ABSTRACT
Human papillomavirus (HPV) vaccines are efficacious against HPV infections and associated lesions in women HPV-naïve at vaccination. However, vaccine efficacy (VE) against oncogenic, high-risk HPV (HR-HPV) types in women infected with any other HR-HPV type at first vaccination (baseline) remains unclear. This post-hoc analysis of a phase II/III study (NCT00779766) evaluated AS04-adjuvanted HPV-16/18 (AS04-HPV-16/18) VE against HR-HPV type infection in 871 Chinese women aged 18–25 years over a 72-month follow-up period. Study participants were DNA-negative at baseline to HR-HPV type(s) considered for VE and DNA-positive to any other HR-HPV type. Initial serostatus was not considered. Baseline DNA prevalence was 14.6% for any HR-HPV type and 10.6% excluding HPV-16/18. In the total vaccinated cohort for efficacy, VE against 6-month and 12-month HPV-16/18 persistent infections (PIs) in women DNA-negative to HPV-16/18 but DNA-positive to any other HR-HPV type at baseline was 100.0% (95% Confidence Interval [CI]: 79.8–100.0) and 100.0% (95%CI: 47.2–100.0), respectively. VE against HPV-16/18 incident infections in women DNA-positive to one vaccine type but DNA-negative to the other one at baseline was 66.8% (95%CI: −18.9–92.5). VE against HPV-31/33/45 incident infections, in women DNA-positive to HPV-16/18 and DNA-negative to the considered HPV type at baseline was 71.0% (95%CI: 27.3–89.8). No HPV-16/18 PIs were observed in vaccinated women with non-vaccine HPV A7/A9 species cervical infection at baseline. These findings indicated that women with existing HR-HPV infection at vaccination might still benefit from the AS04-HPV-16/18 vaccine. However, this potential benefit needs further demonstration in the future.
PLAIN LANGUAGE SUMMARY
What is the context?
Women who are human papillomavirus (HPV) naïve can be protected through HPV vaccines against infections and precancerous lesions caused by oncogenic HPV types.
In China unvaccinated adult women may seed vaccination services on a user-pay basis. These women are more likely to be sexually active and may have ongoing HPV infection at vaccination.
Public health agencies and healthcare professionals need more evidence on HPV vaccines benefit in the population already infected with oncogenic HPV type.
What is new?
We observed high vaccine efficacy of the AS04-HPV-16/18 vaccine against vaccine type HPV-16 and /or -18 infections in 18- to 25-year-old women infected with any other oncogenic HPV type at vaccination.
Some cress-protection against infections with non-vaccine type HPV-30/33/45 was also shown in women infected with any other oncogenic HPV type (including HPV-16/18) at vaccination.
The existence of current infection with phylogenetically related non-vaccine A7/A9 species in the cervix does not seem to impact the vaccine efficacy against endpoints related to HPV-16/18.
What is the impact?
Our results provide evidence of the benefit of AS04-HPV-16/18 vaccination in women already infected with some oncogenic HPV types at the time of vaccination, since the vaccine may still be efficacious against HPV types with which they are not infected.
Introduction
Three prophylactic human papillomavirus (HPV) vaccines have been licensed worldwide for protection against HPV-related diseases and are currently available in over 135 countries. All three vaccines induce strong and sustained protection against anogenital infections and precancerous lesions caused by the vaccine types in subjects HPV-naïve at the time of vaccination.Citation1–4 The AS04-adjuvanted HPV-16/18 (AS04-HPV-16/18) vaccine provides some level of protection against oncogenic, high-risk (HR) non-vaccine types HPV-31/33/45 while a limited cross-protection against HPV-31 is observed for the quadrivalent HPV vaccine.Citation5,Citation6 Most countries have approved the use of at least one HPV vaccine in women up to age 45 years.Citation7 A Chinese domestic HPV vaccine was also approved by the China Food and Drug Administration in December, 2019.Citation8
As of June 2020, HPV vaccines were included in 107 countries worldwide as part of national immunization programs (NIP),Citation9 targeting mainly adolescent girls. Independently, unvaccinated adult women may seek vaccination services on a user-pay basis. In China, for example, low awareness of HPV infections and related diseases and lack of availability of HPV vaccines in the NIP have led to HPV vaccines being mainly administered to adult women.Citation10,Citation11 Adult women are more likely to be sexually active and may thus already have been, or currently be, HPV-infected at the time of first HPV vaccination.
