ABSTRACT
Background
Programmed cell death protein 1 (PD-1) inhibitors are the first-line treatment for advanced non-small-cell lung cancer (NSCLC) patients. However, their efficacy in metastatic NSCLC patients remains controversial.
Aim of the study
The aim of our study was to evaluate the prognosis of advanced metastatic NSCLC patients treated with PD-1 inhibitors, and discuss the predictive effect of metastatic site on the long-term outcome.
Methods
The Embase, Ovid Medline, Cochrane Central Register of Controlled Trials, and PubMed databases were systematically screened up to February 10, 2020. Twenty-five eligible studies, involving 8,067 patients that assessed the impact of metastatic sites on survival outcome were incorporated in our study. Overall survival (OS) and progression-free survival (PFS) were described as hazard ratio (HR) with 95% confidence interval (CI).
Results
Among the advanced NSCLC patients, the median proportion of brain, liver, bone, and adrenal gland metastases were 21%, 17%, 35%, and 21%, respectively. Patients with metastases to the brain, liver, and bone had worse OS compared to patients without these metastases when treated with PD-1 inhibitors. Similarly, patients with metastasis to the brain and liver were more likely to progress when treated with PD-1 inhibitors. Besides, patients with multiple metastatic sites had worse PFS compared to patients with one metastatic site, while no significant difference was found in terms of OS.
Conclusions
Based on the findings of our systematic review and meta-analysis, metastatic sites were independent predictors of the survival outcome for advanced NSCLC patients treated with PD-1 inhibitors.
Introduction
Lung cancer is the most common malignancy worldwide and has the highest mortality rate among all types of malignant tumors.Citation1,Citation2 Non-small-cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancers.Citation3 Due to the lack of early symptoms, 70–80% of patients are diagnosed in advanced stages and lose the opportunity for surgery. Chemo-, radio-, and molecular targeted-therapies remain the main treatment options for such patients, but the 5-y survival rate is <50%.Citation4 The main limitation of chemo- and radiotherapy is that they often cause serious adverse reactions and affect the quality of life of the patient.Citation5 In the past decade, molecular targeted-therapy has become a hot spot with a low incidence of adverse reactions, although the problem of drug resistance has gradually become apparent.Citation6 Due to the limitations of the above treatment modalities, immunotherapy has gained considerable attention for the treatment of NSCLC.
Programmed cell death protein 1 (PD-1) is an important immunosuppressive molecule, which is expressed on T cells, and regulates its activity in the peripheral tissues.Citation7 PD-1 has two ligands, PD-L1 and PD-L2, which are widely expressed on a variety of immune effector, antigen-presenting, and tumor cells.Citation8 According to previous studies, the PD-1/PD-L1 signaling pathway is overexpressed in the tumor microenvironment, and tilts the immune balance in favor of immunosuppression, abnormally strengthens the negative immune effect, and inhibits T cell activation.Citation8,Citation9 Thus, the tumor cells escape from immune cell-mediated cell death, and proliferate and metastasize without being controlled by the body’s defense mechanism.Citation10 In recent years, PD-1/PD-L1 inhibitors have made breakthrough progress in the treatment of NSCLC. Randomized controlled clinical trials such as the CheckMate017, CheckMate057, KEYNOTE010, and OAK have made nivolumab, pembrolizumab, and atezolizumab the second-line treatment options for advanced NSCLC.Citation11–14 Pembrolizumab has also changed the first-line treatment model for advanced lung cancer and has become one of the first-line treatment drugs for NSCLC recommended by the National Comprehensive Cancer Network clinical practice guidelines.Citation15
Currently, PD-1/PD-L1 inhibitors are approved for first-line or second-line treatment of advanced NSCLC, and relevant clinical trials have been carried out widely. However, data pertaining to their safety and effectiveness in the treatment of NSCLC are still contentious. Whether PD-1 treatment is advantageous, especially in patients with metastasized NSCLC, remains controversial. Therefore, in this systematic review and meta-analysis, we aimed to explore the relationship between metastatic sites prior to PD-1 treatment and the long-term survival of those NSCLC patients.
Methods
This study was designed and reported in accordance with the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines.Citation16
Study search strategy and identification
The Embase, Ovid Medline, Cochrane Central Register of Controlled Trials, and PubMed databases were systematically searched up to February 10, 2020. A comprehensive search was performed using the following search terms: PD-1, pembrolizumab, nivolumab, atezolizumab, durvalumab, JS001, IBI308, immune checkpoint inhibitor, immune checkpoint blockade, immune checkpoint therapy, and NSCLC. Both keywords and medical sub-headings terms were used in the search. All studies containing titles and abstracts were imported into Endnote for deleting duplications and screening the literature.
