunity)Citation67 and production of antibodies by differentiated plasma B cells (humoral immunity).Citation68 The immune response is initiated by the interaction between the innate immune cells and adaptive immune system.
Figure 1. Innate and adaptive immune response to a pathogen (Virus/Bacteria/Parasite)
A new live attenuated TB vaccine, named MTBVAC, has been found to induce not only specific T-cell immunity, but also long-term epigenetic and metabolic reprogramming of the cells of innate immune system through a process termed ‘trained immunity.’Citation69 The trained immunity has been found to confer protection against experimental lethal pneumonia infections.Citation70 The modified MTBVAC vaccine promotes proinflammatory genes, and facilitates an enhanced response after nonrelated bacterial stimuli.
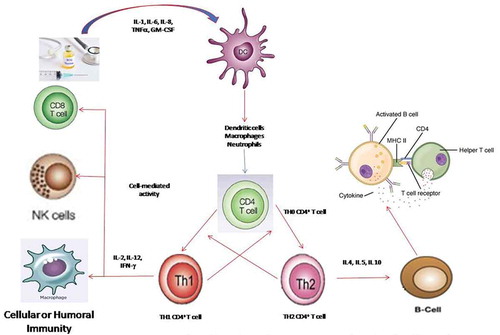
The shift in immune response toward cellular or humoral immunity occurs through stimulation of phagocytes and the cytokine environment , which aids in differentiation of naïve CD4 + T cells into TH1 and/or TH2 cells.Citation69 Appropriate stimulation of TH1 immune responses is needed to achieve the therapeutic outcome of BCG vaccine. Inhibition of TH1 and TH2 immune responses occurs through (interleukin) IL-10 and (interferon-gamma) IFN-γ, respectively. Blocking IL-10 or inducing IFN-γ can lead to TH1-dominated immunity that is essential for BCG-mediated heterologous immunity.Citation15
Figure 2. Schematic representation of BCG-triggered immune response in cell-mediated immunity
BCG vaccine based alternative treatment of COVID-19
Due to the unavailability of a standard and/or specific drug or an appropriate safe and effective approved vaccine to combat in the battle against COVID-19 pandemic, globally researchers are working to find some alternative therapeutics. Usually, the mechanisms outlined for the release of a safe and effective molecule/drug for use in healthcare settings require a long chain of safety, efficacy and feasibility checks. Thus, a drug/vaccine already in use holds better promise in current emergency and global public health crisis. Therefore, scientists and pharmacologists across the globe are searching for vaccines/drugs in use to flatten the explosive graph of SARS-CoV-2-affected population worldwide.
The elderly population is more susceptible than younger people are to many diseases, including influenza, pneumococcal and zoster. In addition, the information on the occurrence of COVID-19 worldwide has provided a strong indication of elderly population more prone to the disease. This increased susceptibility is generally believed to be a result of the aging immune system, which is manifest in lower levels of immune responses to such vaccines in terms of both antibody levels as well as cellular immunity. The figures for age susceptibility pattern are almost similar in SARS-CoV, MERS-CoV, and the current SARS-CoV-2 infections.Citation71 Even if infection is established in young people, symptoms remain mild, and recovery is fast. Salman and SalemCitation72 summarized the influence of routine childhood immunization (usually five to six vaccines) on protection against COVID-19. A few vaccines such as Hepatitis B, rubella, mumps, poliomyelitis, and varicella also lead to development of immunity against SARS-CoV-2, and thereby, provide protection against the invasion of the lung cells. The mechanisms have revealed that TH1 cells (CD4+) are stimulated by killed or inactivated vaccines. The activated TH1 cells then lead to an increase in the production of IFN-γ, IL-2, and IL-12, which aid in maturation of killer T cells and increase the cytotoxic activity of NK cells, thereby, terminating the virus-infected cells.
