ABSTRACT
Two quadrivalent meningococcal conjugate vaccines (MenACWY) that prevent invasive meningococcal disease caused by N. meningitidis serogroups A, C, Y, and W have been licensed in the U.S. in the past 10–15 years. We systematically reviewed published studies conducted in the U.S. to evaluate the real-world safety evidence of meningococcal conjugate vaccines. We performed a literature search in PubMed of publications from 01/01/2005 to 02/29/2020 and identified 18 studies meeting inclusion criteria. Populations included high-risk persons aged 2 months to 10 years, adolescents/adults aged ≥11 years, pregnant populations, and hematopoietic cell transplant recipients. We extracted information about study setting, study design, exposure, outcomes, comparison group, follow-up/look back period, study population, sample size, available demographic/indication information, results, key conclusion, and reference. These published studies found no new significant safety concerns related to MenACWY. Consideration for future research includes a post-licensure safety evaluation of a new MenACWY product approved in April 2020.
Background
Invasive meningococcal disease (IMD) is a severe acute bacterial infection, and in the vast majority of cases is caused by five serogroups of Neisseria meningitidis (A, B, C, W, Y).Citation1 In the U.S., serogroups B, C, and Y are most prevalent, while serogroup A is rarely isolated.Citation2 The overall incidence of meningococcal disease during the ten-year period between 2006 and 2015 was approximately 0.26 cases per 100,000 people in the U.S. with a decrease in incidence by 2015 (0.12 cases per 100,000 people).Citation3 Incidence varies by age; infants younger than one year have the highest incidence of meningococcal disease (0.73 cases per 100,000) and overall case fatality is approximately 15%.Citation3 Morbidity from the disease is also high, with 11 to 27% of survivors experiencing significant sequelae, including neurologic and/or orthopedic disability, allergic complications, myocarditis, and hearing loss.Citation4–7
In the U.S., three quadrivalent meningococcal conjugate vaccines (MenACWY) that prevent invasive meningococcal disease (IMD) caused by N. meningitidis serogroups A, C, Y, and W have been licensed – MenACWY-D (Menactra; Sanofi Pasteur, Swiftwater, PA), MenACWY-CRM (Menveo; GlaxoSmithKline, Middlesex, United Kingdom), and MenACWY-TT (MenQuadfi; Sanofi Pasteur, Swiftwater, PA). MenACWY-D was licensed in 2005 and is approved as a single dose administration to persons 2–55 years of age and as a 2-dose series in children 9–23 months of age.Citation8,Citation9 MenACWY-CRM, a quadrivalent meningococcal oligosaccharide diphtheria CRM197 conjugate vaccine, was licensed in 2010 and is approved as a single dose administration in persons 2–55 years of age and as a multi-dose series in children 2–23 months of age.Citation10 MenACWY-TT, a quadrivalent meningococcal tetanus toxoid-conjugated vaccine, was licensed in April 2020 and is approved as a single dose administration in persons 2 years of age and older.Citation11 However, MenACWY-TT is not yet recommended for use in the U.S. by the Advisory Committee on Immunization Practices (ACIP). Therefore, this paper focuses on MenACYW-D and MenACWY-CRM only, as there were no post-licensure observational studies available for MenACWY-TT.
The Centers for Disease Control and Prevention’s (CDC) ACIP recommends routine vaccination with MenACWY to all persons 11 to 12 years of age with a booster dose at age 16 years.Citation8 However, recommendations for infants, children (2–10 years), and adults (≥19 years) is limited to administration among those at increased risk for meningococcal disease.Citation8 MenACWY-CRM can be administered as early as 2 months of age while MenACWY-D has a minimum age of 9 months. Individuals at increased risk include those who have persistent complement component deficiencies (e.g., C5-C9, properdin, factor H, or factor D); those exposed to a community outbreak attributable to a vaccine serogroup; and those who travel to or reside in countries where meningococcal disease is hyperendemic or epidemic.Citation1,Citation8 Additional groups at increased risk include persons who have functional or anatomic asplenia (including sickle cell disease) or HIV infection. However, among children 23 months and younger with these indications, MenACWY-CRM is the only MenACWY vaccine that is recommended.Citation12
A few reviews of the post-licensure safety of MenACWY have been published. Keshavan et al. (2018) published a review of the clinical experience with MenACWY-CRM that consisted of 4 new safety surveillance studies published since a previous review in 2011.Citation13 Myers and McNeil (2017) published a commentary focusing on the post-licensure safety experience of MenACWY-D and MenACWY-CRM.Citation14 Since these two reports, additional real-worldCitation15 post-licensure safety studies of MenACWY vaccines have been published based on U.S. populations, including the safety experience of infants/toddlers not previously studied, as well as pregnant women. We undertook a systematic review of published studies to evaluate the real-world evidence of MenACWY safety in the U.S., with a focus on the most recent studies.