Inclusion and exclusion criteria
Studies satisfying the following inclusion criteria were selected for the analysis:Citation1 Clinical trials investigating advanced NSCLC treated with PD-1 inhibitors;Citation2 Studies discussing the impact of different metastatic sites on survival outcome;Citation3 Patients diagnosed with metastases prior to treatment with PD-1 inhibitors;Citation5 Overall survival (OS) and progression-free survival (PFS) were described as hazard ratio (HR) with 95% confidence interval (CI) or the HR could be extracted by survival plots;Citation4 If the same population was used by different studies, only study with the longest follow-up time or with the largest sample size was included.
The exclusion criteria were as follows:Citation1 Study type was a review, comments, and case report;Citation2 NSCLC patients were not treated with PD-1 inhibitors;Citation3 Studies included other types of cancer;Citation4 Metastases data or survival outcome data could not be extracted;Citation6 Publication in a language other than English.
Data extraction
Two reviewers independently evaluated the titles and abstracts according to the inclusion and exclusion criteria. Disagreement between the two reviewers was resolved by a third reviewer.
The following information was extracted independently from each included studies: Author, publication year, publication center and country, recruitment period, study type, anti-PD-1 treatment, patient characteristics (patient sample, age, gender, smoking status, histology of cancer, tumor stage), metastatic site and patient number, HRs and 95% CIs associated with OS and PFS from univariate or multivariate COX regression analyses.
Quality assessment
The quality of the prognostic studies was evaluated as reported previously.Citation17 Two researchers (ZL Zhang and WJ Li) independently assessed the quality of the included studies following the criteria:Citation1 Representativeness of the population;Citation2 Exposed cohort;Citation3 Ascertainment of exposure;Citation4 Outcome assessment;Citation5 Appropriate measurement and account;Citation6 Measurement of outcomes;Citation7 Completeness of follow-up. Studies with a score of >7 were regarded as high-quality studies.
Statistical analysis
Statistical analysis was performed using the Stata 15.1 software (Stata Corporation, College Station, Texas, USA). We used the method of random-effects and fixed-effects model to pool outcomes, which is calculated by HR and 95% CI to estimate the predictor value of different metastatic sites on long-term outcome. The I2 statistic and χ2 test were used for heterogeneity assessment between studies. Heterogeneity was considered to exist between studies if I2 ≥ 50% and the random-effect model would be used. A statistical test with P < .05 was considered significant.
Result
Selection of eligible studies
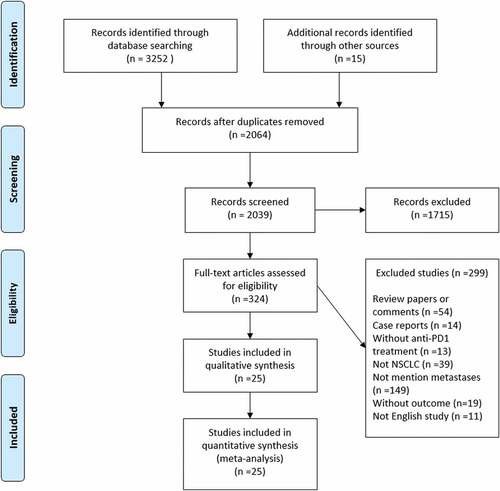
A total of 3,252 articles were identified from the online database search. shows the flowchart of the study selection process. After screening the titles and abstracts, 324 studies were selected for full-text review. Following further assessment of the full-texts, 299 studies were excluded based on the inclusion and exclusion criteria. Finally, 25 studies that met all the criteria were included in the systematic review and meta-analysis.Citation3,Citation7,Citation18–40
Characteristics of the studies included and quality assessment
The baseline characteristics of the 25 studies included in the analysis are listed in . A total of 8,067 patients were included in our systematic review. The search was limited to publications between 2017 and 2020, with the recruitment period ranged from 2013 to 2019. The recruited patients were from Europe, Asia, South and North America, and eight studies were from multicenter data. A total of 67% of the patients (range: 23–80%) were males with a median age of 64 y. In terms of the tumor histology, 27% of patients were diagnosed with squamous NSCLC, and 66% were diagnosed with adenocarcinoma. Among the patients, 19% did not have a history of smoking.
Table 1. The characteristics of included studies
Table 2. The reported metastatic sites of advanced NSCLC in different studies
summarizes the common metastatic sites in advanced NSCLC patients treated with PD-1 inhibitors. The median proportion of brain, liver, bone, and adrenal gland metastases were 21%, 17%, 35%, and 21%, respectively. Five studies reported the proportion of lymph node metastases (median proportion: 57%), three studies reported pleural invasion (median proportion: 30%), and six studies reported intrapulmonary metastases (median proportion: 49%).
The assessment of quality between studies was based on the Newcastle Ottawa Scale, which is shown in . Nine studies were regarded as median quality with scores of 5–6, and remaining sixteen studies were regarded as high-quality with a score of >7.