National screening programs for cervical cancer were in place in >144 countries worldwide by 2018.Citation12 In some countries where HPV DNA testing is used for cervical cancer screening and prevention efforts, women attending screening service may be aware of an eventual HPV infection before vaccination, although screening for presence of HPV prior to vaccination is not required.Citation13 This raised the question about HPV vaccine benefit in this population, especially as the World Health Organization and a growing number of countries are proposing HPV testing as a primary screening tool for cervical cancer.Citation14
Until now, most studies focused on subjects HPV-DNA-negative to vaccine types at baseline,Citation7 and some of them have demonstrated vaccine efficacy (VE) in women having cleared prior HPV vaccine type infection (seropositive but DNA-negative to vaccine type).Citation15,Citation16 It remains unclear whether, among women with cervical HPV infection (HPV DNA-positive) at the time of first vaccination, the residual benefit of preventing infection with other HPV types to which they have not yet been exposed would be sufficient to warrant vaccination. More VE evidence in the HR-HPV infected population is needed for informed decisions by public health authorities and healthcare professionals.
Hence, we performed the current post-hoc analysis to determine VE against infections associated with HPV-16/18 and HPV-31/33/45 types (individually or in combination) in Chinese women aged 18–25 years with cervical HPV infection (DNA-positive) with any other HR-HPV type at the time of first vaccination, irrespective of their serostatus.
summarizes the research, clinical relevance and impact of this study on the patient population.
Methods
Study design
The primary study (www.ClinicalTrials.gov; NCT00779766) design, methods, and results of event-driven analyses have been previously presented.Citation17,Citation18 This post-hoc analysis reports VE against HPV-16/18 and HPV-31/33/45 infections (individually or in combination) in women with cervical infection (DNA-positive) with any HR-HPV type other than the one(s) considered for VE at the time of first vaccination (baseline). Subjects were followed up to 72 months with incident infection and persistent infection (PI) as endpoints.
The trial was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and the International Conference on Harmonization Good Clinical Practice guidelines.
Participants
Healthy women aged 18–25 years were enrolled at four sites (Binhai, Jintan, Lianshui and Xuzhou Centers for Disease Control and Prevention) in Jiangsu Province, China. Women were included in the trial regardless of their HPV DNA status, HPV-16/18 serostatus, or cytology results at baseline. Virgins were not enrolled in the study due to cultural and ethical considerations. Written informed consent was obtained from each participant prior to the performance of any study-specific procedures.
Procedures
Briefly, study participants were randomized in a 1:1 ratio to receive either the AS04-HPV-16/18 vaccine (Cervarix, GSK) or aluminum hydroxide [Al(OH)3] at 0, 1, and 6 month intervals in a double-blind manner. AS04 is a proprietary GSK Adjuvant System containing monophosphoryl lipid A (50 μg MPL; produced by GSK) adsorbed on aluminum salt (500 μg AlCitation3+). Cervical cytology samples for HPV DNA testing were collected every six months at each study visit. Cervical cytology was tested using the ThinPrep PapTest (Cytyc Corporation, Boxborough, MA, USA) and reported according to the Bethesda 2001 classification system. Histopathological analysis was performed by a panel of gynecological pathologists at the Cancer Hospital, Chinese Academy of Medical Sciences (CICAMS), Beijing, China. Final case ascertainment of women assumed to meet the criteria for efficacy endpoints was reviewed in a blinded fashion by an independent review committee. A broad-spectrum PCR assay, SPF10-LiPA25 (version 1 based on licensed Innogenetics SPF10 technology; Labo Biomedical Products, Rijswijk, Netherlands) and type-specific PCR for HPV-16 and HPV-18 DNA were used to test cervical samples and biopsy material for HPV DNA from 14 HR-HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) and 11 non-HR-HPV types.