A study published by the New York Institute of Technology has proposed BCG vaccine as an alternative treatment that is in use to treat TB in humans since 1921.Citation73 In the study, analysis of different countries based on wide variation in the intensity of the disease has revealed that in the countries where BCG vaccine is given to newborns and children to prevent childhood TB, the vaccine seems to offer cross-protection against COVID-19.Citation51,Citation73 The study reported that countries that have either stopped or do not provide universal BCG vaccination to children, including the United States, Italy, Spain, and France, have more COVID-19 cases and deaths as compared to the countries having universal BCG immunization program. Middle-high- and high-income countries that currently have universal BCG policy have reported 0.78 ± 0.40 deaths per million. A significant difference can be noticed while comparing COVID-19 mortality data from middle-high- and high-income countries that never had a universal BCG policy with the data from countries that have BCG vaccination in their regular immunization programme. The former have a significantly higher mortality rate, 16.39 ± 7.33 deaths per million people. Moreover countries like Japan and Russia where the vaccine composition includes the original BCG strain have shown low per capita death rate compared to European countries where modified BCG strain is used for vaccination.Citation74 But Brazil is the exception with having second highest number of cases and mortality. The reason for this high number is not clear. Some of the low income countries with universal BCG policy have reported zero deaths per million.Citation73
In another study, Singh and GandharvaCitation69 analyzed data on BCG vaccination from 81 countries, including Canada, Italy, and the United States. The results of the study revealed that there is higher COVID-19 infectivity (11.2% versus 8.53%), case positivity (1043.9 versus 172.4), and deaths (48.9 versus 4.3) per million population in countries that have not adopted BCG vaccination as compared to those that use BCG vaccines. As stated previously, the higher protection against COVID-19 morbidity and mortality observed in countries that provide BCG vaccination during childhood might be due to provoking of both nonspecific and cross-protective immune responses.Citation15,Citation69,Citation75,Citation76 The trained immunity conferred by BCG vaccination include the enhanced expression of certain resistance factors like IL-β which might have a major role in reducing the level of viremia in infected hosts as shown in case of yellow fever virus administration.Citation68
The number of confirmed cases and deaths due to COVID-19 in the United States, Italy, and Spain has outnumbered that of China, which was the epicenter of the outbreak of the disease first identified in Wuhan city in the beginning of December 2019. Why did COVID-19 spread in China, despite the country having a universal BCG strategy since the 1950s? The reason may be that the virus spread unexpectedly and rapidly without it being recognized as a new virus and one capable of high-level interpersonal spread, while measures were not taken initially to restrain its spread. In addition, banning and weakening of the TB prevention and treatment agencies during Cultural Revolution (1966–1976) may also be responsible. This may have produced a pool of potential hosts that could be affected by SARS-CoV-2 and may have played a viral role in spread of COVID-19 transmission. Currently, the condition in China appears to be improving promisingly.Citation4
In India, childhood BCG vaccination began in 1949, and at present, around 97% of 26 million children born in India are administered free BCG vaccine during their first week of birth.Citation77,Citation78 Among all vaccines, BCG is given in highest number to children under five years of age under the universal immunization programme. It is generally given to newborn babies at birth; however, if vaccination is missed at birth, it can be given any time until the age of one. According to the data from the Ministry of Health and Family Welfare, Government of India, the percentage of children vaccinated with BCG went up from 92% in 2015–16 to the current 97%. BCG vaccination is above 95% in all the states of India. In India, since the first COVID-19 case was confirmed on January 30, 2020 (Kerala), and the first COVID-19 fatality was confirmed on March 11, 2020 (Karnataka), the total number of confirmed cases has reached to approximately 2.1 million with nearly 43,000 deaths in the following 191 days (WHO Situation Report-201, India as on August 8, 2020).Citation5 The question before medical researchers is that will BCG vaccination, which had been started in India long ago, help in preventing COVID-19 cases and reducing the death toll?