Survival outcome based on different metastatic site in advanced NSCLC
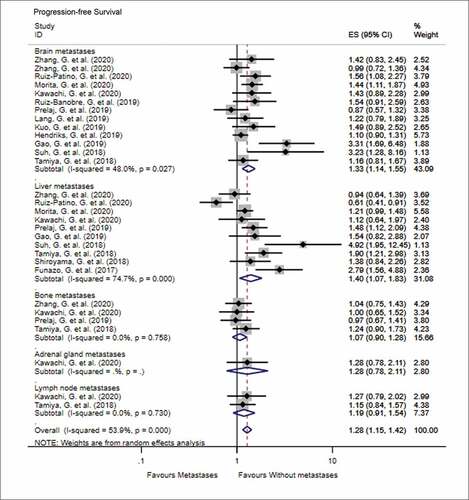
The impact of various metastatic sites in advanced NSCLC, prior to PD-1 inhibitor treatment, on the OS and PFS is shown in (OS) and (PFS). In terms of OS, the number of studies that discussed the predictive impact of brain, liver, bone, and adrenal gland metastases on OS following immune checkpoint inhibitor treatment was 15, 9, 5, and 1, respectively. The results of meta-analysis showed that patients with metastasis to the brain (HR = 1.25, 95% CI = 1.09–1.44, I2 = 43.8%, P < .001), liver (HR = 1.73, 95% CI = 1.35–2.20, I2 = 69.4%, P < .001), and bone (HR = 1.67, 95% CI = 1.30–2.16, I2 = 64.7%, P < .001) had worse OS than patients without these metastases when treated with PD-1 inhibitors. Similarly, patients with metastasis to the brain (HR = 1.33, 95% CI = 1.14–1.55, I2 = 48.0%, P < .001) and liver (HR = 1.40, 95% CI = 1.07–1.83, I2 = 74.7%, P = .015) were more likely to progress than patients without these metastases, when treated with PD-1 inhibitors. There was no significant difference in the PFS when patients were diagnosed with bone, adrenal, or lymph node metastases prior to treatment with PD-1 inhibitors (all P > .05).
Figure 2. Forest plot of the impact of different metastatic sites on overall survival following PD-1 therapy
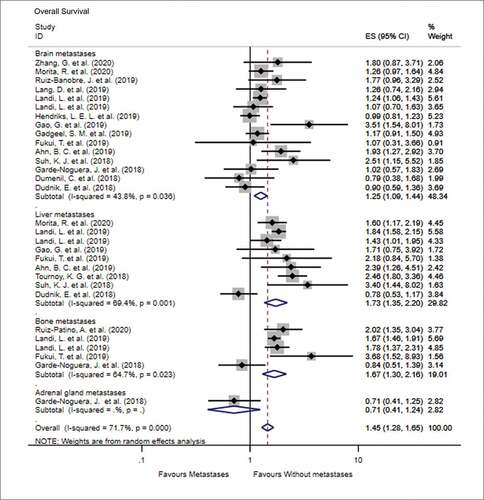
Figure 3. Forest plot of the impact of different metastatic sites on progression-free survival following PD-1 therapy
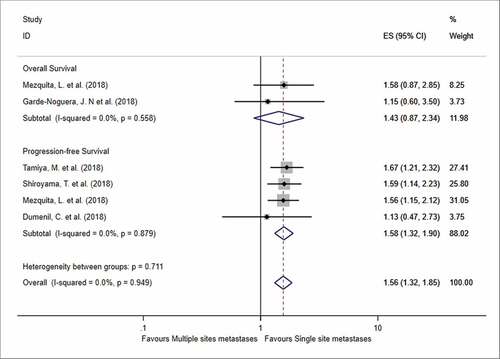
Moreover, we summarized the impact of the number of metastatic sites on the OS and PFS (). Forest plots showed that patients with multiple metastatic sites had worse PFS than patients with one metastatic site (HR = 1.58, 95% CI = 1.32–1.90, I2 = 0%, P < .001), while no significant difference was found in terms of OS (HR = 1.43, 95% CI = 0.87–2.34, I2 = 0%, P = .154). Besides, three studies compared the overall response rate (ORR) in terms of brain metastases, and patients with brain metastases had a lower ORR compared to those without metastases (OR = 1.58, 95% CI = 1.27–1.97, P < .001).
Discussion
To the best of our knowledge, this is the first and largest scale meta-analysis (number of included studies) to analyze the impact of metastatic sites on the prognosis of NSCLC patients. Although several previous studies concluded that tumor metastasis is not a predictor for advanced NSCLC patients when treated with PD-1 inhibitors, Citation3,Citation18,Citation22 in this review we demonstrate that metastatic site is an important factor related to long-term survival, especially in patients with brain and liver metastasis.
The results of our meta-analysis showed that patients with brain, liver, and bone metastases had worse OS. Similarly, patients with brain and liver metastases were more likely to progress than patients without metastases when treated with PD-1 inhibitors. In addition, we found that patients with brain metastases had a lower response rate than patients without metastases. The findings from our analysis suggest that the efficacy of treatments may differ depending on the metastatic site.