Statistical analysis
Previously published reports of this research disclose the efficacy, immunogenicity, and safety analyses in women DNA-negative at baseline (or both at baseline and Month 6), and seronegative at baseline (or irrespective of serostatus for the considered HPV type) in the according to protocol cohort for efficacy (ATP-E) and the total vaccinated cohort for efficacy (TVC-E).Citation17,Citation18 The ATP-E included women with available efficacy data, who received three doses of vaccine or control and had a normal or low-grade cytology at baseline, while TVC-E was similar to ATP-E but included women who received at least one dose of vaccine or control.Citation17,Citation18 Here, we specifically report data from women with HR-HPV infection (i.e. HR-HPV DNA-positive, irrespective of HPV serostatus) at baseline in the TVC-E. Subjects were evaluated for subsequent development of HPV infection with the types they were DNA-negative to at baseline.
Based on the analysis of VE against HPV-16/18 and HPV-31/33/45 infection in women DNA-negative to the considered HPV type and DNA-positive to any of the 14 HR-HPV types (i.e. HPV-16/18/31/33/45/39/45/51/52/56/58/59/66/68) at baseline, further analyses were performed in the following three subsets: (i) VE against HPV-16/18 infection in women DNA-negative to the considered HPV type and DNA-positive to any of the other 12 HR-HPV types (i.e. HPV-31/33/45/39/45/51/52/56/58/59/66/68; including HPV-16/18 coinfections) at baseline; (ii) VE against HPV-16/18 and HPV-31/33/45 infection in women DNA-positive to HPV-16/18 and DNA-negative to the considered HPV type at baseline; (iii) VE against HPV-16 infection in women DNA-positive to non-HPV-16 A9 species (i.e. HPV-31/33/35/52/58), and VE against HPV-18 infection in those DNA-positive to non-HPV-18 A7 species (i.e., HPV-39/45/59/68) at baseline. Besides meeting the above criteria for baseline status, women included in the relevant analyses must have samples available after vaccine dose 1 for the evaluation of the virological endpoints.
The evaluated endpoints were incident infection, 6-month PI and 12-month PI associated with specific HPV type(s). Incident infection was defined as the first detection by PCR of an episode of infection by HPV type(s) in a subject previously negative for the considered HPV type(s) and may have been transient or become persistent. Six-month PI was defined as at least two positive HPV DNA PCR assays for the same HPV type(s) with no negative DNA sample between the two positive DNA samples, over an interval of approximately six months. Twelve-month PI was defined as the detection by PCR of the same HPV type(s) at all available timepoints over an interval of approximately 12 months. Due to the limited sample size and the insufficient number of cervical intraepithelial neoplasia of grade 2 or worse (CIN2+) cases, VE against histological endpoints is not presented.
Endpoint cases were calculated as number of subjects reporting at least one event in each group, and VE was calculated using a conditional exact method, as previously described.Citation17,Citation18 Case counting started on the day after the first dose and ended at the time of an endpoint event or until the end of the 72-month follow-up. Statistical analyses were carried out with SAS 9.4 on the SDD platform. A similar method was used for the analysis in the ATP-E cohort.
Data sharing
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Results
In total, 871 (14.6%) women [444 (14.9%) from the AS04-HPV-16/18 vaccine group and 427 (14.3%) from the control group] from the TVC-E were included in the analysis of ongoing infection with any HR-HPV types, based on the primary objective of this paper (). At baseline, 4.0% (241/5,972) of women in the TVC-E were DNA-positive to HPV-16/18 (with or without coinfections with other HR-HPV types), 10.5% (630/5,972) to any other HR-HPV type without HPV-16/18 and coinfections associated with HPV-16 and/or −18, and only 0.2% (11/5,972) were concomitantly positive to both HPV-16 and HPV-18. The mean follow-up time for this cohort was 60.2 months (standard deviation 19.4). Demographic characteristics and HR-HPV distribution in the vaccine and control groups were similar ().
