ABSTRACT
A novel coronavirus (2019-nCov) emerged in China, at the end of December 2019 which posed an International Public Health Emergency, and later declared as a global pandemic by the World Health Organization (WHO). The International Committee on Taxonomy of Viruses (ICTV) named it SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2), while the disease was named COVID-19 (Coronavirus Disease- 2019). Many questions related to the exact mode of transmission, animal origins, and antiviral therapeutics are not clear yet. Nevertheless, it is required to urgently launch a new protocol to evaluate the side effects of unapproved vaccines and antiviral therapeutics to accelerate the clinical application of new drugs. In this review, we highlight the most salient characteristics and recent findings of COVID-19 disease, molecular virology, interspecies mechanisms, and health consequences related to this disease.
Introduction
Until 2002, human coronaviruses were not widely known to be pathogenic to humans, however, some strains only caused mild infections in immunocompetent individualsCitation1. The devastating epidemic of Severe Acute Respiratory Syndrome (SARS) caused by SARS-CoV in 2002–2003,1,Citation2 and Middle East Respiratory Syndrome (MERS) caused by MERS-CoV in 2012,Citation3 circulated around the world, caused hundreds of mortalities, and posed billions of dollars of economic burden worldwide.Citation4 We could learn from the MERS-CoV epidemic that new and lethal CoVs can emerge anywhere and can cause epidemics or pandemics.Citation4,Citation5 However, the precautionary measures were not taken seriously, and recently (December, 2019), the world encountered another devastating outbreak of Coronavirus Disease-19 (COVID-19)Citation6
Since the emergence of COVID-19 infection, several cases of atypical pneumonia of unexplained etiology were reported in Wuhan, Hubei province, China at the beginning of January 2020Citation7. It was first isolated from the viral metagenomics of bronchoalveolar-lavage (BAL) samples of patients presenting pneumonia-like symptoms in Wuhan.Citation8 Later on, January 30, 2020, novel coronavirus (2019- nCoV) was positioned under the Public Health International Emergency category by the World Health Organization (WHO) due to its rapid spread and high mortality and morbidity rates.Citation6 The International Committee on Taxonomy of Viruses (ICTV) named this virus as SARS-CoV-2 based on its similarity with SARS-CoV.Citation6 It was not known until Dec 31st, 2019 when the WHO was informed and cases were finally reported and entered into the Chinese National Health Database. Although the outbreak in China appears to be in control, this highly contagious disease is now circulating across the globe, with a daily increase in the number of deaths and infected cases and the number of affected countries. COVID-19 disease has already created havoc situation by infecting millions of individuals and causing death of hundreds of thousands of people worldwide.
The addition of information on a daily basis, due to the COVID-19 pandemic, to compile the knowledge and provide facts and evidence of the information speculated earlier is highly important. The present and most probably future emergence of such zoonotic pathogens, their economic impact, and lack of impelling therapeutic strategies have now made it clear that our preparedness to cope or treat the CoV pandemic is very limited. Nevertheless, immediate sequencing of the SARS-CoV-2 genome, development of predictive models, and phylogenetic tracing analysis allowed a better understanding of the virus biology, its rapid monitoring, and response compared to earlier Human CoVs epidemics.Citation1 This review article highlights the current status, recent findings, and scientific evidence, especially epidemiological features, entry mechanism, pathophysiology, antiviral therapies, health consequences, and research progress of continuously evolving COVID-19.
Emergence of COVID-19
Both the SARS-CoV-2 and bat CoV closely resemble each other. The bat is considered as the primary source, while the intermediate host that may exist between bats and humans is still unclearCitation9. Recent studies have shown that for SARS-CoV-2-like CoVs Pangolin can be the natural reservoir, and might be the possible intermediate host between bats and humans. The Pangolin-CoV shares 90.5% and 91.1% similarity to Bat CoV (RaTG13) and SARS-CoV-2, respectively.Citation10 Whereas some of the vital Receptor binding motif (RBM) residues of SARS-CoV are adapted to civet and human Angiotensin-Converting Enzyme 2 (ACE2), and cause partial viral adaptations to two different host species that significantly raised cross-species viral transmission and replication between the two host species. It can be considered that either palm civets did not participate as intermediate hosts for SARS-CoV-2, or they have passed this novel CoV to humans very quickly before adapting to civet ACE2. The phylogenetic analysis of SARS-CoV-2 (bat origin) has indicated a high affinity toward ACE2 derived from diverse animal species, except for rats and mice. These animal species may be possible intermediate hosts or used as animal models for SARS-CoV-2 infections.Citation11 In 2005, the SARS-CoV was isolated in wild palm civets near Wuhan. Knowing the fact that SARS-CoV RBD has already well evolved to civet ACE2 (except for residue 487), the wild animals who dwell near Wuhan should be screened out for SARS-CoV-2.Citation12
Genomic analysis
The SARS-CoV-2 genome sequence available in GenBank on 10th January 2020, by the Shanghai Public Health Clinical Center & School of Public Health, Fudan University, and in a subsequent publication.Citation13 The SARS-CoV-2 is an enveloped, spherical in shape, single-stranded, positive-sense RNA virus. It is the seventh and new member of the genus Betacoronavirus.Citation9,Citation13 The complete genome of SARS-CoV-2 has 29870-bp (excluding the poly-A tail), which contains 15 genes and encodes for 9860 amino acids (). The S gene that codes for the spike glycoprotein located on the surface of the viral envelope is responsible for binding to the host’s cell via ACE2.Citation12,Citation14 Five identified typical Open reading Frame (ORFs) on the same coding strand, including ORF1ab polyprotein (7096-aa), membrane protein (222-aa), an envelope protein (75- aa), nucleocapsid protein (419-aa), and spike glycoprotein (1273-aa).Citation13 It shares less than 50% similarity to HCoV-NL63, −229E, -HKU1, -OC43, and MERS-CoV.Citation9 Due to difference of merely five nucleotides, it is suggested that SARS-CoV-2 has emerged from SARS-CoV.Citation6