How metastatic sites contribute to the patient’s response to PD-1 inhibitors remains unclear. Metastatic spread of cancers to distant organs is the main cause of cancer-related mortality. NSCLC is prone to intrathoracic dissemination, such as intrapulmonary metastases, pleura, and pericardial invasion. The common distant organs of metastases include bone, brain, liver, and adrenal glands. Some studies have indicated that tumors may exhibit four different biological behavior metastases: tumors that mainly metastasize to local lymph nodes; tumors that are mainly involved in direct invasion; tumors that metastasize into the lung, and those that metastasize through systemic seeding.Citation41 Oikawa et al. reported that distant organ metastases in lung cancer are nonrandom, and there may be specific patterns of distant metastases in lung cancer patients.Citation42 Another study showed that when compared to patients with wild-type EGFR, ALK, or KRAS, higher incidence of pericardial, pleural dissemination, and liver metastasis was observed in ALK-positive patients and patients with EGFR mutations had higher incidence of liver metastasis.Citation43 However, there are limited studies on the correlation between metastatic organs, clinicopathological characteristics, and oncogenes, and the correlations remain inconclusive. Thus, considerable heterogeneity exists among studies that evaluated the relationship between metastatic sites and the long-term outcome following PD-1 inhibitor treatment.
Brain is one of the common metastatic sites in NSCLC. Previous studies have shown that brain metastases from NSCLC account for approximately half of all solid tumors metastasized to the brain.Citation44 According to statistics, approximately 16–22% of lung cancer patients develop brain metastases.Citation45 At the same time, brain metastases are also a leading cause of death in patients with NSCLC.Citation46 Previous studies have reported that patients with lung metastases from lung cancer have a very high mortality rate and their 1-y survival rate is low at approximately 10%.Citation45,Citation46 Due to the abundant pulmonary blood vessels and lymphatic networks that exist at the NSCLC development site, the cancer cells can enter the skull through the collateral circulation and carotid artery. Besides, lung cancer has the characteristics of a neutrophilic tissue structure and a strong affinity for the brain. Theoretically, the blood-brain barrier is damaged or affected to a certain extent in patients with brain metastases. Most chemotherapeutic drugs, however, are still unable to enter the brain due to their large molecular structure, and so they remain less effective in treating brain metastases.Citation47 The combination treatment of PD-1 inhibitors with radiotherapy has been a novel attempt at treating brain metastases in NSCLC patients. Schapira et al. conducted a clinical trial in NSCLC patients with brain metastases by treating them with concurrent stereotactic radiosurgery (SRS) and PD-1 inhibitors and showed that the concurrent treatment effectively controlled locoregional disease progression and therefore prolonged long-term OS.Citation45 Similarly, Shepard et al. demonstrated that concurrent treatment with SRS and PD-1 inhibitors was tolerated and provided more rapid brain metastasis regression.Citation48 Although several attempts have been undertaken in treating patients with brain metastases, brain metastases remain a predictor for both progression and OS in our meta-analysis.
Bone is another common metastatic site in NSCLC patients, and the incidence of bone metastasis in NSCLC patients is approximately 30–40%.Citation49 Bone metastases most often involve the central axis bones, including the spine, pelvis, proximal limb bones, and skull, and mainly manifests as osteolytic destruction, which can lead to pain and pathological fractures.Citation49 Following bone metastasis, the median survival time of NSCLC patient is 6–10 months and the 1-y survival rate after treatment is 40–50%.Citation49 The bone metastasis cascade is a multi-step process, which mainly includes the following steps: escape of tumor cells with metastatic ability from the primary tissues and entry into the circulatory system, chemotactic ‘homing’ and ‘settling’ to the bone marrow, with active invasion.Citation50 Besides, the interaction between the metastatic tumor cells and the bone microenvironment plays a vital role in the occurrence and development of bone metastasis.Citation51 It is now clear that bone marrow can supplant the secondary lymphoid tissue as either a primary immune response or memory response. Further, the bone marrow itself serves as an immune regulatory organ, which affects systemic immunity and therapeutic efficacy of PD-1 inhibitors.Citation3 Although we did not find a clear association between bone metastases and either OS or PFS, the risk of bone metastases still needs to be considered when PD-1 inhibitors are used for treatment.
Liver metastasis occurs when NSCLC cells that are shed from the primary site enter the liver through blood circulation and continue to grow following liver colonization. Therefore, liver metastases can be single or multiple nodule metastases. Liver metastasis rate of NSCLC at autopsy is 40–61%. Approximately 38–44% of NSCLC patients develop liver metastases throughout the development and progression of the disease.Citation52 Compared to other metastases, patients with liver metastases respond poorly to chemotherapy, have shorter survival, and poor prognosis. Therefore, liver metastasis is an important factor affecting the prognosis of NSCLC. Currently, several studies have reported that patients with liver metastases may benefit from combined treatment of PD-1 inhibitors with chemotherapy.Citation53,Citation54 However, in our meta-analysis, liver metastases remained a risk factor associated with worse survival outcome.
