ABSTRACT
To improve a DNA vaccine containing the truncated dengue virus serotype 2 (DENV-2) envelope (E) protein and evaluate the influence of precursor membrane (prM) glycoprotein polymorphism on E protein immunogenicity, two vaccine candidates have been constructed by upstream insertion of the DENV-2 and DENV-3 prM genes into the DENV-2 E gene, named pCID2EtD2prM and pCID2EtD3prM, respectively. Both constructs were able to induce antibody production, which were neutralizing against DENV-2 in a murine model. Splenocytes of immunized groups, when challenged with virus, demonstrated Th1 cytokine pattern and proliferation, in addition to the increase of specific T cells. Vaccine candidates pCID2EtD2prM and pCID2EtD3prM confer 70% and 90% protection against DENV-2, respectively. The pCID2EtD3prM plasmid conferred only 40% protection in the lethal challenge with DENV-2. The results demonstrate that DENV-3 prM has a greater influence on the immunogenicity of the E protein and, probably due to its role as a chaperone, these results may be related to the correct folding and, consequently, an increase in the presentation efficiency of produced transcripts.
KEYWORDS:
1. Introduction
The dengue virus belongs to the Flavivirus genus, Flaviviridae family. It can be classified into four genetically related, but antigenically distinct serotypes (DENV-1, DENV-2, DENV-3, and DENV-4), which are etiological agents of Dengue fever. The main vector and reservoir of DENV is the Aedes aegypti mosquito, which transmits the virus to human hosts when feeding on their blood. Other vectors, such as Aedes albopictus, are found in some regions.Citation1 The viral particles show a spherical shape, with diameters ranging from 40 to 60 nm, and are composed of three structural proteins (C, prM/M, and E), and an RNA genome (C protein is the structural unit of the nucleocapsid), surrounded by a lipidic membrane with an E protein embedded in it. Three domains are found in the E protein (ED1, ED2, and ED3), and the third domain contains the major epitopes with capability for the neutralizing antibodies production, as well as for cell surface recognition and virus entry. In the cell, the prM protein is found fused with the E protein ectodomain and serves as a chaperone, helping to produce the correct protein folding in the endoplasmic reticulum. The viral genome is composed of a single positive-sense RNA strand, with a length of 11 kb arranged in only one open reading frame (ORF). This ORF is flanked by two untranslated repetitive regions (UTR), known as 3ʹ UTR and 5ʹ UTR. One unique polyprotein is translated by this ORF, and some cleavages by cellular and viral proteases will give rise to three structural proteins (C, prM/M, and E) and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5).Citation2–7 The structural proteins are used in the assembling of new viral particles, while the non-structural proteins are involved in genomic RNA synthesis, transcription, and translation, as well as immune evasion mechanisms.Citation8
Dengue fever (DF) is a systemic viral disease, common in tropical and subtropical developing countries. The disease is transmitted by Aedes mosquitoes; dengue virus transmission occurs when they bite the human host to feed on their blood.Citation9 Clinical manifestations can be asymptomatic or symptomatic, ranging from a weak self-limited fever (Dengue Fever – DF) to more severe conditions, such as Dengue Hemorrhagic Fever (DHF) or Dengue Shock Syndrome (DSS).Citation10 After primary infection with a DENV-specific serotype, the risk of developing more severe disease manifestations is increased; if a second infection occurs with another serotype, the reactive but non-neutralizing antibodies can bind in another serotype and increase the capture by macrophages and monocytes via FcgR (Fc-g receptors). These infections result in an amplification of the cytokine cascade and complement activation, a phenomenon called Antibody-Dependent Enhancement (ADE).Citation11
Dengue fever represents a major public health problem in 120 countries throughout the world. It is estimated that around 390 million people are infected, and a high number of patients, including children, develop more severe manifestations, requiring hospitalization. Environmental conditions, population growth, urbanization, and globalization are the factors that increase the dispersion of this disease, and, since there is no treatment or vaccine, prevention is focused in vector control using insecticides, elimination of mosquito breeding sites and the use of mosquito traps.Citation12,Citation13
Preventive vaccination is the most powerful alternative to disease control. Currently, several vaccine candidates, using different approaches, are being developed: (i) attenuated chimeras, (ii) DNA vaccines, (iii) subunit vaccines, (iv) inactivated vaccines, and (v) viral vectors. A Live Tetravalent Chimeric Vaccine developed by Sanofi-Pasteur CYD-TDV (Dengvaxia®) is the most advanced candidate for use in humans, presently prequalified by WHO. Dengvaxia trial showed 76% efficacy for seropositive and 39% for seronegative participants aged ≥9 y. However, this vaccine presented adverse effects to seronegative patients, with an increase in hospitalizations and severe illness to unexposed individuals. The more plausible explanation is the antibody-dependent enhancement (ADE), with vaccine acting as fist infection. However, the potential role of the lacking antigen-specific, protective CD8+ T cell immunity could not be ignored. To overcome this bottleneck, World Health Organization (WHO) recently recommends implementing a “pre-vaccination screening strategy”, vaccinating only people who test seropositive. This approach requires a readily available and accurate point-of-care test.Citation14–17 Despite of good results in seropositive patients, this vaccine candidate still suffers from viral interference, and this phenomenon needs to be overcome.