According to a study published in medRxiv, the preprint health sciences server, BCG vaccination seems to be contributing significantly in reduction of number of COVID-19 deaths, with the most substantial reduction in deaths observed in countries that had adopted a BCG vaccination policy earlier, suggesting that there might be less COVID-19 cases and mortality in those countries.Citation73 Though it is early to declare and would need further investigations before a conclusion is reached, the statistical figures on BCG vaccination in India during the early 1950s with target coverage of 80% indicate that the country might benefit from the presence of enhanced acquired innate immunity in a large population. Nevertheless, the low- and middle-income countries have reported 0–0.78 deaths per million, while the countries that adopted a universal BCG policy later than other countries, like Iran in 1984, have exhibited a high death rate, which is contrary to the hypothesis that BCG protects the vaccinated population against COVID-19. The current population of India is 1339.2 million, and to date, India has reported 0.42 deaths per million, which is in accordance with the calculations and range provided by Miller and coworkers.Citation67 Based on the current situation and data available, we can say that BCG vaccination may have an influence on the number of COVID-19 cases and deaths and might provide protection against COVID-19 but to show the actual causality between BCG vaccine and severity of COVID-19, the scientists must confirm the Bradford Hill Criteria which include specificity, reversibility, temporality, and experiment.Citation79 There have been inconsistencies in data related to COVID-19 collected from different countries,; therefore, its important to have a proven result for the use of BCG vaccine in context of COVID-19. Toward this end, it is reported that three clinical trials, with an aim to confirm the role of BCG vaccine in protection against COVID-19, have been intiated.Citation52,Citation53,Citation63,Citation80 The clinical trial planned by Max Plank Institute for Infection Biology in Berlin include the use of genetically modified version of BCG while other two trails have been planned in Australia and Netherland. However, some group also suggest that COVID-19 vaccination should include multiple-doses of BCG vaccine.Citation19
Clinical manifestations of COVID-19 cases (morbidity and moratlity) around the world
The clinical manifestations among the COVID-19 positive cases varies greatly between developed and developing countries.
As per the latest WHO COVID-19 Situation Report-201Citation5 (as on August 8, 2020), the number of laboratory confirmed cases are on the raise among the developed nations and they are currently classified under community transmission phase viz. the United States, and European countries, while the United States is leading the count with more than 4.8 million confirmed positive cases. But on the other hand, the developing countries are still classified under cluster of cases phase. Here, at the developing nations, under-reporting of cases, number of tests carried out for the suspected and confirmed cases, misdiagnosis and missed diagnosis may be considered as part of limitations while comparing the data between developed and developing nations, worldwide.
Similarly, as per the WHO COVID-19 Situation Report-201Citation5 (as on August 8, 2020) have reported the highest number of deaths due to COVID-19 among the developed nations viz. the United States, about 160,000 deaths followed by the Brazil, Mexico, the United Kingdom, Italy, France, Spain, etc. The mortality rate among the developing countries, very low as compared to the developed nations. The data from the developing countries needs to be assessed on a periodic intervals, and as the cause of death also need to be confirmed before declaring the same amid COVID-19 crisis.
R&D/Clinical trials on BCG vaccination among health-care workers against COVID-19
In the United States, a group of researchers from the academic and research institutions have initiated a Clinical trial titled “BCG Vaccine for Health Care Workers (HCW) as Defense Against COVID 19 (BADAS)”.Citation81,Citation82 [U.S. National Library of Medicine, ClinicalTrials.gov website: https://clinicaltrials.gov/ct2/show/NCT04348370]. In summary the clinical trials confirms the investigators hypothesize that BCG vaccination can reduce HCW infection and disease severity during the epidemic phase of SARS-CoV-2. Similar studies are underway in many countries including Murdoch Children’s Research Institute in Australia (MCRI), and India. Since the studies are ongoing, we have to wait to get the concrete conclusion till the end of the study with analyzed data and report with a positive hope to mitigate COVID-19, which is on top priority, globally to avert this public health crisis.
Conclusion and prospects
Due to the enormous impact of the COVID-19 pandemic worldwide, the early research that was mainly focused on the epidemiology and clinical manifestation has turned toward early and accurate diagnosis as well as timely development of an appropriate therapeutic methods and vaccines to control the escalating SARS-CoV-2 infections. Until a suitable cure is available, alternatives like BCG vaccine, which is used for preventing childhood TB, may also be used to provide additional cross-protection against COVID-19 and is a topic of discussion. The initial observations from the countries that have stopped and do not provide childhood BCG vaccination, including the United States, Italy, Spain, and France, revealed that these countries are reporting more COVID-19 cases and deaths than the countries that provide universal BCG vaccination. This higher protection against COVID-19 from the BCG vaccine is assumed to be due to nonspecific and cross-protective immunity.
The signifying advantage of BCG vaccination, an early life immunization with unique formulations, is an unanticipated reduction (∼50%) in all-cause mortality, especially in low-birth weight males, greatly exceeding that attributable to TB prevention. This mortality benefit has been related to prevention of sepsis and respiratory infections, suggesting that BCG vaccine induces “heterologous” cross-protection against unrelated pathogens. The nonavailability of a suitable vaccine or therapeutic option to mitigate the COVID-19 pandemic is forcing researchers and public health experts to be assertive on the use of BCG vaccine to control the COVID-19 disease caused by SARS-CoV-2 especially the adult and old-age population. It is unclear whether the vaccine given to old-age population will make adequate or similar immunological response as delivered to the young age population. The ongoing clinical trials of BCG in health care workers are anticipated to provide an insight on the usage of TB vaccine in old-age population against COVID-19.