The CoVs error-prone RNA-dependent RNA polymerases (RdRP) help them in adaptive evolution, allows mutations, subspecies diversity, and recombination events to occur frequently. Mutation in the SARS-CoV genome during the 2002–2004 epidemic increased its affinity for host cellular receptors and enhanced its replication in human host cells, cumulating in higher virulence.Citation15 The cryo-electron microscopy, transmission electron microscopy, and Scanning electron microscopy images have shown that changes have occurred in spike glycoprotein of SARS-CoV-2.Citation6 The genetic mutations can potentially affect the spread and severity of the SARS-CoV-2.Citation16 In SARS-CoV, even a single N501T mutation corresponding to the S487T mutation can enhance the binding affinity of SARS-CoV-2 RBD to human ACE2. Recently, population genetics-based analysis has indicated that different viral strains differ at orf1ab and ORF8 regions, while new variations of the spike protein in SARS-CoV-2 are linked to natural selections and mutations. Therefore, further studies are necessary to evaluate the impacts of these mutations on transmission, symptoms development, and recovery from COVID-19 disease.Citation17 Any mutation events in SARS-CoV-2 patients occurred at the 501 positions (also the 494 positions) should be monitored properly.Citation11 From December-2019 to April 5th-2020, a total of 95 complete genome sequences of SARS-CoV-2 are available in GenBank, National Microbiology Data Center (NMDC), and NGDC Genome Warehouse. In total 116 mutations were found after a genomic analysis, out of which 3 most common mutations are present in 28144 T > C in ORF8 gene, 8782 C > T in ORF1ab gene, and 29095 C > T in the N gene.Citation16
Transmission
The previous controversy existed over the chances of COVID-19 infection transmission during the incubation period and before the onset of symptoms. The epidemiological studies have now confirmed the transmission of infection during the incubation period.Citation18 The 4 days estimated median serial interval of SARS-CoV-2 is close or shorter than its median incubation period, which illustrates that chances of secondary transmission exist even prior to the onset of illness.Citation19 Other routes of viral transmission include direct transmission through coughing, sneezing, virus-laden aerosol inhalation, infected person`s saliva,Citation9 and if body fluids are exposed to mucous membranes of mouth, eyes, or nose, close contact, and fomites.Citation15 During the previous outbreak of SARS-CoV, viral load was detected in the cerebrospinal fluid of acutely infected patients. Thus, the brain is also vulnerable to viral entry as ACE2 receptors are found over neurons and glial cells. According to a recent report, 78 out of 214 patients of COVID-19 have shown neurological manifestations, claiming that novel SARS-CoV-2 can damage the brain. However, the role of SARS-CoV-2 against the neurotropic potential is yet to be established.Citation20 Viral RNA has been found in the plasma of 15% of the most severely affected patients.Citation21 The viral nucleic acids have been found in anal swabs and fecal samples, suggesting that possibility of fecal-oral transmission in COVID-19 infection should not be ruled out.Citation13 The virus is also reported in the sewage system and could reflect disease burden in the community and guide the policymakers to decide for public health measures.Citation22 The estimated reproduction number (R0) value for COVID-19 disease is 3.6–4.0, which shows that 72–75% of transmissions must be avoided in order to deal with the infection increasing trend.Citation15
Binding receptors and entry mechanism
Coronaviruses proteins expression in the host cell leads the coronavirus-host interplay either by changing host gene expression or by effecting host’s antiviral defense mechanisms.Citation23 The CoV spike protein mediates its entry into host cells by attaching itself with the host receptor and then fuse the viral membrane with the host cell membrane.Citation11 Changes occurring at spikes on receptor binding ligands cause cross-species transmission and zoonotic spillover.Citation6 The spike proteolytic activation controls the release of the fusion peptide into host cellular membranes. Thus, several host proteases have been modified to proteolytically activate the spike protein including, trypsin cell surface transmembrane protease/serine (TMPRSS) proteases, endosomal cathepsins and furin.Citation24 However, due to large size genome, complicated genomic expression, the knowledge about mechanism and replication cycle of CoVs is still in an early stage compared to some other positive RNA viruses.Citation23
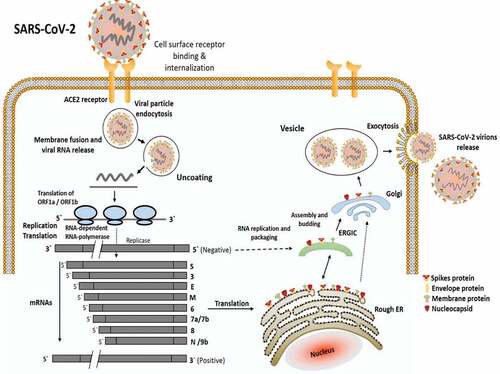
Different coronaviruses bind to different types of host receptors, such as ACE2 and CD26 for SARS-CoV and MERS-CoV, respectively.Citation6 In the case of SARS-CoV-2, its RBD is structurally similar to SARS-CoV,Citation25 however, its binding affinity for ACE2 is 10–20 folds higher as compared to SARS-CoV.Citation20 The structure of SARS-CoV RBD shows that it has a core structure with RBM, which binds to the protruded claw-like structure of ACE2. Certain amino acids (at 442, 472, 479, 480, and 487 positions) have shown to modify viral attachment with human ACE2 while others at the same amino acid positions can increase viral attachment with civet ACE2. Notably, human receptor ACE2 favoring residues combined into one RBD could bind to human ACE2 with greater affinity and mediate viral entry very efficiently into human host cells.Citation11 The clear mechanism of SARS-CoV-2 attachment and infection to the human cell is illustrated in .