Methods
We performed a literature search in PubMed of publications from January 1, 2005 to February 29, 2020. Study inclusion criteria included studies conducted in the U.S that examined the observational post-licensure safety of any of the two MenACWY vaccines (MenACWY-D and MenACWY-CRM) recommended by the ACIP (MenACWY-TT had not yet been licensed and was not included). We first used the terms ‘meningococcal’ ‘conjugate’ ‘vaccine’ ‘safety’ to search all fields. Second, we conducted a separate targeted search of the title/abstract using the terms ‘meningococcal’ ‘vaccine’ ‘safety’ ‘united states’ excluding hits with ‘serogroup B’ ‘serogroup C’ and ‘trial.’ Finally, we manually searched the references cited by Myers et al. for additional references not captured in our systematic PubMed search findings.Citation14
One author (TAB) conducted the systematic review of the literature and assessed study eligibility. One other author (ZS) completed a quality control check to verify the study eligibility of 1) a 20% random sample of those publications identified in the first step of the systematic search, and 2) all publications determined by the first author to meet study inclusion criteria. Any discrepancies by the two authors were brought to the other authors for a final decision to include/exclude publications for review (HT, LSS).
We extracted information regarding study setting, study design, exposure, outcomes, comparison group, follow-up/look back period, study population, sample size, available demographic/indication information, results, key conclusion, and reference (author, publication year).
Results
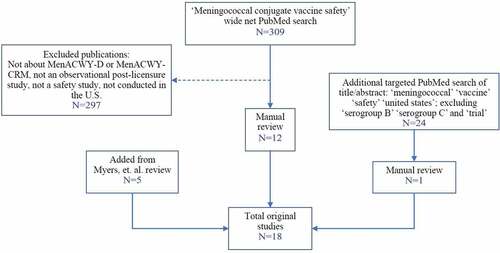
The PubMed search resulted in 309 published papers that were manually reviewed for eligibility. Of these, 12 original studies met the eligibility criteria (). Studies were excluded because they were not post-licensure, not about a meningococcal vaccine approved for use in the U.S., or were studies based outside of the U.S. One additional study was identified from the targeted PubMed search. Five additional studies were added from the Myers et al. review that were not captured in the original PubMed search because they reflected larger vaccine safety assessments and did not exclusively evaluate safety of MenACWY vaccines.
Of the 18 studies selected in the first review, the secondary review identified 2 studies that did not meet inclusion criteria because they were broader safety evaluations of vaccines and it was unclear whether specific MenACWY vaccine safety analyses were conducted. However, the authors decided to include the papers after tertiary review. The papers reported on specific safety outcomes after meningococcal vaccination, but sample size limited the ability to conduct further analyses. In the random sample of papers identified in the first step (n = 60), the secondary reviewer confirmed appropriate exclusion of all the excluded papers (). Of the included publications, 67% (n = 12) were observational studies (e.g., cohort, self-controlled, case-centered, or tree-temporal scan designs) and 22% (n = 4) were analyses of spontaneous reports from the Vaccine Adverse Event Reporting System (VAERS). VAERS is a national vaccine safety surveillance system co-administered by the CDC and the U.S. Food and Drug Administration (FDA) and is reliant on passive reporting of inadvertent exposures and/or adverse events.