The data and statistics available from limited investigations have revealed a correlation between the use of BCG vaccination and protection from COVID-19. However, further studies are required to judge the prophylactic and/or therapeutic significance of BCG vaccine against COVID-19 and to strengthen the evidence of the molecular mechanisms proving this hypothesis. Considering the global need to restrain the COVID-19 emergence and transmission, scholars at Murdoch Children’s Research Institute in Australia (MCRI) are underway to explore the use of BCG vaccine-generated cross-protection in countering COVID-19.
Some important factors that must be considered include the shortage of BCG vaccines, appropriate time of vaccination for such protections and longivity of heterologous immunity conferred by BCG vaccine. In this regard, Japanese Society for Vaccinology recently raised a concern with a view that indiscriminate use of BCG vaccine might create a shortage of the vaccine for regular immunization schedule which may lead to the spread of tuberculosis. This review has provided information that might help to control the current COVID-19 pandemic; however, further extensive and comprehensive research is required for the development of valid and reliable methods for managing the current public health pandemic in both short- and long-term aspects for the benefit of the global population on the whole.
Author contributions
All the authors substantially contributed to the conception, compilation of data, checking and approving the final version of the manuscript, and agree to be accountable for its contents.
Acknowledgments
All the authors acknowledge and thank their respective Institutes and Universities. YSM acknowledges the Education Division, ICAR, GoI for National Fellowship.
Disclosure statement
All authors declare that there exist no commercial or financial relationships that could, in any way, lead to a potential conflict of interest.
Additional information
Funding
References
- Paules CI, Marston HD, Fauci AS. Coronavirus infections-more than just the common cold. JAMA. 2020;323(8):707. [published online ahead of print, 2020 Jan 23]. doi:https://doi.org/10.1001/jama.2020.0757.
- de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nature Rev Microbiol. 2016;14(8):523. doi:https://doi.org/10.1038/nrmicro.2016.81.
- Malik YS, Sircar S, Bhat S, Khan S, Dhama K, Dadar M, Tiwari R, Chaicumpa W. Emerging novel coronavirus (2019-nCoV)—current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Quart. 2020a;40(1):68–76. doi:https://doi.org/10.1080/01652176.2020.1727993.
- Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS, Singh KP, Chaicumpa W, Bonilla-Aldana K, Morales AJR. Coronavirus disease 2019–COVID-19. Preprints. 2020b:2020030001. doi:https://doi.org/10.20944/preprints202003.0001.v2.
- WHO Coronavirus disease (COVID-19) Situation Report No. 201 ( As on 8th August 2020). [accessed 2020 Aug 9]. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200808-covid-19-sitrep-201.pdf?sfvrsn=121bb855_2
- Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, Baghbanzadeh M, Aghamohammadi N, Zhang W, Haque U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. 2020;dyaa033. [published online ahead of print, 2020 Feb 22]. doi:https://doi.org/10.1093/ije/dyaa033.
- Malik YS, Sircar S, Bhat S, Vinodhkumar OR, Tiwari R, Sah R, Rabaan AA, Morales AJR, Dhama K. Emerging coronavirus disease (COVID-19), a pandemic public health emergency with animal linkages: current status update. Preprints. 2020b:2020030343. doi:https://doi.org/10.20944/preprints202003.0343.v1.
- Chan JF, Yuan S, Kok K-H, To KKW, Chu H, Yang J, Xing F, Liu J, Yip CCY, Poon RWS, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–23. doi:https://doi.org/10.1016/S0140-6736(20)30154-9.
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–79. doi:https://doi.org/10.1056/NEJMc2001737.
- Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng O-T, Marimuthu K, Ang LW, Mak TM, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–94. [published online ahead of print, 2020 Mar 3] [published correction appears in JAMA. doi: https://doi.org/10.1001/jama.2020.4372]. doi:https://doi.org/10.1001/jama.2020.3204.