DNA vaccines present some advantages, such as stability at high temperatures, lower production costs, and more safety than live-attenuated vaccines. The structural proteins prM and E and the non-structural protein NS1 have been the main target in DNA vaccine design. Initial results of clinical tests of the DNA vaccine based on prM/E gene have shown reduced efficacy against serotype 2 (DENV-2).Citation18,Citation19
Our work group has previously reported the expression of the truncated envelope (E) protein in VERO cells by two constructions which have a prM of the two Dengue virus serotype (DENV-2 and DENV-3) genes upstream of the E gene.Citation20 Higher yield was obtained by the vaccine candidate which expresses the prM from the DENV-3 serotype (around 67% more) (data not shown), which suggests that this protein is a better chaperone than the polymorphic prM from DENV-2. In the present study, vaccine candidates were assessed for their capability to generate specific immune response against DENV-2 in a murine model. Results show that the candidates are effective at generating an immune response at a sufficient level for ensuring the protection of animals. However, the construction with the prM gene from DENV-3 now gives more effectiveness than the DENV-2 polymorphic gene.
2. Material and methods
2.1. Cell, virus, plasmid and animals
Vero cells were cultivated in Eagle´s Minimum Essential Medium (MEM) (Merck, Darmstadt, Germany) supplemented with 10% Fetal Bovine Serum (FBS) (Merck, Darmstadt, Germany) and an antibiotic mix (penicillin 10.000 UI/mL and streptomycin 10 mg/mL) at 37°C and 0.5% CO2 atmosphere. C6/36 cells were maintained at 28°C in the Leibovitz L15 Medium (Himedia, Mumbai, India) supplemented with 10% FBS and the same antibiotic mix. The pGEM-T Easy vector system (Promega Corporation, Madison, WI) was used as a cloning plasmid. For expression, genes of the DENV-2 and DENV-3 prM proteins were cloned into the pCI expression plasmid (Promega Corporation, Madison, WI), in which the DENV-2 truncated protein E gene, called pCID2Et, had already been inserted. Constructions were placed under the control of the CMV promoter, present in the plasmid. These cassettes for DENV-2 and DENV-3 were called pCID2EtprMD2 and pCID2EtprMD3, respectively.Citation20,Citation21
The DENV-2 strain New Guinea C (ATCC™ VR-1584™) and the DENV-3 strain H87 (ATCC™ VR-1256_FD™) were propagated in C6/36 cells. In the experiments were used Swiss mice, which were fed ad libitum and maintained under standard conditions. All experiments were carried out according to the protocols approved by the Ethics Committee of the Federal University of Viçosa, Viçosa, Minas Gerais state, Brazil.
2.2. Immunization of mice
Four groups of ten 3-week-old female Swiss mice were intramuscularly immunized with 100 μg of the vaccine plasmids (pCID2EtprMD3 and pCID2EtprMD2) and the controls (pCID2Et and pCI empty), for a total of 3 doses (a primer and two boosters) at intervals of 15 d between them. The animals´ blood was collected 1 d before each vaccination and 15 d after the last vaccination. Serum samples were processed and stored at −70°C.
2.3. ELISA
Antibodies specific to the DENV-2 E protein were detected using the indirect ELISA with 96-well plates sensitized with 5 μg of purified E protein, washed, blocked, and incubated at 37°C for 60 min. Serum samples were diluted to 1:10 in 1% PBS, added to the sensitized plate, and incubated for 120 min at 37°C. After the incubation period, the plates were washed 10 times with 0.05% Tween 20 phosphate buffered solution (PBS) (PBS-T 0.05%) and then incubated with peroxidase-labeled anti-mouse IgG antibodies (Merck, Darmstadt, Germany) diluted to 1:200 in 1X PBS for 120 min at 37°C. The plates were washed 10 more times with 0.05% PBS-T, and the substrate SIGMAFAST™ OPD (o-Phenylenediamine dihydrochloride) (Merck, Darmstadt, Germany), prepared according to the manufacturer’s instructions, was added to each well. The plate was incubated with the substrate until color was observed and the optical density (OD) of each sample was read at 450 nm. Samples whose ODs were higher than the mean OD of the negative control (pCI group) plus 2 standard deviations were considered positive.