Table 1. Summary of baseline demographic characteristics of women DNA-positive to at least one HR-HPV species at baseline in the TVC-E
Figure 2. Analysis flowchart
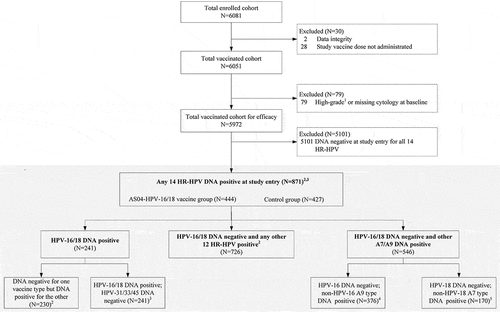
VE against HPV-16/18 infections in women with HR-HPV infections at baseline
Overall, the vaccine had high efficacy against incident infection, 6-month and 12-month PIs associated with HPV-16 and/or HPV-18 in women infected with any of the 14 HR-HPV types at baseline: 74.7% (95% Confidence Interval [CI]: 52.2 to 87.5), 100.0% (95% CI: 79.8 to 100.0) and 100.0% (95% CI: 47.2 to 100.0), respectively. Consistently, high VE was observed in women DNA-negative to HPV-16/18 but DNA-positive to any other 12 HR-HPV types ().
Table 2. Vaccine efficacy against HPV-16/18 infectiona stratified by baseline DNA infection status (TVC-E)
We also analyzed the efficacy against infection related to HPV-16/18 in women with HPV-16 or HPV-18 infection at baseline. In 223 women DNA-negative at baseline to one vaccine type but DNA-positive to the other one, VE against incident infection endpoints associated with HPV-16/18 was 66.8% (95% CI: −18.9 to 92.5). There were not enough cases to evaluate 6- or 12-month PI-related endpoints in this subgroup ().
Two subsets were analyzed to investigate the impact of infection with non-vaccine A9 and A7 HPV species at baseline on VE against virological endpoints associated with HPV-16/18. VE against incident infection, 6- and 12-month PIs associated with HPV-16 in women DNA-positive to non-HPV-16 A9 species at baseline was 69.9% (95% CI: 0.6 to 92.9), 100% (95% CI: 0.8 to 100) and 100% (95% CI: −119.6 to 100), respectively. Similarly, VE against incident and 6-month PI associated with HPV-18 in women DNA-positive to non-HPV-18 A7 species at baseline was 100% (95% CI: 45.3 to 100) and 100% (95% CI: −3,137 to 100), respectively. VE against 12-month HPV-18 PI could not be determined as no cases were reported during the duration of the study ().
Similar results were obtained for the ATP-E cohort (Supplementary table 1).
VE against HPV-31/33/45 infections in women with HR-HPV infections at baseline
Cross-protective efficacies against incident infections with HPV-31 and HPV-31/33/45 (any or in combination) in women DNA-positive to any other HR-HPV type at baseline were 63.2% (95% CI: 28.0 to 82.3) and 55.5% (95% CI: 30.3 to 72.1), respectively. VEs against 6-month and 12-month PIs are presented in .
Table 3. Vaccine efficacy against HPV-31/33/45 infectiona stratified by baseline DNA infection status (TVC-E)
In women with HPV-16/18 infection at baseline, high cross-protective VE against incident infections associated with HPV-31/33/45 was observed: 71.0% (95% CI: 27.3 to 89.8). Cross-protective VE values for 6- and 12-month PIs were 37.3% (95% CI: −191.4 to 87.6) and −59.6% (95% CI: −1,664 to 77.1), respectively ().
Similar results were obtained for the ATP-E cohort (Supplementary table 2).
HPV-16/18 associated CIN2± in women with HR-HPV infections at baseline
In all 871 subjects DNA-positive to any 14 HR-HPV types at baseline, irrespective of serostatus, only five CIN2+ cases associated with HPV-16 and/or −18 (all five cases were associated with HPV-16) were reported in subjects DNA-negative to the corresponding HPV type at baseline.
Discussion
As part of the debate on the added-value of prophylactic HPV vaccination in already sexually active women infected with HPV, this post-hoc analysis supplied data on Chinese 18–25-year-old women who, at the time of first vaccination, had cervical HR-HPV infection (regardless of their serostatus). It evidences that the AS04-HPV-16/18 vaccine may provide preventive benefit to this population.