- Peiris JS, Yuen KY, Osterhaus AD, Stöhr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349(25):2431–41. doi:https://doi.org/10.1056/NEJMra032498.
- Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020;1–7. [published online ahead of print, 2020 Mar 18]. doi:https://doi.org/10.1080/21645515.2020.1735227.
- Zhang YZ, Holmes EC. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020;181(2):223–27. doi:https://doi.org/10.1016/j.cell.2020.03.035.
- Yi Y, Lagniton PNP, Ye S, Li E, Xu RH. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16(10):1753–66. Published 2020 Mar 15. doi:https://doi.org/10.7150/ijbs.45134.
- Shann F. Nonspecific effects of vaccines and the reduction of mortality in children. Clin Therapeut. 2013;35(2):109–14. doi:https://doi.org/10.1016/j.clinthera.2013.01.007.
- Moorlag SJCFM, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25(12):1473–78. doi:https://doi.org/10.1016/j.cmi.2019.04.020.
- Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, et al. Bacille calmette-guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA. 2012;109(43):17537–42. doi:https://doi.org/10.1073/pnas.1202870109.
- Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, Duan G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 2020;12(4):E372. Published 2020 Mar 27. doi:https://doi.org/10.3390/v12040372.
- Ayoub BM. COVID-19 vaccination clinical trials should consider multiple doses of BCG. Pharmazie. 2020;75(4):159. doi:https://doi.org/10.1691/ph.2020.0444.
- Alakwaa FM, Gilbert JA. Repurposing Didanosine as a Potential Treatment for COVID-19 Using Single-Cell RNA Sequencing Data. Msystems. 2020;5(2):e00297–20. doi:https://doi.org/10.1128/mSystems.00297-20.
- Zhang TY, Zhong B. Meeting the Potential Emergency Global Drug Supply Challenge of Hydroxychloroquine for COVID-19. Med Drug Discov. 2020;5:100036. doi:https://doi.org/10.1016/j.medidd.2020.100036.
- Picot S, Marty A, Bienvenu A-L, Blumberg LH, Dupouy-Camet J, Carnevale P, Kano S, Jones MK, Daniel-Ribeiro CT, Mas-Coma S, et al. Coalition: advocacy for prospective clinical trials to test the post-exposure potential of hydroxychloroquine against COVID-19. One Health. 2020;9:100131. [published online ahead of print, 2020 Apr 4]. doi:https://doi.org/10.1016/j.onehlt.2020.100131.
- Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. [published online ahead of print, 2020 Apr 3]. doi:https://doi.org/10.1016/j.antiviral.2020.104787.
- Patrì A, Fabbrocini G. Hydroxychloroquine and ivermectin: A synergistic combination for COVID-19 chemoprophylaxis and treatment? J Am Acad Dermatol. 2020;S0190-9622(20):30557. [published online ahead of print, 2020 Apr 10]. doi:https://doi.org/10.1016/j.jaad.2020.04.017.
- Damle B, Vourvahis M, Wang E, Leaney J, Corrigan B. Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19. Clin Pharmacol Ther. 2020;108(2):201–11. [published online ahead of print, 2020 Apr 17]. doi:https://doi.org/10.1002/cpt.1857.
- Ford N, Vitoria M, Rangaraj A, Norris SL, Calmy A, Doherty M. Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID-19: initial assessment. J Int AIDS Soc. 2020;23(4):e25489. doi:https://doi.org/10.1002/jia2.25489.
- Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systemic review. J Med Virol. 2020;92(5):479–90. doi:https://doi.org/10.1002/jmv.25707.
- Yavuz S, Ünal S. Antiviral treatment of COVID-19. Turkish J Med Sci. 2020;50(SI–1):611–19. doi:https://doi.org/10.3906/sag-2004-145.
- Salvi R, Patankar P. Emerging pharmacotherapies for COVID-19. Biomed Pharmacother. 2020 May;14:110267. doi:https://doi.org/10.1016/j.biopha.2020.110267.
- Bachanova V, Bishop MR, Dahi P, Dholaria B, Grupp SA, Lattin BH, Janakiram M, Maziarz RT, McGuirk JP, Nastoupil LJ. CAR T Cell Therapy During the COVID-19 Pandemic. Biol Blood Marrow Trans. 2020;26(7):1239–46. doi:https://doi.org/10.1016/j.bbmt.2020.04.008.