2.4. Plaque reduction neutralization test – PRNT
The presence of neutralizing antibodies against DENV-2 was analyzed by the Plaque Reduction Neutralization Test as described by Russell, Nisalak.Citation22 For this, monolayers of VERO cells were infected with 100 PFU of DENV-2, previously incubated at 37° for 1 h with different dilutions of sera from the immunized mice (1:2 to 1:512). Reduction in the number of the lysis plates was calculated for each dilution and compared to the control, in which the DENV-2 was preincubated with pre-immune serum. The highest dilution showing a 50% or greater decrease in plate number was considered to be the neutralizing antibody titer.
2.5. Cytokine detection
To analyze the cytokine profile, 5 × 106 cells/well from the spleens of the immunized animals were incubated in 24-well plates with DENV-2 at two different MOIs (0.1 and 0.5) for 12 and 24 hours each at 37°C. For control, the cells were incubated with 2 μg of Concanavalin A (ConA) or RPMI medium supplemented with 10% FBS as positive and negative control, respectively. Total cell RNA was extracted, and the cDNA was made with GoScript according to the manufacturer´s recommendations (GoScript™ Reverse Transcription System, Promega, Madison, WI, USA). The cytokine profiles for Th-1 (interleukin-2 and interferon-γ) or Th-2 (IL-4 and IL-10) cellular immune response were performed using the Real-Time PCR technique (Eco Real-Time System, Illumina), using primers specific for the amplification of the genes of β-actin (endogenous control), IL-2, INF-γ, IL-4, and IL-10.
2.6. Cell phenotyping
Splenocytes from immunized animals were phenotypically assessed by flow cytometry. Briefly, the cells were cultured at 37°C for 48 h under stimulation with the virus at different MOIs (0.1 and 0.5) of DENV-2, and in 2 μg of Concanavalin A or RPMI medium supplemented with 10% FBS as positive and negative controls, respectively. Afterward, the cells were collected and incubated with a mixture containing anti-CD4/anti-CD44 or anti-CD8/anti-CD44 antibodies and evaluated on a Guava® easyCyte Flow Cytometer (Merck, Darmstadt, Germany), and the data was analyzed using the Guava CytoSoft software.
2.7. Lymphoproliferation
Lymphocyte proliferation through DENV-2 stimulation was determined according to the MTT (Thiazolyl Blue Tetrazolium Bromide) protocol. Then, 0.5 × 106 splenocytes/well from the immunized animals were stimulated with 0.1 and 0.5 MOI of DENV-2, with 2 μg of Concanavalin A (positive control) or RPMI medium with 10% of FBS (negative control). The plates were incubated for 44 hours at 37°C and then MTT (5 µg/ml) was added and incubated at 37°C for 4 more hours. The product of the metabolism of the MTT (formazan crystals) was suspended in 200 μL of DMSO (Dimethyl sulfoxide) and absorbance was measured at 560 nm.
2.8. Challenge
Groups of ten 3-week-old female Swiss mice were immunized with 100 μg of the recombinant plasmids pCID2EtprMD3, pCID2EtprMD2, pCID2Et, and pCI in 20% sucrose. Vaccine plasmids were inoculated intramuscularly into the quadriceps in a total of three doses (a primer and two boosters) with 15-d intervals between them. Fifteen days after the third inoculation, the mice were intracerebrally challenged with 100 DL50/ml of DENV-2 (1x105 PFU/ml) and the animals were monitored daily for 21 d.Citation23–25
3. Results
3.1. Neutralizing antibodies (ELISA and PRNT)
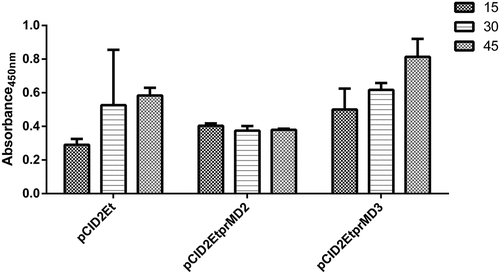
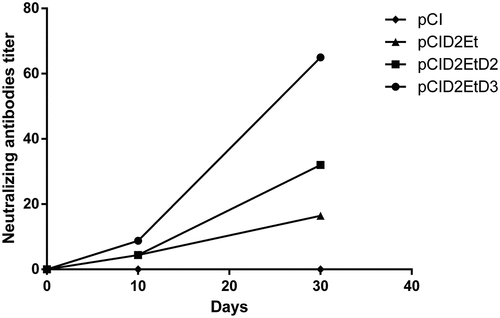
The presence of specific antibody anti-protein E induced by the vaccine candidates was assessed by indirect ELISA assay. As shown in , the animals that were immunized with the pCID2EtD3prM construct presented higher seroconversion than the other candidates evaluated, especially after 45 d following the first immunization. In addition, the induction of neutralizing antibodies against DENV-2 by the studied candidates was also evaluated. The plasmid pCID2EtD3prM induced a higher production of such antibodies compared to other constructs, which was already evident in the serum collected 30 d after the prime immunization .