The FUTURE II (NCT00092534), VIVIANE (NCT00294047) and PATRICIA (NCT00122681) studies have already demonstrated significant VE against vaccine type HPV infection or related neoplasia in subjects with previous or existing HPV infection but DNA-negative for the targeted HPV type at baseline.Citation16,Citation19,Citation20 The FUTURE II study, using the quadrivalent HPV vaccine, provided results from women seropositive or DNA-positive to one to three HPV vaccine types before vaccination.Citation19 The VIVIANE study, using the AS04-HPV-16/18 vaccine, had a subset analysis consisting of women with a history of previous HPV infection or disease.Citation20 However, seropositivity is considered to be associated with prior HPV exposure even though the infection has been cleared, while DNA status informs more about ongoing HR-HPV infection, which significantly affects VE. Moreover, HPV antibody titers are not routinely tested. Thus, our study merely included subjects who were DNA-positive at the time of first vaccination (regardless of their serostatus). The findings provide real-world practical and instructive implications for both clinicians and infected women.
In women DNA-positive to one of the HPV-16/-18 types at baseline but DNA-negative to the other type, our results displayed a statistically non-significant VE against HPV-16/18 incident infections. The PATRICIA study group also analyzed VE against CIN2+ associated with HPV-16/18 in women who were HPV DNA-positive to one vaccine type at baseline but DNA-negative and seronegative to the other vaccine type. The VE was 90.0% (95% CI: 31.8 to 99.8) for HPV-16/18.Citation16 Considering DNA status might have more impact on efficacy compared with serostatus, the negative lower bound of the CI for the VE observed in our analysis could be explained by the limited sample size available. It is also possible that efficacy is higher against high-grade lesions than against infections.
As demonstrated in our study, among women DNA-positive to the vaccine types (HPV-16/18), including those simultaneously infected with both types, cross-protection against HPV-31/33/45 incident infections is evident. We also observed decreasing VEs against HPV-31/33/35 infections that became persistence; VE for incident infection > VE for 6-month PI > VE for 12-month PI. These changes in VE estimates may be linked to the underestimation of non-vaccine HPV types in the control group due to the broad-spectrum HPV PCR methodology used. This method is known to potentially underestimate the prevalence of genotypes present at low relative concentrations in multiple infections, a scenario that is more likely to arise in the control group when considering vaccine and cross-protected HPV types.Citation21 This bias is stronger for 12-month PI than for 6-month PI and incident infection, and may explain the decreasing VEs observed in this study.Citation21
The L1 protein of non-vaccine A9 species (HPV-31/33/35/52/58) and non-vaccine A7 species (HPV-39/45/59/68) display amino acid homology with L1 proteins of HPV-16 and HPV-18 types, respectively.Citation22 Based on the phylogenetic relatedness, it is interesting to explore the impact of the preexistence of non-vaccine A7/A9 species infections at first vaccination on VE against endpoints related to HPV-16/18. However, most published studies have focused on cross-protective efficacy and a possible cross-reactive immune response related to A7/A9 species.Citation22,Citation23 In contrast, our data indicate high VE against HPV-16 or HPV-18 infections in women DNA-positive to any other non-vaccine HR-HPV A9 or A7 species at vaccination, respectively.
HPV prevalence studies among Chinese women have shown two peaks in HR-HPV infections: a first peak in those aged 15–24 years, soon after the onset of sexual debut, and a second in those aged >40 years.Citation24 The second peak is thought to be associated with new HPV exposure and/or the reactivation of a latent infection, and coincides with the peak of cervical cancer incidence observed in women up to 50–55 years old in China.Citation25 As evidenced by our study, vaccinating women with preexisting HR-HPV infection could reduce the risk of infection with and transmission of HPV type to which they have not yet been exposed. Moreover, even though HPV vaccines have no therapeutic impact on preexisting infection,Citation19 vaccination could prevent eventual re-infection later in life. Vaccination of infected women on an individual basis would confer protection to women from the second attack peak, protect their partners from possible infection and may slow down the progression of the HPV infectionCitation26 at the scale of the Chinese population. Further evidence is required on this point.