- Zhao RC. Stem Cell-Based Therapy for Coronavirus Disease 2019. Stem Cells Dev. 2020;29(11):679–81. [published online ahead of print, 2020 Apr 17]. doi:https://doi.org/10.1089/scd.2020.0071.
- Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, Van Tongeren SA, Dong L, Retterer CJ, Eaton BP, Pegoraro G, et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014 Apr 17;508(7496):402–05. doi:https://doi.org/10.1038/nature13027.
- Kawase M, Shirato K, van der Hoek L, Taguchi F, Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 2012 Jun;86(12):6537–45. doi:https://doi.org/10.1128/JVI.00094-12.
- Kang S, Peng W, Zhu Y, Lu S, Zhou M, Lin W, Wu W, Huang S, Jiang L, Luo X, et al. Recent progress in understanding 2019 novel coronavirus (SARS-CoV-2) associated with human respiratory disease: detection, mechanisms and treatment. Int J Antimicrob Agents. 2020 Mar;29(5):105950. doi:https://doi.org/10.1016/j.ijantimicag.2020.105950.
- Jiang L, Wang N, Zuo T, Shi X, Poon KM, Wu Y, Gao F, Li D, Wang R, Guo J, et al. Potent neutralization of MERS-CoV by human neutralizing monoclonal antibodies to the viral spike glycoprotein. Sci Transl Med. 2014 Apr 30;6(234):234ra59. doi:https://doi.org/10.1126/scitranslmed.3008140.
- Jawhara S. Could Intravenous Immunoglobulin Collected from Recovered Coronavirus Patients Protect against COVID-19 and Strengthen the Immune System of New Patients? Int J Mol Sci. 2020;21(7):E2272. Published 2020 Mar 25. doi:https://doi.org/10.3390/ijms21072272.
- Moderna Announces First Participant Dosed in NIH-led Phase 1 Study of mRNA Vaccine (mRNA-1273) Against Novel Coronavirus [accessed May 19, 2020]. https://investors.modernatx.com/node/8466/pdf
- Zhu FC, Li YH, Guan XH, Hou L-H, Wang W-J, Li J-X, Wu S-P, Wang B-S, Wang Z, Wang L, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–54. doi:https://doi.org/10.1016/S0140-6736(20)31208-3.
- Smith TRF, Patel A, Ramos S, Elwood D, Zhu X, Yan J, Gary EN, Walker SN, Schultheis K, Purwar M, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11(1):2601. Published 2020 May 20. doi:https://doi.org/10.1038/s41467-020-16505-0.
- Angelidou A, Diray-Arce J, Conti MG, Smolen KK, van Haren SD, Dowling DJ, Husson RN, Levy O. BCG as a case study for precision vaccine development: lessons from vaccine heterogeneity, trained immunity, and immune ontogeny. Front Microbiol. 2020;11:332. Published 2020 Mar 11. doi:https://doi.org/10.3389/fmicb.2020.00332.
- Raviglione MC, Pio A. Evolution of WHO policies for tuberculosis control, 1948-2001. Lancet. 2002;359(9308):775–80. doi:https://doi.org/10.1016/s0140-6736(02)07880-7.
- Hooft van Huijsduijnen R, Kojima S, Carter D, Okabe H, Sato A, Akahata W, Wells TNC, Katsuno K. Reassessing therapeutic antibodies for neglected and tropical diseases. PLoS Negl Trop Dis. 2020;14(1):e0007860. Published 2020 Jan 30. doi:https://doi.org/10.1371/journal.pntd.0007860.
- Al Abri S, Kasaeva T, Migliori GB, Goletti D, Zenner D, Denholm J, Al Maani A, Cirillo DM, Schön T, Lillebæk T, et al. Tools to implement the world health organization end TB strategy: addressing common challenges in high and low endemic countries. Int J Infect Dis. 2020;92S:S60–S68. doi:https://doi.org/10.1016/j.ijid.2020.02.042.
- Tsang JS, Dobaño C, VanDamme P, Moncunill G, Marchant A, Othman RB, Sadarangani M, Koff W, Kollmann TR. Improving vaccine-induced immunity: can baseline predict outcome? Trends Immunol. 2020;41(6):457-465. doi:https://doi.org/2020;doi:10.1016/j.it.2020.04.001.
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367(9517):1173–80. doi:https://doi.org/10.1016/S0140-6736(06)68507-3.