Currently, PD-1/PD-L1 inhibitors are used to treat several types of malignancies. In melanoma, the main research focus is currently on PD-1 inhibitors as single agent or combined with Ipilimumab for pre- and post-(neo) adjuvant treatment of high-risk melanoma of stage IIB to III. Although many Phase III clinical trials are still in progress, the current results of PD-1 inhibitors used in adjuvant treatment of middle-advanced resectable melanoma significantly prolonged PFS and reduced the incidence of grade 3 to 4 adverse reactions.Citation55 More recently, Warner et al. conducted a cohort study and summarized that majority of melanoma patients with confirmed complete response chose to discontinue the PD-1 treatment and the complete response was mostly durable.Citation56 In bladder cancer, several PD-1 inhibitors were approved by FDA as a second-line treatment in 2017.Citation57 A Phase I trial (NCT01375842) involving 95 patients with metastatic bladder cancer showed that the partial response rate of the patients was 26%, the median PFS was 2.7 months, and the median survival time was 10.1 months. Among them, 40% had PD-LI expression of ≥5%, and their median survival time was 14.6 months, Citation58 which indicated that detection of PD-L1 expression level is of considerable significance for tumor immunotherapy. More recently, Phase 1b trials published the efficacy of combination of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma, which indicated that the combination treatment could prolong the lifespan of advanced liver cancer patients with median PFS of 9.3 months.Citation59 Besides, a single-arm, Phase 1b-2 trial (CP-MGAH22-05) was conducted to treat HER2-positive gastroesophageal adenocarcinoma patients and the combination of anti-HER2 agent and anti-PD-1 checkpoint blockade was found to be safe and well tolerated, and ORR was observed in 18% of the patients with malignancy.Citation60 Nevertheless, treatment with PD-1/PD-L1 inhibitors remains promising although attention must be paid to the associated toxicity.
Some limitations of our study need to be acknowledged. First, although we used the random-effect model to decrease the effect of weight in different studies, we could not entirely eliminate the heterogeneity between studies. The heterogeneity existed in two parts: (a) PD-1 expression may be different between primary tumors and metastatic sites. Generally, the inclusion criteria in different studies may consider tumors expressing higher PD-1 expression were, the better the response may the patients have. However, majority of advanced stage patients were biopsied in the primary tumors. Thus, primary tumors with high expression of PD-1 may be cured by PD-1 inhibitors, however, whether the metastatic sites have the same expression of PD-1 as the primary tumor remains controversial. (b) Metastatic sites may have a different genetic profile compared to the primary site. Different PD-1 inhibitors may be effective in different kinds of mutations or mismatches. Thus, the primary tumors may benefit from one PD-1 inhibitor, while the metastatic sites may benefit from another. Second, most of the studies were retrospective studies and very few studies were prospectively designed for advanced metastatic NSCLC patients treated with PD-1 inhibitor. Besides, chemotherapy and radiotherapy were used in the treatment in some studies, which may increase the bias between different studies. Further multicenter and prospective studies need to be undertaken in NSCLC patients with metastases.
Conclusion
Our systematic meta-analysis suggests that metastatic sites are independent predictors of survival outcome in advanced NSCLC patients treated with PD-1 inhibitors. Combination of treatments is needed to target the different metastatic sites.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author’s contribution
Design of the meta-analysis: Yangyun Huang, Lihuan Zhu and Xiaojie Pan
Literature screening: Tianxing Guo and Wenshu Chen
Quality assessment: Zhenlong Zhang and Wujin Li
Statistics analysis: Yangyun Huang and Lihuan Zhu
Write and revise: Yangyun Huang, Lihuan Zhu, Tianxing Guo, Wenshu Chen, Zhenlong Zhang, Wujin Li, and Xiaojie Pan
References
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA: a cancer journal for clinicians 2016;66(2):115–132. doi:10.3322/caac.21338.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
- Landi L, D’Inca F, Gelibter A, Chiari R, Grossi F, Delmonte A, Passaro A, Signorelli D, Gelsomino F, Galetta D, et al. 2019. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J ImmunoTher Cancer. 7(1). (no pagination)(316). doi:10.1186/s40425-019-0793-8.
- Thomas A, Liu SV, Subramaniam DS, Giaccone G. Refining the treatment of NSCLC according to histological and molecular subtypes. Nat Rev Clin Oncol. 2015;12(9):511–26. doi:10.1038/nrclinonc.2015.90.
- Belani CP, Langer C. First-line chemotherapy for NSCLC: an overview of relevant trials. Lung Cancer. 2002;38:13–19. doi:10.1016/S0169-5002(02)00394-X.
- Lin Y, Wang X, Jin H. EGFR-TKI resistance in NSCLC patients: mechanisms and strategies. Am J Cancer Res. 2014;4:411.
- Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L, Audigier-Valette C, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4(3):351–57. doi:10.1001/jamaoncol.2017.4771.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi:10.1146/annurev.immunol.26.021607.090331.
- Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21(1):24–33. doi:10.1016/j.molmed.2014.10.009.
- Santarpia M, González-Cao M, Viteri S, Karachaliou N, Altavilla G, Rosell R. Programmed cell death protein-1/programmed cell death ligand-1 pathway inhibition and predictive biomarkers: understanding transforming growth factor-beta role. Transl Lung Cancer Res. 2015;4:728.
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123–35. doi:10.1056/NEJMoa1504627.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. doi:10.1056/NEJMoa1507643.
- Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, Molina J, Kim J-H, Arvis CD, Ahn M-J, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet. 2016;387(10027):1540–50. doi:10.1016/S0140-6736(15)01281-7.
- Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. The Lancet. 2016;387(10030):1837–46. doi:10.1016/S0140-6736(16)00587-0.
- Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, D’Amico TA, DeCamp MM, Dilling TJ, Dobelbower M, et al. Non–small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Nat Compr Cancer Network. 2017;15(4):504–35. doi:10.6004/jnccn.2017.0050.
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
- Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144(6):427–37. doi:10.7326/0003-4819-144-6-200603210-00010.
- Zhang G, Cheng R, Wang H, Zhang Y, Yan X, Li P, Zhang M, Zhang X, Yang J, Niu Y, et al. Comparable outcomes of nivolumab in patients with advanced NSCLC presenting with or without brain metastases: a retrospective cohort study. Cancer Immunol Immunother 2020;69(3):399–405.
- Zhang F, Huang D, Li T, Zhang S, Wang J, Zhang Y, Wang G, Zhao Z, Ma J, Wang L, et al. Anti-PD-1 therapy plus chemotherapy and/or Bevacizumab as second line or later treatment for patients with advanced non-small cell lung cancer. J Cancer. 2020;11(3):741–49. doi:10.7150/jca.37966.
- Ruiz‐Patiño A, Arrieta O, Cardona AF, Martín C, Raez LE, Zatarain‐Barrón ZL, Barrón F, Ricaurte L, Bravo‐Garzón MA, Mas L, et al. Immunotherapy at any line of treatment improves survival in patients with advanced metastatic non-small cell lung cancer (NSCLC) compared with chemotherapy (Quijote-CLICaP). Thoracic Cancer. 2020;11(2):353–61. doi:10.1111/1759-7714.13272.
- Morita R, Okishio K, Shimizu J, Saito H, Sakai H, Kim YH, Hataji O, Yomota M, Nishio M, Aoe K, et al. Real-world effectiveness and safety of nivolumab in patients with non-small cell lung cancer: A multicenter retrospective observational study in Japan. Lung Cancer. 2020;140:8–18. doi:10.1016/j.lungcan.2019.11.014.
- Kawachi H, Tamiya M, Tamiya A, Ishii S, Hirano K, Matsumoto H, Fukuda Y, Yokoyama T, Kominami R, Fujimoto D, et al. Association between metastatic sites and first-line pembrolizumab treatment outcome for advanced non–small cell lung cancer with high PD-L1 expression: a retrospective multicenter cohort study. Invest New Drugs. 2020;38(1):211–18. doi:10.1007/s10637-019-00882-5.
- Ruiz-Banobre J, Areses-Manrique MC, Mosquera-Martinez J, Cortegoso A, Afonso-Afonso FJ, de Dios-álvarez N, Fernández-Núñez N, Azpitarte-Raposeiras C, Amenedo M, Santomé L, et al. Evaluation of the lung immune prognostic index in advanced nonsmall cell lung cancer patients under nivolumab monotherapy. Transl Lung Cancer Res. 2019;8(6):1078–85. doi:10.21037/tlcr.2019.11.07.
- Ren F, Zhao T, Liu B, Pan L. Neutrophil-lymphocyte ratio (NLR) predicted prognosis for advanced non-small-cell lung cancer (NSCLC) patients who received immune checkpoint blockade (ICB). Onco Targets Ther. 2019;12:4235–44. doi:10.2147/OTT.S199176.
- Prelaj A, Ferrara R, Rebuzzi SE, Proto C, Signorelli D, Galli G, De Toma A, Randon G, Pagani F, Viscardi G, et al. Epsilon: A prognostic score for immunotherapy in advanced non-small-cell lung cancer: A validation cohort. Cancers. 2019;11(12):1954. (no pagination)(1954). doi:10.3390/cancers11121954.
- Lang D, Huemer F, Rinnerthaler G, Horner A, Wass R, Brehm E, Akbari K, Granitz M, Hutarew G, Kaiser B et al: Therapy Line and Associated Predictors of Response to PD-1/PD-L1-Inhibitor Monotherapy in Advanced Non-small-Cell Lung Cancer: A Retrospective Bi-centric Cohort Study. Targeted oncology. 2019;14(6):707–717.