Figure 2. The mechanism associated with the infectiousness of SARS-CoV-2 in the human body. After attachment to the ACE2 receptor of human cells, SARS-CoV-2 enters the cell and releases its nucleic material (RNA). Using human cell machinery, the viral genome undergoes transcription and translation, followed by RNA replication and packaging. During packaging, spike protein and other enveloping materials bind to the outer surface of newly synthesized virions. Once absolute nascent virions are formed, they are released from the cell and the cycle is repeated in the new healthy cells
Incubation period and symptomatic conditions
Initially, the incubation period for COVID-19 infected individuals was estimated to range from 1 to 14 days (minimum 3–10 days),Citation15 and at least 14 days quarantine was recommended for the infected patients.Citation26 Later, it was estimated that the incubation period may range from 0 to 24 days.Citation27 The most common clinical presentation observed at the onset of COVID-19 disease includes headache (6.5%), dry cough (45.8%), fever (82.1%), dyspnea (6.9%), and fatigue (26.3%).Citation28 Whereas shortness of breath, sneezing, rhinorrhea, diarrhea, muscle ache, sore throat, vomiting, nausea, and acute respiratory distress syndrome are reported in other cases. Critically ill patients also suffered from septic shock and respiratory and vital organ failure.Citation29 According to CDC China, the majority of asymptomatic patients (80.9%) presented with mild pneumonia heavily released viruses at the initial phase of infection, which made viral spread containment very challenging.Citation27
COVID-19 and human health
The outcomes for SARS-CoV-2 seemed to be very high as compared to SARS-CoV and MERS-CoV.Citation30 However, the placentas delivered from infected pregnant women resulted in negative for SARS-CoV-2 detection tests, with no morphological changes in the placenta.Citation31 Another case of COVID-19 infected pregnant woman delivered at the 35th week of pregnancy in a negative-pressure operating room did not show any mother-to-child transmission.Citation32 Psychological stress and anxiety are the major outcomes of viral outbreaks.Citation33 Mental illnesses have been reported to alter immunity and increase disease vulnerability,Citation34 thus, it can be related to high morbidity and mortality cases overall. Nevertheless, restricted exposure to the outside environment, long-term quarantine, lost income, no access to masks and preventive measures, fear of contracting the infection, and lack of facilities have fueled the severity of mental illness.Citation35 As China is the first country to encounter the COVID-19 infection, studies show that individuals faced disturbed sleep-wake behaviors due to constant exposure to electronic devices and minimal physical activities. Especially, children can easily be affected by altering circadian rhythms and are prone to develop mental illnesses.Citation36 Recently, a wide range of online surveys related to mental health is conducted by researchers in China, including medical staff fighting against COVID-19 infection, students, and the general population. The online psychological counseling services are also available for them.Citation37 COVID-19 pandemic has also adversely affected individuals with clinical histories, such as diabetes, Cardiac problems, or blood pressure issues.
Therapeutics and drugs
Currently, no specific therapeutic agents and licensed vaccines are available for preventing and controlling the CoV infection. Drugs used during SARS-CoV and MERS-CoV epidemics such as, Remdesivir, Baricitinib, Hydroxychloroquine, and Favipiravir have been found effective against COVID-19, while several other potential options are required to be tested.Citation38 Many other anti-viral drugs including, Nafamostat, Ribavirin, Ritonavir, and Arbidol; broad-spectrum antibiotics and interferons-α nebulization have also exhibited intermediate results against COVID-19.Citation39,Citation40 Despite the increasing trend of COVID-19 infection, no drug is significantly validated by conducting large-scale clinical studies that can effectively reduce the SARS-CoV-2 viral load.Citation41 An antiviral agent remdesivir, which effectively inhibits RNA-dependent RNA polymerase (RdRp) activity, evades viral exoribonuclease proofreading, and prevents viral replication both in vitro and in SARS-CoV mouse model has shown promising results against COVID-19 infection.Citation42 In a clinical trial conducted to analyze the efficacy of remdesivir, a loading dose of 200 mg on day 1, followed by 100 mg once daily for the next 9 days was administered intravenously to hospitalized COVID-19 infected adult patients having lower respiratory tract infection. The results have suggested that patients receiving remdesivir recovered around 3 days earlier as compared to the placebo group, resulting in early discharge from the hospital.Citation43 Although remdesivir is a potential therapeutic option, further studies are required to analyze its uncertain negative side effects including, hepatic toxicity, vomiting, nausea, and rectal hemorrhage among others.Citation39 Favipiravir (FPV), another RdRp inhibitor drug, when used against RNA viruses it transforms into an active phosphoribosylated form that acts as a substrate of viral RNA polymerase. FPV is approved to use against SARS-CoV-2 infection in China after successful clinical studies against COVID-19 infected Chinese patients.Citation41 A randomized experimental trial was conducted to examine the therapeutic efficacy of FPV plus interferon- α (IFN-α) versus Ritonavir (RTV)/Lopinavir (LPV) against confirmed COVID-19 infected patients. One group of patients received an oral dose of FPV (1:1600 mg twice on day-1, and 14: 600 mg twice on day-2) plus IFN-α. Whereas the second group of patients received a dosage of RTV/LPV (14: 400 mg/100 mg twice per day) plus IFN-α. The results have demonstrated that FPV showed faster viral clearance and significant improvement in chest imaging, in addition to less harmful effects on patients internal health.Citation44