- Sumner T, Scriba TJ, Penn-Nicholson A, Hatherill M, White RG. Potential population level impact on tuberculosis incidence of using an mRNA expression signature correlate-of-risk test to target tuberculosis preventive therapy. Sci Rep. 2019;9(1):11126. Published 2019 Jul 31. doi:https://doi.org/10.1038/s41598-019-47645-z.
- Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PEM, Rodrigues LC, Smith PG, Lipman M, Whiting PF, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58(4):470–80. doi:https://doi.org/10.1093/cid/cit790.
- Abubakar I, Pimpin L, Ariti C, Beynon R, Mangtani P, Sterne J, Fine P, Smith PG, Lipman M, Elliman D, et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guérin vaccination against tuberculosis. Health Technol Assess. 2013;17(37):1–vi. doi:https://doi.org/10.3310/hta17370.
- Roy P, Vekemans J, Clark A, Sanderson C, Harris RC, White RG. Potential effect of age of BCG vaccination on global paediatric tuberculosis mortality: a modelling study. Lancet Glob Health. 2019;7(12):e1655–e1663. doi:https://doi.org/10.1016/S2214-109X(19)30444-9.
- Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG world atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8(3):e1001012. doi:https://doi.org/10.1371/journal.pmed.1001012.
- Butkeviciute E, Jones CE, Smith SG. Heterologous effects of infant BCG vaccination: potential mechanisms of immunity. Future Microbiol. 2018;13(10):1193–208. doi:https://doi.org/10.2217/fmb-2018-0026.
- Redelman-Sidi G. Could BCG be used to protect against COVID-19? Nat Rev Urol. 2020:1‐2. doi:https://doi.org/10.1038/s41585-020-0325-9.
- Kumar J, Meena J. Demystifying BCG Vaccine and COVID-19 Relationship. Indian Pediatr. 2020;PII:S097475591600168. [published online ahead of print, 2020 Apr 30]
- Covián C, Fernández-Fierro A, Retamal-Díaz A, Díaz FE, Vasquez AE, Lay MK, Riedel CA, González PA, Bueno SM, Kalergis AM, et al. BCG-induced cross-protection and development of trained immunity: implication for vaccine design. Front Immunol. 2019;10:2806. Published 2019 Nov 29. doi:https://doi.org/10.3389/fimmu.2019.02806.
- Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180–83. doi:https://doi.org/10.1016/S0022-5347(17)58737-6.
- Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer—a current perspective. Nat Rev Urol. 2014;11(3):153–62. doi:https://doi.org/10.1038/nrurol.2014.15.
- Alexandroff AB, Jackson AM, O’Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353(9165):1689–94. doi:https://doi.org/10.1016/S0140-6736(98)07422-4.
- Kawai K, Miyazaki J, Joraku A, Nishiyama H, Akaza H. Bacillus Calmette-Guerin (BCG) immunotherapy for bladder cancer: current understanding and perspectives on engineered BCG vaccine. Cancer Sci. 2013;104(1):22–27. doi:https://doi.org/10.1111/cas.12075.
- Netea MG, Schlitzer A, Placek K, Joosten LAB, Schultze JL. Innate and adaptive immune memory: an evolutionary continuum in the host’s response to pathogens. Cell Host Microbe. 2019;25(1):13–26. doi:https://doi.org/10.1016/j.chom.2018.12.006.
- Van’t Wout JW, Poell R, van Furth R. The role of BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice. Scand J Immunol. 1992;36(5):713–19. doi:https://doi.org/10.1111/j.1365-3083.1992.tb03132.x.
- Andersen P, Kaufmann SHE. Novel vaccination strategies against tuberculosis. Cold Spring Harb Perspect Med. 2014;4(6):a018523. doi:https://doi.org/10.1101/cshperspect.a018523.
- Bertholet S, Ireton GC, Kahn M, Guderian J, Mohamath R, Stride N, Laughlin EM, Baldwin SL, Vedvick TS, Coler RN, et al. Identification of human T cell antigens for the development of vaccines against mycobacterium tuberculosis. J Immunol. 2008;181(11):7948–57. doi:https://doi.org/10.4049/jimmunol.181.11.7948.
- O’Neill LAJ, Netea MG. BCG- induced trained immunity: can it offer protection against COVID-19? Nature Rev. 2020. doi:https://doi.org/10.1038/s41577-020-0337-.