The HPV-FASTER protocol proposed offering HPV vaccination to women in a broad age range (9–45 years) to contribute to faster and greater population impact, based on the results of high VE from phase III clinical trials among adult women. However, vaccination of HR-HPV infected women has been controversial.Citation27–29 The results of our analysis lend support to this concept, suggesting vaccinating all women, irrespective of their HPV DNA status. Within the context of global investment and commitments toward the elimination of cervical cancer, this would allow to achieve maximum health benefit and equity to the entire population.
The main strength of our analysis was the specific focus on HR-HPV DNA-positive women at baseline, ignoring confounding factor of serostatus that is moreover not examined routinely, apart from clinical trial testing. An unavoidable limitation is that the findings were generated from a post-hoc analysis of a randomized controlled trial, which was not designed to analyze efficacy in women HR-HPV positive at vaccination, and randomization was not controlled for HPV-infection at baseline. Besides, the small number of women with ongoing HR-HPV infection available for inclusion in the present analysis (i.e., 15% of the total cohort) limits its statistical power. Also, due to insufficient endpoint cases, we evaluated efficacy mainly on a virologic perspective in the form of incident infections, with some subsets based on 6-month and 12-month PIs. However, as demonstrated in previous clinical trials, efficacy against PI is an acceptable surrogate endpoint for efficacy in the prevention of cervical cancer.Citation30
In conclusion, our analysis indicated that women with existing HR-HPV infection at the time of first vaccination might still benefit from the AS04-HPV-16/18 vaccine. However, this potential benefit needs further demonstration in the future.
Trademark statement
Cervarix is a trademark owned by or licensed to the GSK group of companies.
ThinPrep PapTest is a trademark owned by or licensed to Cytyc Corporation.
Innogenetics is a trademark owned by or licensed to Innogenetics N.V.
SAS is a trademark owned by or licensed to SAS Institute Inc.
Supplemental Material
Download MS Word (80.9 KB)Acknowledgments
The authors would like to thank all trial participants, study nurses, and other staff members for contributing to this study and all investigators from the different institutions. The authors also thank Dorota Borys (Clinical & Epidemiology Research & Development Project Lead, GSK) and Valérie Berlaimont (Medical Affairs, GSK) for their inputs during the review of the manuscript.
Authors would also like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Bruno Baudoux (Business & Decision Life Sciences c/o GSK) coordinated manuscript development and editorial support. Stefan Amisten (Freelance c/o GSK) provided medical writing assistance in the intermediate stage of manuscript writing on behalf of GSK.
Disclosure statement
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare the following potential conflicts of interest: The institutions of Fang-Hui Zhao, Shangying Hu, Ying Hong, Yue-Mei Hu, Xun Zhang, Qin-Jing Pan, Wen-Hua Zhang, Cheng-Fu Zhang, Xiaoping Yang, Jia-Xi Yu, Jiahong Zhu, Yejiang Zhu, Feng Chen, Xiaoqian Xu, Shuang Zhao and Feng-Cai Zhu received grants/investigator fees from the GSK group of companies for the conduct and follow-up of this study. Fang-Hui Zhao, Feng-Cai Zhu and Yue-Mei Hu received support for travel to meetings related to the study from the GSK group of companies. Naveen Karkada, Haiwen Tang, Dan Bi are employees of the GSK group of companies. Frank Struyf was an employee of the GSK group of companies at the time the study was performed and is currently an employee of Janssen, Pharmaceutical companies of Johnson & Johnson. Haiwen Tang, Dan Bi, and Frank Struyf hold shares in the GSK group of companies.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2020.1829411.