- Kuo CHS, Wang CC, Huang YC, Pavlidis S, Liu C-Y, Ko H-W, Chung F-T, Lin T-Y, Wang C-L, Guo Y-K, et al. Comparison of a combination of chemotherapy and immune checkpoint inhibitors and immune checkpoint inhibitors alone for the treatment of advanced and metastatic non-small cell lung cancer. Thoracic Cancer. 2019;10(5):1158–66. doi:10.1111/1759-7714.13057.
- Hendriks LEL, Henon C, Auclin E, Mezquita L, Ferrara R, Audigier-Valette C, Mazieres J, Lefebvre C, Rabeau A, Le Moulec S, et al. Outcome of patients with non-small cell lung cancer and brain metastases treated with checkpoint inhibitors. J Thoracic Oncol. 2019;14(7):1244–54. doi:10.1016/j.jtho.2019.02.009.
- Gao G, Jia K, Zhao S, Li X, Zhao C, Jiang T, Su C, Ren S, Zhou F, Zhou C, et al. Analysis of the association between prior chemotherapy regimens and outcomes of subsequent anti-PD-(L)1 monotherapy in advanced non-small cell lung cancer. Transl Lung Cancer Res. 2019;8(6):920–28. doi:10.21037/tlcr.2019.11.25.
- Gadgeel SM, Lukas RV, Goldschmidt J, Conkling P, Park K, Cortinovis D, de Marinis F, Rittmeyer A, Patel JD, von Pawel J, et al. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: exploratory analyses of the phase III OAK study. Lung Cancer. 2019;128:105–12. doi:10.1016/j.lungcan.2018.12.017.
- Fukui T, Okuma Y, Nakahara Y, Otani S, Igawa S, Katagiri M, Mitsufuji H, Kubota M, Hiyoshi Y, Ishihara M, et al. Activity of nivolumab and utility of neutrophil-to-lymphocyte ratio as a predictive biomarker for advanced non-small-cell lung cancer: a prospective observational study. Clin Lung Cancer. 2019;20(3):208–14.e2. doi:10.1016/j.cllc.2018.04.021.
- Ahn BC, Pyo KH, Xin CF, Jung D, Shim HS, Lee CY, Park SY, Yoon HI, Hong MH, Cho BC, et al. Comprehensive analysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice. J Cancer Res Clin Oncol. 2019;145:1613–23. doi:10.1007/s00432-019-02899-y.
- Tournoy KG, Thomeer M, Germonpre P, Derijcke S, De Pauw R, Galdermans D, Govaert K, Govaerts E, Schildermans R, Declercq I, et al. Does nivolumab for progressed metastatic lung cancer fulfill its promises? An efficacy and safety analysis in 20 general hospitals. Lung Cancer. 2018;115:49–55. doi:10.1016/j.lungcan.2017.11.008.
- Tamiya M, Tamiya A, Inoue T, Kimura M, Kunimasa K, Nakahama K, Taniguchi Y, Shiroyama T, Isa S-I, Nishino K, et al. Metastatic site as a predictor of nivolumab efficacy in patients with advanced non-small cell lung cancer: A retrospective multicenter trial. PLoS ONE [Electronic Resource]. 2018;13(2):e0192227. doi:10.1371/journal.pone.0192227.
- Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim TM, Kim D-W, Heo DS, Lee JS. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2018;67(3):459–70. doi:10.1007/s00262-017-2092-x.
- Shiroyama T, Suzuki H, Tamiya M, TAMIYA A, TANAKA A, OKAMOTO N, NAKAHAMA K, TANIGUCHI Y, ISA S-I, INOUE T, et al. Clinical characteristics of liver metastasis in nivolumab-treated patients with non-small cell lung cancer. Anticancer Res. 2018;38(8):4723–29. doi:10.21873/anticanres.12779.
- Garde-Noguera J, Martin-Martorell P, De Julian M, Perez-Altozano J, Salvador-Coloma C, García-Sanchez J, Insa-Molla A, Martín M, Mielgo-Rubio X, Marin-Liebana S, et al. Predictive and prognostic clinical and pathological factors of nivolumab efficacy in non-small-cell lung cancer patients. Clin Transl Oncol. 2018;20(8):1072–79. doi:10.1007/s12094-017-1829-5.
- Dumenil C, Massiani MA, Dumoulin J, Giraud V, Labrune S, Chinet T, Giroux Leprieur E. Clinical factors associated with early progression and grade 3-4 toxicity in patients with advanced non-small-cell lung cancers treated with nivolumab. PLoS ONE [Electronic Resource]. 2018;13(4):e0195945. doi:10.1371/journal.pone.0195945.
- Dudnik E, Moskovitz M, Daher S, Shamai S, Hanovich E, Grubstein A, Shochat T, Wollner M, Bar J, Merimsky O, et al. Effectiveness and safety of nivolumab in advanced non-small cell lung cancer: the real-life data. Lung Cancer. 2018;126:217–23. doi:10.1016/j.lungcan.2017.11.015.
- Funazo T, Nomizo T, Kim YH. Liver metastasis is associated with poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. J Thoracic Oncol. 2017;12(9):e140–e1. doi:10.1016/j.jtho.2017.04.027.