Ritonavir (RTV) and Lopinavir (LPV) are protease inhibitors (PIs) that target 3 C-like protease and papain-like protease of CoVs.Citation41 The papain-like protease (PLpro) and 3 C-like protease (3CLpro) are coronaviral proteases essential for its replication that can be used as antiviral drug targets.Citation4 In a clinical trial (Chinese Clinical Trial Register number, ChiCTR2000029308) conducted on the LPV/RTV treatment, 400/100 mg dose was administered orally twice per day to clinically confirmed COVID-19 patients for 14 days. The patients induced adverse gastrointestinal effects while, no decrease in viral load was observed; however, a slight decline in the death rate of severely infected SARS-CoV-2 patients was noticed in the LPV/RTV group as compared to the standard care group.Citation45 Thus, It is suggested that a higher concentration than the serum level might be required to inhibit the replication of pulmonary SARS-CoV-2 when treating with LPV/RTV alone.Citation46 Combination treatment of LPV/RTV plus ribavirin has also demonstrated effective results against SARS-CoV patients, and in vitro.Citation41 The combinational therapy of LPV + RTV, and LPV + RTV ritonavir + IFN-β, have also displayed improved clinical symptoms and reduced viral loads.Citation1 During the COVID-19 epidemic in China, the use of traditional Chinese medicines (TCM) together with antivirals or antibiotics, has significantly blocked the SARS-CoV-2 replication and clinically recovered infected patients.Citation39 The most commonly used TCM are Glycyrrhizae Radix Et Rhizoma (Gancao), Saposhnikoviae Radix (Fangfeng), Fructus forsythia (Lianqiao), Astragali Radix (Huangqi), Atractylodis Macrocephalae Rhizoma (Baizhu) and Lonicerae Japonicae Flos.Citation47
Immunotherapeutics
Passive immunization of infected patients by administering plasma from seroconverted patients who have fully recovered from pathogenic infection has a very long history.Citation48 Convalescent plasma or Serum therapy has been reportedly used against several viral infections as a final resort to improve infected patients’ survival rates. In serum therapy, antibodies in the blood plasma of recovered COVID-19 patients, ‘human convalescent serum,’ are injected to combat infection in severely infected patients.Citation49 Shen et al. (2020) reported that convalescent plasma obtained from SARS-CoV-2 recovered patients when administered together with methylprednisolone and antiviral agents, improved the condition of patients with respiratory distress syndrome (ARDS) and laboratory-confirmed COVID-19 infection. Overall, the improvement observed in patients’ health included successful weaning from mechanical ventilation, rise in PaO2/FiO2, body temperature normalization, the decline in Sequential Organ Failure Assessment score, resolution of ARDS, decrease in viral loads and increase in neutralizing antibody titers.Citation50 To evaluate convalescent plasma efficacy against COVID-19 infection, FDA has encouraged randomized clinical trials and emphasized an investigation of novel drug applications as well.Citation48 However, highly specific substitutes of serum plasma, broadly active neutralizing monoclonal antibodies (MAbs) have been recently developed against various viruses.Citation51
Currently, MAbs are widely considered as a treatment option against COVID-19 disease.Citation48 It is considered that the administration of MAbs directly to the infected individuals may play a crucial role in controlling CoV, as SARS recovered patients display highly potent NAb responses.Citation51 The S-protein-specific neutralizing antibodies (NAbs) S309, isolated from a patient’s memory B cells who recovered from SARS-CoV in 2003, neutralized both SARS-CoV-1 and SARS-CoV −2 by ligating the RBD. The binding assays and Cryo-electron microscopy images illustrated that the conserved epitope of S309 consisted of glycans, and instead of MAb specificity for the RBD, it has no interference with ACE2 binding.Citation52 The cross-neutralization affinity of SARS-CoV RBD-specific neutralizing MAbs highly depends on the resemblance of their RBDs. For instance, RBD-specific Abs of SARS-CoV can cross-neutralize bat-SARS-like-CoV strain ‘WIV1 RBD’ (8 amino acid differences to SARS-CoV), but not to ‘SHC014ʹ strain (24 amino acid differences). Such a cross-neutralizing affinity of RBD-specific mAbs of SARS-CoV should be further analyzed for SARS-CoV-2. For this purpose, analysis between SARS-CoV-2 and SARS-CoV RBD is required to identify the RBD-specific MAbs to evaluate further by clinical trials. Regeneron is currently working on identifying specific MAbs effective against SARS-CoV-2. The efficacy of combination therapy of MAbs and remdesivir could be further evaluated as an ideal therapy for COVID-19.Citation51
An antigen-presenting cell called dendritic cell (DC) induces a potential immune response via antigen presentation.Citation53 DCs resides in the periphery, and are more active at engulfing and presenting exogenous antigens in an immature state, but become mature when come across the DAMPs and PAMPs.Citation54 DC vaccination, an alternate form of immunotherapy is a simple injection with tolerating therapy. Most cases reported zero adverse effects of DC vaccination. In short, DC vaccination is a Targeted Therapy, which means the DC is trained against the patient’s infection or disease. For the investigation of a potential Immunotherapeutic against SARS-CoV-2, engineering technology has been used for the synthetic minigenes conserved domains of polyprotein protease and structural proteins of the virus. The SARS-CoV-2 invading via spike protein attachment to ACE2 receptor, and replication depends on all these viral proteins molecular mechanism. Shenzhen Geno-Immune Medical Institute (SGIMI) developed a lentivirus vector-based candidate vaccine called LV-SMENP-DC. The LV-SMENP DC vaccine was synthesized by dendritic cell modification with the SARS-CoV-2 immune-modulatory gene and minigene SMENP expressing lentivirus vectors (NHP/TYF). On March 24, 2020, the clinical trial phase 1 was conducted involving 100 patients and the expected date of completion of the study is December 31, 2024 (NCT04276896). In the current study, the efficacy and safety of the COVID-19 proposed LV-SMENP DC vaccine will be investigated along with activated antigen-specific cytotoxic T cell (TC cell) vaccine.Citation10,Citation55,Citation56 The objectives of this study are (i) to inject and infuse LV-SMENP DC and TC cell vaccines to SARS-CoV-2 infected patients and healthy volunteers to evaluate the safety, and (ii) to investigate the LV-SMENP DC and TC cells vaccine efficacy.Citation57