3.2. Cytokine profile
Splenocytes extracted from the immunized animals were stimulated with DENV-2. The data obtained demonstrated a predominant Th1 pattern, with high expression of INF-γ. IL-10 expression was also detected; however, it did not exceed the expression of INF-γ .
Table 1. Expression of IL-4, IL-10, and INF-γ compared to the control group, after stimulation with Dengue-2 virus
3.3. Cell phenotyping
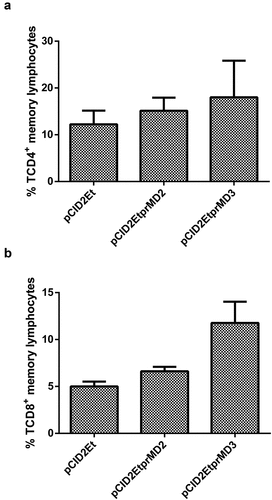
The results presented in and point to a greater induction of both types of activated/memory cells (CD4+/CD44+ and CD8+/CD44+) in animals immunized with the plasmid pCID2EtprMD3, when compared with groups vaccinated with pCID2Et and pCID2EtprMD2. This pattern was maintained in relation to the number of CD8+ T lymphocytes, and the total amount of T lymphocytes (CD4 + CD8). However, CD4+ T lymphocytes induced by immunization with the constructed plasmids were compared, and animals immunized with pCID2EtprMD3 and pCID2EtprMD2 showed similar amounts of this cell type.
Table 2. Percentage of lymphocytes induced in animals immunized with plasmid constructs
3.4. Lymphoproliferation
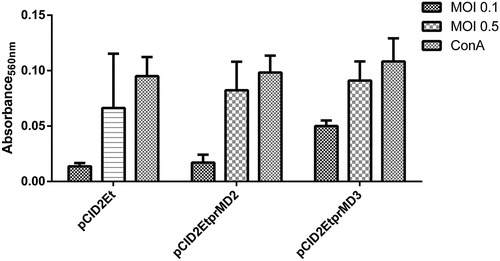
A lymphoproliferation assay was performed to evaluate the response of lymphoid cells elicited by DENV-2 stimulus. Cells from all groups proliferated, however, the results showed greater activation of spleen lymphoid cells from the animals vaccinated with pCID2EtprMD3. This difference becomes more evident when an MOI of 0.1 is used. Both pCID2Et and pCID2EtprMD2 presented similar values of lymphoproliferation .
3.5. Challenge
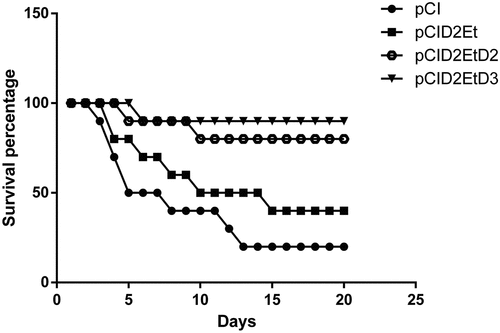
The vaccine candidates pCID2Et, pCID2EtprMD2, and pCID2EtprMD3 were evaluated according to their abilities to induce a protective immunity against DENV-2 during a lethal challenge. The plasmid pCID2EtprMD3 conferred a 90% survival rate to animals challenged with DENV-2. However, animals immunized with the candidates pCID2EtprMD2 and pCID2Et had survival rates of 70% and 40%, respectively, .