Additional information
Funding
Notes on contributors
Fanghui Zhao
Shangying HU: Conceptualization and/or Methodology, Investigation, Resources, Formal analysis, Writing - Original Draft Xiaoqian XU: Conceptualization and/or Methodology, Resources, Formal analysis, Writing - Original Draft Fengcai ZHU: Conceptualization and/or Methodology, Investigation, Formal analysis, Writing - Review & Editing Ying HONG: Conceptualization and/or Methodology, Investigation, Formal analysis, Writing - Review & Editing Yuemei HU: Conceptualization and/or Methodology, Investigation, Formal analysis, Writing - Review & Editing Xun ZHANG: Conceptualization and/or Methodology, Investigation, Formal analysis, Writing - Review & Editing Qinjing PAN: Conceptualization and/or Methodology, Investigation, Formal analysis, Writing - Review & Editing Wenhua ZHANG: Conceptualization and/or Methodology, Investigation, Formal analysis, Writing - Review & Editing Chengfu ZHANG: Conceptualization and/or Methodology, Investigation, Resources, Writing - Review & Editing Xiaoping YANG Investigation, Writing - Review & Editing Jiaxi YU: Investigation, Writing - Review & Editing Jiahong ZHU: Conceptualization and/or Methodology, Investigation, Resources, Writing - Review & Editing Yejiang ZHU: Investigation, Writing - Review & Editing Feng CHEN: Conceptualization and/or Methodology, Investigation, Formal analysis, Writing - Review & Editing Shuang ZHAO: Conceptualization and/or Methodology, Formal analysis, Writing - Review & Editing Naveen KARKADA: Formal analysis, Writing - Review & Editing Haiwen TANG: Conceptualization and/or Methodology, Resources, Formal analysis, Writing - Review & Editing Dan BI: Conceptualization and/or Methodology, Resources, Formal analysis, Writing - Review & Editing Frank STRUYF: Conceptualization and/or Methodology, Investigation, Formal analysis, Writing - Review & Editing Fanghui ZHAO: Conceptualization and/or Methodology, Investigation, Resources, Formal analysis, Writing - Original Draft.
References
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi:10.1016/S0140-6736(09)61248-4.
- Huh WK, Joura EA, Giuliano AR, Iversen OE, de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN, Hirschberg AL, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet. 2017;390:2143–59. doi:10.1016/S0140-6736(17)31821-4.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi:10.1056/NEJMoa061741.
- Beachler DC, Kreimer AR, Schiffman M, Herrero R, Wacholder S, Rodriguez AC, Lowy DR, Porras C, Schiller JT, Quint W, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV infection. J Natl Cancer Inst. 2016;108:pii: djv302. doi:10.1093/jnci/djv302.
- World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017-recommendations. Vaccine. 2017;35:5753–55. doi:10.1016/j.vaccine.2017.05.069.
- Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:Cd009069.
- Black LL, Zimet GD, Short MB, Sturm L, Rosenthal SL. Literature review of human papillomavirus vaccine acceptability among women over 26 years. Vaccine. 2009;27:1668–73. doi:10.1016/j.vaccine.2009.01.035.
- National Medical Products Administration. The first domestic recombinant human papillomavirus vaccine was approved into market. 2019, Dec 31. [Accessed 2020 Apr 17]. http://www.nmpa.gov.cn/WS04/CL2056/373049.html. [In Chinese].
- World Health Organization. Vaccines in National Immunization Programme Update. 2020, June. [Accessed 2020 June 19]. https://www.who.int/immunization/monitoring_surveillance/VaccineIntroStatus.pptx.
- Zhang Y, Wang Y, Liu L, Fan Y, Liu Z, Wang Y, Nie S. Awareness and knowledge about human papillomavirus vaccination and its acceptance in China: a meta-analysis of 58 observational studies. BMC Public Health. 2016;16:216. doi:10.1186/s12889-016-2873-8.
- Zhao FH, Tiggelaar SM, Hu SY, Zhao N, Hong Y, Niyazi M, Gao XH, Ju LR, Zhang LQ, Feng XX, et al. A multi-center survey of HPV knowledge and attitudes toward HPV vaccination among women, government officials, and medical personnel in China. Asian Pac J Cancer Prev. 2012;13:2369–23678.
- World Health Organization. Global health observatory data repository. Cervical cancer screening: Response by country. [Accessed 2020 Apr 17]. https://apps.who.int/gho/data/view.main.UHCCERVICALCANCERv.
- World Health Organization. Guide to introducing HPV vaccine into national immunization programmes. [Accessed 2020 Apr 17]. https://www.who.int/immunization/diseases/hpv/HPV_vaccine_intro_guide_draft_Oct2016.pdf.