A recent study has identified that MAb (CR3022) can potentially bind with S-RBD of SARS-CoV-2, because, antibody’s epitope does not overlap with the divergent ACE2 RBM. CR3022 can be considered as the potential therapeutic candidate to treat COVID-19 infection either alone or in combination with other Nabs.Citation2 Moreover, the soluble version of ACE2, fused with the FC domain of an immunoglobulin (ACE2-Fc), may block the viral entry and provide a NAbs with high breath to avoid any viral escape. It will not only recruit the immune system to build lasting immunity but also balance the ACE2 level declined during lung infection and treat acute respiratory distress pathophysiology simultaneously. Such a treatment would help infected patients before the availability of commercially available protective vaccines.Citation58 Among various other sources of Abs, chicken egg yolk antibodies (IgY) can be considered as a substitute to mammalian Abs that have been studied against SARS-CoV. Chicken egg yolk antibodies (IgY) have higher binding affinity to certain antigens, the extraction and production cost is low and possesses greater pathogen-neutralizing activity in lungs as compared to mammalian Abs.Citation59 It is also suggested that phage display methodology can be effectively used against SARS-CoV-2 by using highly specific MAbs such as chicken egg yolk antibodies (IgY).Citation60
Chicken MAbs IgY has proved to be highly specific compared to polyclonal Abs while recognizing a unique epitope. Hence, SARS CoV-2 epitope identified for spike protein (S) could be a significant candidate for Abs-based vaccine, as immunotherapeutic drugs play a vital role in treating and combating infections both clinically and scientifically. Additionally, the application of chicken scFv IgY against SARS CoV-2 spike protein (S) by phage display method can be considered as an effective model for highly specific MAbs (IgY) for mass production and use in long term.Citation59 From a panel of several human MAbs, Zost et al. identified those which can target S glycoprotein, exhibit neutralizing activity, and capable of fully blocking S protein RBD from interaction with human ACE2. Two NMAbs COV2-2196 and COV2-2130 can potentially recognize non-overlapping sites, significantly bind to S and neutralize SARS-CoV-2 virus. The SARS-CoV-2 infected mouse models, when passively immunized with COV2-2130 or COV2-2196 MAbs alone or in combination, simultaneously protected mice from abrupt weight loss, reduced viral load and inflammation in lungs. Additionally, passive immunization of MAbs COV2-2196 or COV2-2381, as a monotherapy to rhesus macaques models infected with SARS-CoV-2 infection developed immunity against the virus. These results provide a structure-based platform for coherent vaccine design and the selection of effective immunotherapeutics.Citation61
Large gaps exist in our understanding of epidemiological risk factors, immunopathology risk with SARS-CoV-2, and pathological immune mediators during CoVs infections. Carefully designed human infection models, by using less virulent CoVs, such as human CoV OC43, may provide a safe avenue to identify the probability of immune response enhancement in CoV infected models.Citation62 Nevertheless, the development of clinical drugs for coronaviruses is challenging because the repeatedly emerging novel coronaviruses with diverse features require the specific drug for each newly emerged virus. Therefore, designing and development of novel broad-spectrum antiviral drugs that can potentially target all coronaviruses, in general, maybe the only treatment option against reemerging and circulating coronaviruses.
Vaccines
There is no currently approved vaccine available for COVID-19, though some pharmaceutical companies and research centers are working to rapidly develop a vaccine. Of the structural proteins of SARS-CoV-2, S protein is the foremost intermediator of viral entry into the cell upon attachment to ACE2 receptor and the leading factor to determine the virus pathogenicity, host range, and virus-neutralizing antibodies, which is why it is considered to be a key target element to design a vaccine against SARS-CoV-2.Citation63 S-protein has two subunits (i) N-terminal S1 (RBD) and (ii) C-terminal S2, because of the high conservation and non-mutational S2 part, become a critical target part for the design a potent vaccine.Citation64,Citation65 On the bases of the above discussion, different vaccines have been developed (in clinical trials, see ) against the S protein by genetic engineering of the RNA or DNA that is responsible for the synthesis of S protein copies.
Table 1. Different approaches applied by various pharmaceutical industries and research centers to develop efficient vaccine for COVID-19
Stermina therapeutics, GeoVax-BravoVax industry, Clover Biopharmaceuticals, Chinese Center for Disease Control and Prevention, US National Institute of Allergy and Infectious Diseases, Vir Biotechnology, Johnson & Johnson, Pfizer, Merck Sanofi, and Novavax and many other companies are making their efforts to develop a vaccine by using various techniques such as mRNA-based vaccine, DNA-based vaccine, inactivated virus vaccine, and protein subunit-trimer-based vaccine. Some of these vaccines are now progressed to phase 2 and 3 trails.Citation2,Citation71,Citation78 Recently, a vaccine named as ‘PiCoVacc’ developed by Sinovac Biotech using inactivated virus was effective against 11 various strains of SARS. The vaccine was tested on various animal models such as mice, rats, and non-human primates. The vaccine effectively blocked the replication of all 10 strains of SARS-CoV-2 in infected rats, mice, and picramates.Citation84,Citation89 Shenzhen GenoImmune Medical Institute has also prepared a vaccine named LV-SMENP-DC. The vaccine is prepared from dendritic cells modified with artificial antigens that can produce antibodies against SARS-CoV-2, the vaccine is also in an animal trial version.Citation55 Further, the details of various vaccines against SARS-CoV-2, production strategies, and manufacturers are enlisted in .