4. Discussion
Dengue is the main arboviruses that affects humans today and represents a major public health threat in tropical and subtropical regions.Citation26 Thus, there is a consensus in the scientific community concerning the need for an effective vaccine which can be readily available for the prevention of diseases. Several vaccine candidates are currently being developed in the world using different strategies, with the Dengvaxia vaccine being the most advanced candidate, which has been authorized in several countries in Asia and Latin America (including public programs in Brazil), and some other tetravalent vaccines, TV003/TV005, and TDV, are undergoing phase 3 trials. However, the WHO recommends that countries should consider the introduction of the Dengvaxia vaccine only where epidemiological data indicate persistent and high intensity of transmission (prior infection in this population should ideally be 70% or higher, as measured by seroprevalence to any DENV serotype), with the main reason for this recommendation being the differential performance (efficacy, and possibly safety) of vaccine by dengue serostatus at the time of first vaccination.Citation27 Thus, the most effective action in disease prevention is still control of the mosquito vector.
Although mice are generally used in initial test of vaccine prototype, no murine model presenting all aspects of dengue fever are disponible to vaccine evaluation. Dengue viruses are unable to subvert IFN-α/β response in immunocompetent mice and the use of immunocompromised mice present several concerns. Therefore, the uses of intracerebral inoculum rise as interesting alternative, since a systemic effect of the DENV-2 inoculation in BALB/c mice was observed.Citation28 In this work was evaluated the immunogenicity of three DNA vaccines against DENV-2, in order to observe whether the presence of the prM protein in a vaccine plasmid expressing the E protein would represent an increase in its capacity to generate immune response in mice, supported by observation in our laboratory that this construct presented an increase in E protein expression in vitro. Studies demonstrated that the E protein co-expressing with the prM protein leads to the induction of secretion of the neutralizing antibody, and consequent protection, in mice.Citation29 Others work suggest that there is a correlation between the levels of E protein expression and the induction of neutralizing antibodies.Citation30–33
Jimenez and da FonsecaCitation21 also demonstrated that the low protection of a DNA vaccine against DENV-2 may be due to poor activation of the immune system as a consequence of an imperfect secretion of the truncated E protein due to the absence of prM. Moreover, it is known that much of the research involving plasmid constructs expressing DENV-3 prM/E protein showed better results than those in which the plasmids contained the DENV-2 prM/E expression cassette.Citation20,Citation25,Citation34 These observations suggest that prM/DENV-3 protein maybe have a greater capacity as chaperone than prM/DENV-2, since it is known that prM plays a fundamental role in the synthesis, processing, and correct conformation of E protein.Citation35,Citation36
In order to investigate this possibility, two plasmids were constructed by insertion of the prM/DENV-2 and prM/DENV-3 genes into the plasmid vector pCID2Et, previously constructed to express the truncated E/DENV-2 protein.Citation21 An indirect ELISA assay was performed with sera from animals immunized with all these plasmids. The results indicate increased seroconversion in animals that received the plasmid containing the prM/DENV-3, with 40% more specific anti-DENV-2 antibodies than other groups. No increase in the number of specific antibodies was observed after the second dose of the vaccine in the pCID2EtprMD2 group, in contrast to the other two groups. The PRNT assay, which is in accordance with the ELISA, demonstrated greater seroneutralization of the animals vaccinated with the plasmid pCID2EtD3prM. This is probably due to increased expression of the E protein, corroborating the results obtained in vitro .Citation20 Despite high values found in ELISA assay , neutralizing antibodies titers were low . These results were observed to an inactivated H7 Influenza virus vaccine, which was able to confer protection and can be related to the non-neutralizing antibodies production. To seasonal viruses have been long known that antibodies titers of 40 or higher are related to protection, and titers of 80 or more present similar protection.Citation37 Leclerc, DeriaudCitation38 and Tighe, CorrCitation39 found a Th1 pattern of immune response, with IFN-γ production, when used intramuscular injection way.
High proliferation of specific lymphocytes was also observed in animals vaccinated with pCID2EtprMD3. In this case, the difference was more evident when the stimulus was made with an MOI of 0.1, where the proliferation reached 280% more in relation to pCID2Et and 194% in relation to pCID2EtprMD2. When an MOI of 0.5 was used, pCID2EtprMD3 continued to respond better, but was less dissimilar to the others. Moreover, both constructs containing prM showed better proliferation responses when compared to the candidate pCID2Et, indicating that the presence of the prM protein is important for the induction of a more efficient immune response.