- van Kooyk Y, Engering A, Lekkerkerker AN, Ludwig IS, Geijtenbeek TB. Pathogens use carbohydrates to escape immunity induced by dendritic cells. Curr Opin Immunol. 2004;16(4):488–93. doi:https://doi.org/10.1016/j.coi.2004.05.010.
- Akira S. Innate immunity and adjuvants. Philos Trans R Soc Lond B Biol Sci. 2011;366(1579):2748–55. doi:https://doi.org/10.1098/rstb.2011.0106.
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–95. doi:https://doi.org/10.1126/science.1183021.
- Gyssens IC, Netea MG. Heterologous effects of vaccination and trained immunity. Clin Microbiol Infect. 2019;25(12):1457–58. doi:https://doi.org/10.1016/j.cmi.2019.05.024.
- Tanner R, Villarreal-Ramos B, Vordermeier HM, McShane H. The humoral immune response to BCG vaccination. Front Immunol. 2019;10:1317. Published 2019 Jun 11. doi:https://doi.org/10.3389/fimmu.2019.01317.
- Guerra-Maupome M, Vang DX, McGill JL, Basu J. Aerosol vaccination with bacille calmette-guerin induces a trained innate immune phenotype in calves. PLoS One. 2019;14(2):e0212751. Published 2019 Feb 22. doi:https://doi.org/10.1371/journal.pone.0212751.
- Tarancón R, Domínguez-Andrés J, Uranga S, Ferreira AV, Groh LA, Domenech M, González-Camacho F, Riksen NP, Aguilo N, Yuste J, et al. New live attenuated tuberculosis vaccine MTBVAC induces trained immunity and confers protection against experimental lethal pneumonia. PLoS Pathog. 2020;16(4):e1008404. Published 2020 Apr 2. doi:https://doi.org/10.1371/journal.ppat.1008404.
- Rabaan AA, Al-Ahmed SH, Haque S, Sah R, Tiwari R, Malik YS, Dhama K, Yatoo MI, Bonilla-Aldana DK, Rodriguez-Morales AJ, et al. SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Infez Med. 2020;28(2):174–84.
- Salman S, Salem ML. Routine childhood immunization may protect against COVID-19. Med Hypotheses. 2020;140:109689. doi:https://doi.org/10.1016/j.mehy.2020.109689.
- Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Yan L, Otazu GH. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv. 2020. doi:https://doi.org/10.1101/2020.03.24.20042937.
- Iwasaki A, rubau ND. Why does Japan have so few cases of COVID-19? EMBO Mol Med. 2020;12(5):e12481. doi:https://doi.org/10.15252/emmm.202012481.
- Singh BR, Gandharva R. Are BCG vaccination, population density, median age and poverty important determinants of COVID-19 pandemic spread, morbidity and mortality? Researchgate. 2020. doi:https://doi.org/10.13140/RG.2.2.21116.49282.
- Iqbal NT, Hussain R. Non-specific immunity of BCG vaccine: a perspective of BCG immunotherapy. Trials Vaccinol. 2014;3:143–49. doi:https://doi.org/10.1016/j.trivac.2014.08.002.
- Udani PM. BCG vaccination in India and tuberculosis in children: newer facets. Indian J Pediat. 1994;1994(61):451–62. doi:https://doi.org/10.1007/BF02751703.
- Lahariya C. A brief history of vaccines & vaccination in India. Indian J Med Res. 2014;139:491.
- Kuroda N. Demand for BCG vaccine due to unproven claims of its role in preventing COVID-19 is causing shortages of vaccines for infants in Japan. Pediatr Infect Dis J. 2020:Publish Ahead of Print. doi:https://doi.org/10.1097/INF.0000000000002724.
- O’Connor E, The J, Kamat AM, Lawrentschuk N. Bacillus Calmette Gu´erin (BCG) vaccination use in the fight against COVID-19 – what’s old is new again? Future Oncol. 2020;16(19):1323–25. (Epub ahead of print). doi:https://doi.org/10.2217/fon-2020-0381.
- Lawton G. Trials of BCG vaccine will test for covid-19 protection. New Sci. 2020;246(3280):9. doi:https://doi.org/10.1016/S0262-4079(20)30836-8.
- U.S. National Library of Medicine, ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04348370