Multiple platforms including RNA and DNA-based platforms, next-generation sequencing, autologous dendritic cell-based vaccine, inactive virus vaccine, replicating or non-replicating viral-vectored vaccines, and reverse genetics are being used to develop vaccines against the SARS-CoV-2.Citation90,Citation91 During the pandemic, these potential platforms can cut the development time of more conventional vaccines. However, still, there are several challenges such as optimizing antigen design and investigating the potential duration of immunity.Citation90 According to WHO, more than 137 candidates undergoing preclinical phase and 23 in the early clinical phase.Citation92 However, vaccine development is a lengthy and expensive process and takes many years to manufacture a licensed vaccine.Citation93 For example, the first vaccine against Ebola was approved 43 years after the virus was discovered. On average, it takes about 10 years to develop a vaccine. However, due to the significant global crisis, everyone is hoping that this time it will be different.Citation94 Some experts argued that 18 months for developing the first vaccine is an extremely aggressive schedule while others believe that it might be ready by the end of 2020.Citation94 An ideal vaccine against the SARS-CoV-2 would confer immunity for a minimum of 6 months, effective after one or two vaccinations, reduce onward transmission of the pathogen, and would protect target populations such as immunocompromised individuals, older adults, and those with comorbidities.Citation95 Even if the vaccine is approved, it will bring its difficulties such as production, delivery, and accessibility. Several obstacles like geographical, commercial, and nationalistic factors could stand in the way of maximizing vaccine utility and effectiveness in the field.Citation94
Conclusion and prospect
SARS-CoV-2 pandemic not only imposed physical illness and deaths around the globe, but also affected the mental health of the kids, adults, and disabled subjects who are locked down at homes, many of them are suffering from xenophobia and insomnia. Due to the lack of any effective and approved vaccine, health workers are treating patients with a higher and frequent dose of previously used broad-spectrum antibiotics and antivirals, though the patients have recovered from COVID-19, many of them are facing severe adverse effects. Remdesivir, Chloroquine, and related salts, and Arbidol have caused severe complications in patients, such as mental illness, cardiac, hepatic, and kidney-related issues. Several multinational pharmaceutical companies are in the race to develop and commercialize the COVID-19 vaccine as quickly as possible. Besides pharmaceutical industries, many research centers and departments of several universities are also working to develop an efficient vaccine by using various approaches. There are more than 40 strains of SARS-CoV-2 reported until now; therefore, the developed vaccine must be effective against all of these strains. Vaccines production should not be limited to few strains but to all occurring strains to eradicate SARS-CoV-2 from this world.
Funding
The authors acknowledge the postdoctoral Science Foundation grant number“2020M672291” (for S.K), operating grant support from the National Natural Science Foundation of China (grants no: 81870942, 81471174 and 81520108011), National Key Research and Development Program of China (grant no: 2018YFC1312200), and Innovation Scientists and Technicians Troop Constructions Projects of Henan Province of China (for MX).
Disclosure of potential conflicts of interest
The authors of this manuscript declare that there is no conflict of interest.
Additional information
Funding
References
- Bonilla-Aldana DK, Quintero-Rada K, Montoya-Posada JP, Ramírez-Ocampo S, Paniz-Mondolfi A, Rabaan AA, Sah R, Rodríguez-Morales AJ. SARS-CoV, MERS-CoV and now the 2019-novel CoV: have we investigated enough about coronaviruses?–A bibliometric analysis. Travel Med Infect Dis. 2020;33:101566. doi:10.1016/j.tmaid.2020.101566.
- Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi:10.1016/j.jare.2020.03.005.
- Gao H, Yao H, Yang S, Li L. From SARS to MERS: evidence and speculation. Front Med. 2016;10(4):377–82. doi:10.1007/s11684-016-0466-7.
- Báez-Santos YM, John SES, Mesecar AD. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi:10.1016/j.antiviral.2014.12.015.
- Khan M, Shereen MA, Khokhar M, Kamil A, Rahman H A novel effective therapeutic approach for treatment of Leishmania tropica through Miltefosine Loaded Chitosan Nanoparticles. 2020.
- Rodriguez-Morales AJ, Bonilla-Aldana DK, Tiwari R, Sah R, Rabaan AA, Dhama K. COVID-19, an emerging coronavirus infection: current scenario and recent developments-an overview. J Pure Appl Microbiol. 2020;14(1):6150. doi:10.22207/JPAM.14.1.02.
- Khan S, Nabi G, Han G, Siddique R, Lian S, Shi H, Bashir N, Ali A, Shereen MA. Novel coronavirus: how things are in Wuhan. Clinical Microbiol Infect. 2020;26(4):399. doi:10.1016/j.cmi.2020.02.005.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. New England J Med. 2020;382(8):727–33. doi:10.1056/NEJMoa2001017.
- Phan T. Novel coronavirus: from discovery to clinical diagnostics. Infec Gen Evol. 2020;79:104211. doi:10.1016/j.meegid.2020.104211.
- Zhang T, Wu Q, Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Current Biol. 2020;30(7):1346–1351.e2. doi:10.1016/j.cub.2020.03.022.
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127-20.
- Xiong C, Jiang L, Chen Y, Jiang Q. Evolution and variation of 2019-novel coronavirus. Biorxiv. 2020.
- Gao QY, Chen YX, Fang JY. 2019 novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;30(3):1346–1351.e2. doi:10.1111/1751-2980.12851.
- Chan JF-W, Kok K-H, Zhu Z, Chu H, To KK-W, Yuan S, Yuen K-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Micro Infect. 2020;9(1):221–36. doi:10.1080/22221751.2020.1719902.
- Chen J. Pathogenicity and transmissibility of 2019-nCoV—a quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22(2):69–71. doi:10.1016/j.micinf.2020.01.004.
- Khailany RA, Safdar M, Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020;19:100682. doi:10.1016/j.genrep.2020.100682.
- Khan S, Liu J, Xue M. Transmission of SARS-CoV-2, required developments in research and associated public health concerns. Front Med. 2020;7:310.
- Yu P, Zhu J, Zhang Z, Han Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J Infect Dis. 2020;221(11):1757–61. doi:10.1093/infdis/jiaa077.
- Nishiura H, Linton NM, Akhmetzhanov AR. Serial interval of novel coronavirus (COVID-19) infections. Inter J Infect Dis. 2020;93:284‒286.
- Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–98. doi:10.1021/acschemneuro.0c00122.