Animals vaccinated with plasmids containing the prM protein showed a higher percentage of memory T lymphocytes; therefore, it seems clear that the prM protein plays a key role in this process.Citation6,Citation7 The induction of memory T cells was significantly increased in the animals vaccinated with pCID2EtprMD3, mainly in relation to the TCD8 + population, while in the CD4 + T lymphocyte population, this increase occurred less markedly. The pattern of cellular immune response induced by the vaccine candidates demonstrated Th1 pattern predominance, which agrees with other studies using monovalent DNA vaccines.Citation25 The plasmids pCID2EtprMD2 and pCID2EtprMD3 were able to induce a higher interferon-γ production than the plasmid pCID2Et, but the induction of this cytokine by the vaccine candidate containing prM/DENV-3 was markedly greater than that induced by the plasmid containing prM/DENV-2.
The plasmids pCID2EtprMD2 and pCID2EtprMD3 showed greater protection capacity, conferring 70% and 90% survival, respectively, against only 40% of that of the plasmid pCID2Et. These results indicate that the methodology employed is promising, because the plasmid pCID2EtprMD3 was shown to be more effective than other DNA vaccines presented in other studies, which confer only 60% and 80% protection.Citation7,Citation25,Citation40 Although, the full-length E protein expression is related to a better protein production and immunogenicity, due to correct folding or virus-like particles VLP formation, Citation41,Citation42 our results have shown that the coexpression of the chaperone-like protein prM can improve the immunogenicity and yield of truncated protein. Thus, the co-expression of prM/DENV-3 with the E proteins of the other serotypes could be used to create a more effective tetravalent vaccine.
Based on the obtained data, it is possible to infer that the prM protein of DENV-3 performs the function of an E protein folding agent more efficiently, consequently increasing its expression and presentation to the T cells, improving the immune response. Finally, we concluded that the immunological response induced by the plasmids pCID2EtprMD2 and pCID2EtprMD3 was higher than that induced by the preexisting vaccine pCI2Et.
Acknowledgments
This work was funding by the “Fundação de Amparo à Pesquisa do Estado de Minas Gerais. – FAPEMIG”, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Additional information
Funding
References
- Li J, Gao N, Fan D, Chen H, Sheng Z, Fu S, Liang G, An J. Cross-protection induced by Japanese encephalitis vaccines against different genotypes of Dengue viruses in mice. Sci Rep. 2016;6(1):19953. doi:10.1038/srep19953.
- Castillo JA, Castrillón JC, Diosa-Toro M, Betancur JG, St Laurent G, Smit JM, Urcuqui-Inchima S. Complex interaction between dengue virus replication and expression of miRNA-133a. BMC Infect Dis. 2016;16(1):29. doi:10.1186/s12879-016-1364-y.
- Lindenbach B, Thiel H, Rice C. Flaviviridae: The viruses and their replication. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields virology. Philadelphia (PA): Lippincott Williams & Wilkins; 2007. p. 1101–52.
- Suzuki Y, Chin W-X, Han Q, Ichiyama K, Lee CH, Eyo ZW, Ebina H, Takahashi H, Takahashi C, Tan BH, et al. Characterization of RyDEN (C19orf66) as an interferon-stimulated cellular inhibitor against Dengue Virus replication. PLoS Pathog. 2016;12(1):e1005357. doi:10.1371/journal.ppat.1005357.
- Kulkarni MR, Numoto N, Ito N, Kuroda Y. Modeling and experimental assessment of a buried Leu-Ile mutation in dengue envelope domain III. Biochem Biophys Res Commun. 2016;471(1):163–68. doi:10.1016/j.bbrc.2016.01.159.
- Idris F, Muharram SH, Diah S. Glycosylation of dengue virus glycoproteins and their interactions with carbohydrate receptors: Possible targets for antiviral therapy. Arch Virol. 2016;161(7):1751–60. doi:10.1007/s00705-016-2855-2.
- Roby JA, Hall RA, Setoh YX, Khromykh AA. Post-translational regulation and modifications of flavivirus structural proteins. J Gen Virol. 2015;96(7):1551–69. doi:10.1099/vir.0.000097.
- Gack MU, Diamond MS. Innate immune escape by Dengue and West Nile viruses. Curr Opin Virol. 2016;20:119–28. doi:10.1016/j.coviro.2016.09.013.
- Galula JU, Shen W-F, Chuang S-T, Chang GJJ, Chao D-Y. Virus-like particle secretion and genotype-dependent immunogenicity of dengue virus serotype 2 DNA vaccine. J Virol. 2014;88(18):10813–30. doi:10.1128/JVI.00810-14.