- Chrysostomou AC, Stylianou DC, Constantinidou A, Kostrikis LG. Cervical cancer screening programs in Europe: the transition towards HPV vaccination and population-based HPV testing. Viruses. 2018;10:pii: E729. doi:10.3390/v10120729.
- Olsson SE, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5:696–704. doi:10.4161/hv.5.10.9515.
- Szarewski A, Poppe WA, Skinner SR, Wheeler CM, Paavonen J, Naud P, Salmeron J, Chow SN, Apter D, Kitchener H, et al. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in women aged 15–25 years with and without serological evidence of previous exposure to HPV-16/18. Int J Cancer. 2012;131:106–16. doi:10.1002/ijc.26362.
- Zhu FC, Chen W, Hu YM, Hong Y, Li J, Zhang X, Zhang YJ, Pan QJ, Zhao FH, Yu JX, et al. Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18–25 years: results from a randomized controlled trial. Int J Cancer. 2014;135:2612–22. doi:10.1002/ijc.28897.
- Zhu FC, Hu SY, Hong Y, et al. Efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine in Chinese women aged 18–25 years: event-triggered analysis of a randomized controlled trial. Cancer Med. 2017;6:12–25. doi:10.1002/cam4.869.
- Future II Study Group. Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis. 2007;196:1438–46. doi:10.1086/522864.
- Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, Garland SM, Chatterjee A, Lazcano-Ponce E, Salmerón J, McNeil S, Stapleton JT, Bouchard C, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis. 2016;16:1154–68. doi:10.1016/S1473-3099(16)30120-7.
- Struyf F, Colau B, Wheeler CM, Naud P, Garland S, Quint W, Chow SN, Salmerón J, Lehtinen M, Del Rosario-Raymundo MR, et al. Post hoc analysis of the PATRICIA randomized trial of the efficacy of human papillomavirus type 16 (HPV-16)/HPV-18 AS04-adjuvanted vaccine against incident and persistent infection with nonvaccine oncogenic HPV types using an alternative multiplex type-specific PCR assay for HPV DNA. Clin Vaccine Immunol. 2015;22:235–44.
- Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Tay EH, Garcia P, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199:926–35. doi:10.1086/597307.
- Wheeler CM, Castellsague X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmerón J, Chow SN, Apter D, Kitchener H, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–10. doi:10.1016/S1470-2045(11)70287-X.
- Zhao FH, Lewkowitz AK, Hu SY, Chen F, Li LY, Zhang QM, Wu RF, Li CQ, Wei LH, Xu AD, et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: a pooled analysis of 17 population-based studies. Int J Cancer. 2012;131:2929–38.
- Gu XY, Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen WQ, He J. Incidence and mortality of cervical cancer in China, 2014. Zhonghua Zhong Liu Za Zhi. 2018;40:241–46.
- Ryser M, Berlaimont V, Karkada N, Mihalyi A, Rappuoli R, van der Most R. Post-hoc analysis from phase III trials of human papillomavirus vaccines: considerations on impact on non-vaccine types. Expert Rev Vaccines. 2019;18:309–22. doi:10.1080/14760584.2019.1579647.
- Castellsague X, Munoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, Luna J, Myers E, Mallary S, Bautista OM, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br J Cancer. 2011;105:28–37. doi:10.1038/bjc.2011.185.
- Skinner SR, Szarewski A, Romanowski B, Garland SM, Lazcano-Ponce E, Salmerón J, Del Rosario-Raymundo MR, Verheijen RH, Quek SC, da Silva DP, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 4-year interim follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet. 2014;384:2213–27. doi:10.1016/S0140-6736(14)60920-X.
- Bosch FX, Robles C, Diaz M, Arbyn M, Baussano I, Clavel C, Ronco G, Dillner J, Lehtinen M, Petry KU, et al. HPV-FASTER: broadening the scope for prevention of HPV-related cancer. Nat Rev Clin Oncol. 2016;13:119–32. doi:10.1038/nrclinonc.2015.146.
- IARC HPV Working Group. Primary end-points for prophylactic HPV vaccine trials. IARC Working Group Reports. 2014;7.