- Vetter P, Eckerle I, Kaiser L. Covid-19: a puzzle with many missing pieces. BMJ. 2020:m627. doi:10.1136/bmj.m627.
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–69.
- de Wilde AH, Snijder EJ, Kikkert M, van Hemert MJ. Host factors in coronavirus replication. Roles Host Gene Non Cod RNA Exp Virus Infec Springer. 2017;419:1–42.
- Millet JK, Whittaker GR. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–34. doi:10.1016/j.virusres.2014.11.021.
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–63. doi:10.1126/science.abb2507.
- Linton NM, Kobayashi T, Yang Y, Hayashi K, Akhmetzhanov AR, Jung S-M, Yuan B, Kinoshita R, Nishiura H. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med. 2020;9(2):538. doi:10.3390/jcm9020538.
- Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures. J Med Virol. 2020;92(6):568–76. doi:10.1002/jmv.25748.
- Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, Chen H, Wang D, Liu N, Liu D, et al. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–06. doi:10.1016/j.jinf.2020.02.018.
- Shanmugaraj B, Malla A, Phoolcharoen W. Emergence of novel coronavirus 2019-nCoV: need for rapid vaccine and biologics development. Pathogens. 2020;9(2):148. doi:10.3390/pathogens9020148.
- Favre G, Pomar L, Musso D, Baud D. 2019-nCoV epidemic: what about pregnancies? Lancet (London, England). 2020;395(10224):e40. doi:10.1016/S0140-6736(20)30311-1.
- Chen S, Huang B, Luo DJ, Li X, Yang F, Zhao Y, Nei X, Huang BX. Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases. Zhon Bing Li Xue Za Zhi Chin J Pathol. 2020;49:E005–E.
- Li Y, Zhao R, Zheng S, Chen X, Wang J, Sheng X, Zhou J, Cai H, Fang Q, Yu F, et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus. Emerg Infect Dis. 2020;2:1335‒36.(China).
- Lee SM, Kang WS, Cho A-R, Kim T, Park JK. Psychological impact of the 2015 MERS outbreak on hospital workers and quarantined hemodialysis patients. Compr Psychiatry. 2018;87:123–27. doi:10.1016/j.comppsych.2018.10.003.
- Chiang JJ, Cole SW, Bower JE, Irwin MR, Taylor SE, Arevalo J, Fuligni AJ. Depressive symptoms and immune transcriptional profiles in late adolescents. Brain Behav Immun. 2019;80:163–69. doi:10.1016/j.bbi.2019.03.004.
- Liem A, Wang C, Wariyanti Y, Latkin CA, Hall BJ. The neglected health of international migrant workers in the COVID-19 epidemic. Lancet Psych. 2020;7(4):e20. doi:10.1016/S2215-0366(20)30076-6.
- Zitting K-M, Münch MY, Cain SW, Wang W, Wong A, Ronda JM, Aeschbach D, Czeisler CA, Duffy JF. Young adults are more vulnerable to chronic sleep deficiency and recurrent circadian disruption than older adults. Sci Rep. 2018;8(1):1–14. doi:10.1038/s41598-018-29358-x.
- Liu S, Yang L, Zhang C, Xiang Y-T, Liu Z, Hu S, Zhang B. Online mental health services in China during the COVID-19 outbreak. Lancet Psych. 2020;7(4):e17–e8. doi:10.1016/S2215-0366(20)30077-8.
- Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther. 2020;14(1):58–60. doi:10.5582/ddt.2020.01012.
- Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):1–14. doi:10.1038/s41467-019-13940-6.
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–71. doi:10.1038/s41422-020-0282-0.
- Jean -S-S, Lee P-I, Hsueh P-R. Treatment options for COVID-19: the reality and challenges. J Micro Bio Immunol Infec. 2020;53(3):436–43. doi:10.1016/j.jmii.2020.03.034.
- Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018:9(2).
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, et al. Remdesivir for the treatment of Covid-19—preliminary report. New England J Med. 2020. doi:10.1056/NEJMoa2007764.
- Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, Liao X, Gu Y, Cai Q, Yang Y, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020. doi:10.1016/j.eng.2020.03.007.
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. New England J Med. 2020;382(19):1787–99. doi:10.1056/NEJMoa2001282.
- Baden LR, Rubin EJ. Covid-19—the search for effective therapy. N Engl J Med. 2020; 382:1851‒1852
- Luo H, Tang Q-L, Shang Y-X, Liang S-B, Yang M, Robinson N, Liu JP. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin J Integr Med. 2020;17:1–8.
- Klasse PJ, Moore JP. Antibodies to SARS-CoV-2 and their potential for therapeutic passive immunization. Elife. 2020.
- Derebail VK, Falk RJ. ANCA-associated vasculitis—refining therapy with plasma exchange and glucocorticoids. N Engl J Med 2020; 382:671‒673
- Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang, March F, Li D, Yang M, et al. Posting date. Treatment of 5 critically ill patients with COVID19 with convalescent plasma. JAMA. 2020;323(16):1582. doi:10.1001/jama.2020.4783.
- Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 20202;16(6):1232‒1238.
- Pinto D, Park Y-J, Beltramello M, Walls AC, Tortorici MA, Bianchi S, Jaconi S, Culap K, Zatta F, De Marco A. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;18:1–6.
- Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol. 2020;7:577–93. doi:10.1016/j.it.2017.05.006.
- Nace G, Evankovich J, Eid R, Tsung A. Dendritic cells and damage-associated molecular patterns: endogenous danger signals linking innate and adaptive immunity. J Innate Immun. 2012;4(1):6–15. doi:10.1159/000334245.
- Le TT, Andreadakis Z, Kumar A, Roman RG, Tollefsen S, Saville M, Mayhew S. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–06. doi:10.1038/d41573-020-00073-5.
- Sherley JL, Prentice D An ethics assessment of COVID-19 vaccine programs.