- Kim SH, Kim YN, Truong TT, Thu Thuy NT, Mai LQ, Jang Y-S. Development of a monoclonal antibody specific to envelope domain III with broad-spectrum detection of all four dengue virus serotypes. Biochem Biophys Res Commun. 2016;473(4):894–98. doi:10.1016/j.bbrc.2016.03.146.
- Kirkpatrick BD, Whitehead SS, Pierce KK, Tibery CM, Grier PL, Hynes NA, Larsson CJ, Sabundayo BP, Talaat KR, Janiak A, et al. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci Transl Med. 2016;8(330):330ra36. doi:10.1126/scitranslmed.aaf1517.
- Orellano PW, Reynoso JI, Stahl H-C, Salomon OD. Cost-utility analysis of dengue vaccination in a country with heterogeneous risk of dengue transmission. Vaccine. 2016;34(5):616–21. doi:10.1016/j.vaccine.2015.12.040.
- Porter KR, Raviprakash K. Nucleic acid (DNA) immunization as a platform for dengue vaccine development. Vaccine. 2015;33(50):7135–40. doi:10.1016/j.vaccine.2015.09.102.
- Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, Savarino S, Zambrano B, Moureau A, Khromava A, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018;379(4):327–40. doi:10.1056/NEJMoa1800820.
- Rosenbaum L. Trolleyology and the dengue vaccine dilemma. N Engl J Med. 2018;379(4):305–07. doi:10.1056/NEJMp1804094.
- Galula JU, Salem GM, Chang GJJ, Chao D-Y. Does structurally-mature dengue virion matter in vaccine preparation in post-Dengvaxia era? Hum Vaccin Immunother. 2019;15(10):2328–36. doi:10.1080/21645515.2019.1643676.
- Chiang C-Y, Liu S-J, Hsieh C-H, Chen M-Y, Tsai J-P, Liu -H-H, Chen I-H, Chong P, Leng C-H, Chen H-W, et al. Recombinant lipidated dengue-3 envelope protein domain III stimulates broad immune responses in mice. Vaccine. 2016;34(8):1054–61. doi:10.1016/j.vaccine.2016.01.009.
- Putri DH, Sudiro TM, Yunita R, Jaya UA, Dewi BE, Sjatha F, Konishi E, Hotta H, Sudarmono P. Immunogenicity of a candidate DNA vaccine based on the prM/E genes of a dengue type 2 virus cosmopolitan genotype strain. Jpn J Infect Dis. 2015;68(5):357–63. doi:10.7883/yoken.JJID.2014.313.
- Abrao EP, Espósito DLA, Lauretti F, Fonseca BALD. Dengue vaccines: What we know, what has been done, but what does the future hold? Rev Saude Publica. 2015;49:60. doi:10.1590/S0034-8910.2015049006146.
- Oliveira MDD, et al. Enhancement of dengue-2 E protein expression by the expression of the precursor membrane protein (Prm) of the dengue-3 virus. J Vaccines Vaccine. 2013;4:182. doi:10.4172/2157-7560.1000182.
- Jimenez RO, da Fonseca BAL. Recombinant plasmid expressing a truncated dengue-2 virus E protein without co-expression of prM protein induces partial protection in mice. Vaccine. 2000;19(6):648–54. doi:10.1016/S0264-410X(00)00247-4.
- Russell PK, et al. A plaque reduction test for dengue virus neutralizing antibodies. J Immunol. 1967;99(2):285–90.
- Azevedo AS, Gonçalves AJS, Archer M, Freire MS, Galler R, Alves AMB. The synergistic effect of combined immunization with a DNA vaccine and chimeric yellow fever/dengue virus leads to strong protection against dengue. PLoS One. 2013;8(3):e58357. doi:10.1371/journal.pone.0058357.
- Falgout B, Bray M, Schlesinger JJ, Lai CJ. Immunization of mice with recombinant vaccinia virus expressing authentic dengue virus nonstructural protein NS1 protects against lethal dengue virus encephalitis. J Virol. 1990;64(9):4356–63. doi:10.1128/JVI.64.9.4356-4363.1990.
- De Paula SO, et al. A DNA vaccine candidate expressing dengue-3 virus prM and E proteins elicits neutralizing antibodies and protects mice against lethal challenge. Arch Virol. 2008;153(12):2215–23. doi:10.1007/s00705-008-0250-3.
- Prompetchara E, et al. Induction of neutralizing antibody response against four dengue viruses in mice by intramuscular electroporation of tetravalent DNA vaccines. PLoS One. 2014;9(6):e92643. doi:10.1371/journal.pone.0092643.