- Detterbeck FC, Tanoue LT, Boffa DJ. Anatomy, biology and concepts, pertaining to lung cancer stage classification. J Thoracic Oncol. 2009;4(4):437–43. doi:10.1097/JTO.0b013e31819852ed.
- Oikawa A, Takahashi H, Ishikawa H, Kurishima K, Kagohashi K, Satoh H. Application of conditional probability analysis to distant metastases from lung cancer. Oncol Lett. 2012;3(3):629–34. doi:10.3892/ol.2011.535.
- Doebele RC, Lu X, Sumey C, Maxson DA, Weickhardt AJ, Oton AB, Bunn PA, Barón AE, Franklin WA, Aisner DL, et al. Oncogene status predicts patterns of metastatic spread in treatment‐naive nonsmall cell lung cancer. Cancer. 2012;118(18):4502–11. doi:10.1002/cncr.27409.
- Patel RR, Mehta MP. Targeted therapy for brain metastases: improving the therapeutic ratio. Clin Cancer Res. 2007;13(6):1675–83. doi:10.1158/1078-0432.CCR-06-2489.
- Schapira E, Hubbeling H, Yeap BY, Mehan WA, Shaw AT, Oh K, Gainor JF, Shih HA. Improved overall survival and locoregional disease control with concurrent PD-1 pathway inhibitors and stereotactic radiosurgery for lung cancer patients with brain metastases. Int J Radiat Oncol Biol Phys. 2018;101(3):624–29. doi:10.1016/j.ijrobp.2018.02.175.
- Crino L, Bronte G, Bidoli P, Cravero P, Minenza E, Cortesi E, Garassino MC, Proto C, Cappuzzo F, Grossi F, et al. Nivolumab and brain metastases in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer. 2019;129:35–40. doi:10.1016/j.lungcan.2018.12.025.
- Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, Shanley R, Yeh N, Gaspar LE, Braunstein S, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA oncol. 2017;3(6):827–31. doi:10.1001/jamaoncol.2016.3834.
- Shepard MJ, Xu Z, Donahue J, Eluvathingal Muttikkal TJ, Cordeiro D, Hansen L, Mohammed N, Gentzler RD, Larner J, Fadul CE, et al. Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non-small cell lung cancer to the brain: a matched cohort study. J Neurosurg. 2019:1–8. doi:10.3171/2019.4.JNS19822
- Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop Relat Res. 2008;466(3):729–36. doi:10.1007/s11999-007-0051-0.
- Raubenheimer E, Noffke C. Pathogenesis of bone metastasis: a review. J Oral Pathol Med. 2006;35(3):129–35. doi:10.1111/j.1600-0714.2006.00360.x.
- Santini D, Pantano F, Vincenzi B, Tonini G, Bertoldo F. The role of bone microenvironment, vitamin D and calcium. Recent Results Cancer Res 2012;192:33–64
- Yang N, Jiang Y, Zhang H, Sun B, Hou C, Zheng J, Liu Y, Zuo P. Active targeting docetaxel-PLA nanoparticles eradicate circulating lung cancer stem-like cells and inhibit liver metastasis. Mol Pharm. 2015;12(1):232–39. doi:10.1021/mp500568z.
- Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387–401. doi:10.1016/S2213-2600(19)30084-0.
- Vokes EE, Ready N, Felip E, Horn L, Burgio MA, Antonia SJ, Arén Frontera O, Gettinger S, Holgado E, Spigel D, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Annals Oncol. 2018;29(4):959–65. doi:10.1093/annonc/mdy041.
- Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–35. doi:10.1056/NEJMoa1709030.
- Betof Warner A, Palmer JS, Shoushtari AN, Goldman DA, Panageas KS, Hayes SA, Bajwa R, Momtaz P, Callahan MK, Wolchok JD, et al. Long-term outcomes and responses to retreatment in patients with melanoma treated with PD-1 blockade. J clin oncol. 2020;38(15):1655–63. doi:10.1200/JCO.19.01464.
- Bellmunt J, Powles T, Vogelzang NJ. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: the future is now. Cancer Treat Rev. 2017;54:58–67. doi:10.1016/j.ctrv.2017.01.007.
- Petrylak DP, Powles T, Bellmunt J, Braiteh F, Loriot Y, Morales-Barrera R, Burris HA, Kim JW, Ding B, Kaiser C, et al. Atezolizumab (MPDL3280A) monotherapy for patients with metastatic urothelial cancer: long-term outcomes from a phase 1 study. JAMA oncol. 2018;4(4):537–44. doi:10.1001/jamaoncol.2017.5440.
- Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2020;38(26):2960–70.
- Catenacci DVT, Kang Y-K, Park H, Uronis HE, Lee K-W, Ng MCH, Enzinger PC, Park SH, Gold PJ, Lacy J, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol. 2020;21(8):1066–76. doi:10.1016/S1470-2045(20)30326-0.