- ClinicalTrials.gov. Immunity and safety of Covid-19 synthetic minigene vaccine. 2020.
- Kruse RL. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Research. 2020;9:72.
- Somasundaram R, Choraria A, Antonysamy M. An approach towards development of monoclonal IgY antibodies against SARS CoV-2 spike protein (S) using phage display method: A review. Int Immunopharmacol. 2020;85:106654. doi:10.1016/j.intimp.2020.106654.
- Palaniyappan A, Das D, Kammila S, Suresh MR, Sunwoo HH. Diagnostics of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid antigen using chicken immunoglobulin Y. Poult Sci. 2012;91:636–42. doi:10.3382/ps.2011-01916.
- Zost SJ, Gilchuk P, Case JB, Binshtein E, Chen RE, Nkolola JP, Schäfer A, Reidy JX, Trivette A, Nargi RS, et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020:584:443‒9.
- de Alwis R, Chen S, Gan ES, Ooi EE. Impact of immune enhancement on Covid-19 polyclonal hyperimmune globulin therapy and vaccine development. EBioMedicine. 2020:102768. doi:10.1016/j.ebiom.2020.102768.
- He C, Qin M, Sun X. Highly pathogenic coronaviruses: thrusting vaccine development in the spotlight. Acta Pharm Sin B. 2020;10(7):1175–91. doi:10.1016/j.apsb.2020.05.009.
- Guo Y, Sun S, Wang K, Zhang S, Zhu W, Chen Z. Elicitation of immunity in mice after immunization with the S2 subunit of the severe acute respiratory syndrome coronavirus. DNA Cell Biol. 2005;24:510–15. doi:10.1089/dna.2005.24.510.
- Li J, Ulitzky L, Silberstein E, Taylor DR, Viscidi R. Immunogenicity and protection efficacy of monomeric and trimeric recombinant SARS coronavirus spike protein subunit vaccine candidates. Viral Immunol. 2013;26:126–32. doi:10.1089/vim.2012.0076.
- McKay B Drugmakers rush to develop vaccines against china virus. The Wall Street Journal Available online: https://www wsj com/articles/drugmakers-rush-to-develop-vaccines-against-china-virus-11579813026 (accessed on 23 March 2020).
- Yi C, Yi Y, Li J. mRNA vaccines: possible tools to combat SARS-CoV-2. Virol Sin. 2020;35:259‒62.
- Smith J CureVac bids to develop first mRNA coronavirus vaccine. 2020.
- Park A. Inside the company that’s hot-wiring vaccine research in the race to combat the coronavirus. Time. 2020;2020.
- de Vries APJ, Alwayn IPJ, Hoek RAS, van den Berg AP, Ultee FCW, Vogelaar SM, Haase-Kromwijk BJ, Heemskerk MB, Hemke AC, Nijboer WN. Coronavirus dashboard (COVID-19)-update log. J Am Soc Nephrol. 2020.
- Inovio IP Inovio selected by cepi to develop vaccine against new coronavirus inovio.
- Technology P. University of Waterloo to develop nasal vaccine for Covid-19. Pharmaceutical Technology; 2020.
- Onyeador O Cobra and the KI collaborate to develop COVID-19 vaccine. PRODUCT & INDUSTRY NEWS, 2020.
- ClinicalTrials.gov. Evaluating the Safety, Tolerability and Immunogenicity of bacTRL-Spike Vaccine for Prevention of COVID-19. 2020.
- Cheung E China coronavirus: hong Kong researchers have already developed vaccine but need time to test it, expert reveals. 2020.
- News GR. GeoVax and BravoVax (Wuhan, China) to collaborate on development of coronavirus vaccine. 2020.
- Biopharmaceuticals C. Clover initiates development of recombinant subunit-trimer vaccine for wuhan coronavirus (2019-nCoV). 2020.
- Johnson. Our COVID-19 response efforts. 2020.
- Codagenix I. Codagenix and Serum Institute of India Initiate Co-Development of a Scalable, Live-Attenuated Vaccine Against the 2019 Novel Coronavirus, COVID-19. 2020.
- Hennessy J. Australia’s been asked to make a coronavirus vaccine at ‘unprecedented speed. Sci Alert. 2020.
- Vaxart. Vaxart announces initiation of coronavirus vaccine program. 2020.
- Mukherjee S The first coronavirus drug candidate is set for testing in China. 2020.
- Radcliffe S Here’s exactly where we are with vaccines and treatments for COVID-19. 2020.
- Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, et al. Rapid development of an inactivated vaccine for SARS-CoV-2. bioRxiv. 2020.
- Liu A China’s CanSino Bio advances COVID-19 vaccine into phase 2 on preliminary safety data. 2020.
- Sanofi. Sanofi’s Response in the Fight against COVID-19. SanofiFacts. 2020.
- Stanton D. Merck betting on ‘swish-and-swallow’ and single administration COVID vaccines. Bioprocess Int. 2020.
- Technology P. Coronavirus: vir Biotechnology and Novavax announce vaccine plans. 2020.
- Mestrovic T PiCoVacc vaccine candidate for COVID-19 effective in animal trials. 2020.
- Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. New England J Med. 2020;382:1969–73. doi:10.1056/NEJMp2005630.
- Rogers J CEPI-funded COVID-19 vaccine candidates progress to clinical trials. 2020.
- World Health O. DRAFT landscape of COVID-19 candidate vaccines. World; 2020.
- Gouglas D, Le TT, Henderson K, Kaloudis A, Danielsen T, Hammersland NC, Robinson JM, Heaton PM, Røttingen J-A. Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Global Health. 2018;6:e1386–e96. doi:10.1016/S2214-109X(18)30346-2.
- Mullard A. COVID-19 vaccine development pipeline gears up. Lancet. 2020;395:1751–52. doi:10.1016/S0140-6736(20)31252-6.
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bitaye M, Clutterbuck AE, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020.