- Wichmann O, Vannice K, Asturias EJ, de Albuquerque Luna EJ, Longini I, Lopez AL, Smith PG, Tissera H, Yoon I-K, Hombach J, et al. Live-attenuated tetravalent dengue vaccines: The needs and challenges of post-licensure evaluation of vaccine safety and effectiveness. Vaccine. 2017;35(42):5535–42. doi:10.1016/j.vaccine.2017.08.066.
- Amorim JFS, et al. Dengue infection in mice inoculated by the intracerebral route: Neuropathological effects and identification of target cells for virus replication. Sci Rep. 2019;9(1):17926. doi:10.1038/s41598-019-54474-7.
- Heinz FX, Allison SL, Stiasny K, Schalich J, Holzmann H, Mandl CW, Kunz C. Recombinant and virion-derived soluble and particulate immunogens for vaccination against tick-borne encephalitis. Vaccine. 1995;13(17):1636–42. doi:10.1016/0264-410X(95)00133-L.
- Konishi E, Fujii A, Mason PW. Generation and characterization of a mammalian cell line continuously expressing Japanese encephalitis virus subviral particles. J Virol. 2001;75(5):2204–12. doi:10.1128/JVI.75.5.2204-2212.2001.
- Konishi E, Terazawa A, Fujii A. Evidence for antigen production in muscles by dengue and Japanese encephalitis DNA vaccines and a relation to their immunogenicity in mice. Vaccine. 2003;21(25–26):3713–20. doi:10.1016/S0264-410X(03)00376-1.
- Konishi E, et al. Induction of protective immunity against Japanese encephalitis in mice by immunization with a plasmid encoding Japanese encephalitis virus premembrane and envelope genes. J Virol. 1998;72(6):4925–30.
- Konishi E, et al. A DNA vaccine expressing dengue type 2 virus premembrane and envelope genes induces neutralizing antibody and memory B cells in mice. Vaccine. 2000;18(11–12):1133–39. doi:10.1016/S0264-410X(99)00376-X.
- Blair PJ, KOCHEL T, RAVIPRAKASH K, GUEVARA C, SALAZAR M, WU S, OLSON J, PORTER K. Evaluation of immunity and protective efficacy of a dengue-3 pre-membrane and envelope DNA vaccine in Aotus nancymae monkeys. Vaccine. 2006;24(9):1427–32. doi:10.1016/j.vaccine.2005.09.032.
- Lindenbach BD, Rice CM. Molecular biology of flaviviruses. Adv Virus Res. 2003;59:23–61.
- Lu H, Xu X-F, Gao N, Fan D-Y, Wang J, An J. Preliminary evaluation of DNA vaccine candidates encoding dengue-2 prM/E and NS1: Their immunity and protective efficacy in mice. Mol Immunol. 2013;54(2):109–14. doi:10.1016/j.molimm.2012.11.007.
- Kamal RP, et al. Inactivated H7 influenza virus vaccines protect mice despite inducing only low levels of neutralizing antibodies. J Virol. 2017;91(20). doi:10.1128/JVI.01202-17.
- Leclerc C, Dériaud E, Rojas M, Whalen RG. The preferential induction of a Th1 immune response by DNA-based immunization is mediated by the immunostimulatory effect of plasmid DNA. Cell Immunol. 1997;179(2):97–106. doi:10.1006/cimm.1997.1161.
- Tighe H, Corr M, Roman M, Raz E. Gene vaccination: Plasmid DNA is more than just a blueprint. Immunol Today. 1998;19(2):89–97. doi:10.1016/S0167-5699(97)01201-2.
- Lima DM, Paula SOD, França RFDO, Palma PVB, Morais FR, Gomes-Ruiz AC, Aquino MTPD, Fonseca BALD. A DNA vaccine candidate encoding the structural prM/E proteins elicits a strong immune response and protects mice against dengue-4 virus infection. Vaccine. 2011;29(4):831–38. doi:10.1016/j.vaccine.2010.10.078.
- Raviprakash K, et al. Immunogenicity of dengue virus type 1 DNA vaccines expressing truncated and full length envelope protein. Vaccine. 2000;18(22):2426–34. doi:10.1016/S0264-410X(99)00570-8.
- Xiao S, Shiloach J, Betenbaugh MJ. Engineering cells to improve protein expression. Curr Opin Struct Biol. 2014;26:32–38. doi:10.1016/j.sbi.2014.03.005.