Table 1. Real-world post-licensure studies of the safety of MenACWY in the U.S
MenACWY during pregnancy
Although the vaccines are not approved for use in pregnant women, inadvertent exposure can occur, often in the early gestational period when the status of pregnancy may be unknown. Four studies to date have evaluated the safety of MenACWY vaccines during pregnancy, two of which were reviewed by Myers et al.Citation16–19
One of the earlier studies, conducted by Zheteyeva et al., looked at adverse events during pregnancy after inadvertent exposure to MenACWY-D and found 17 (16.5%) spontaneous abortions and one congenital anomaly (1%; aqueductal stenosis and severe ventriculomegaly) among 103 exposed pregnant women reported to the VAERS.Citation16 The authors stated that the patterns were not concerning based on background prevalence of the outcomes. The other study was also a VAERS analysis of 14 pregnant women exposed to MenACWY-CRM, in which only 3 reports included limited information on birth outcomes that were in all cases normal.Citation18
After the Myers et al. review, there was a third study, a large evaluation of MenACWY-D safety conducted within a large integrated health care system that identified 25 pregnancy exposures. Of these, 18 had available outcome data: 12 live births (including birth of an infant with a dermoid cyst), 5 elective abortions, and 1 fetal death.Citation19 The authors stated that this was not suggestive of an increased risk of pregnancy complications or an unusual pattern of adverse events among their infants, although this study included very few exposed pregnancies. The latest study evaluated MenACWY-CRM exposure in 92 pregnant women within a large integrated health care system. It was based on a cohort of vaccinated young women 11–21 years old who consequently became pregnant within 28 days of vaccination or were pregnant at the time of vaccination.Citation17 In this study, the prevalence of spontaneous and induced abortions, preterm birth, low birth weight, and major congenital malformations were comparable with U.S. background prevalence estimates.Citation17
MenACWY in infants and toddlers
Only two studies have assessed the post-licensure safety of MenACWY vaccines specifically in infants and toddlers, one in MenACWY-D recipients and the other in MenACWY-CRM recipients.Citation20,Citation21 Both studies evaluated emergency department (ED) visits and hospitalizations as outcomes. Becerra-Culqui et al. assessed all-cause ED visits/hospitalizations and associated diagnoses up to 6 months post MenACWY-CRM vaccination in 138 high-risk individuals 2–23 months of age and found comparable rates of ED/hospitalization visits in available published data, suggesting no safety concerns.Citation20 Hansen et al. assessed 30-day ED visits/hospitalizations, pre-specified conditions in the outpatient setting, and death following MenACWY-D vaccination in 116 infants/toddlers 9–23 months of age. Compared to outcomes in a 31–75 days post-vaccination period, they found no new safety concerns in relation to outcomes reported in clinical trials.Citation21
MenACWY in children 2–10 years
Two studies have specifically reviewed safety events among children 2–10 years old. In addition to evaluating outcomes in infant/toddlers, Hansen et al. reviewed safety outcomes following MenACWY-D among children 2–10 years old.Citation21 The only significantly elevated outcome was cellulitis/abscess in the inpatient setting, although the two cases did not appear near the injection site and thus were determined to be unrelated to vaccination. Tartof et al. evaluated safety outcomes following MenACWY-CRM in 327 children.Citation22 Among pre-specified events of interest, they found 1 confirmed new onset case of asthma, although it was 237 days post-vaccination. Only 4 children experienced events in the ED or inpatient settings within 30 days of vaccination. Findings were consistent with the events reported after vaccination with MenACWY-CRM from clinical trials described in the MenACWY-CRM package insert.Citation10
MenACWY in adolescents and adults
Several studies have evaluated the safety of MenACWY vaccines in adolescents and young adults, and many of these studies have been reviewed by Myers et al.Citation14 Briefly, early reports raised concerns about an association between MenACWY-D and Guillain-Barre syndrome (GBS).Citation23 It was estimated in 2012 that if such a risk existed, it was no more than 0.66 excess cases per 1 million adolescents vaccinated.Citation24 In the same year, Velentgas et al. published an evaluation of the risk of GBS among 1.4 million Men-ACWY-D vaccinations in 5 U.S. health plans, finding 0 GBS cases occurring within a 42-day window post-vaccination. They concluded that there was no evidence of an increased risk of GBS associated with MenACWY-D.Citation25 Baxter et al. measured the recurrence of GBS following any vaccination, including 7 individuals who had GBS prior to MenACWY-D vaccination. They found 0 recurrences of GBS after MenACWY-D vaccination.Citation26 In 2017, Myers et al. reported finding 4 confirmed cases of GBS out of 2,614 VAERS data reports after MenACWY-CRM vaccination.Citation18 However, 2 individuals had a viral illness prior to onset of GBS symptoms, and the authors stated that they did not observe disproportionate reporting for this condition in the data mining analysis. No cases of GBS were observed in evaluations of 48,499 MenACWY-CRM recipients and 31,561 MenACWY-D recipients in large integrated health care systems.Citation19,Citation27
Postvaccination syncope was reported to VAERS in 2005–2007 following vaccination with MenACWY-D and other routine adolescent vaccines (Tdap, HPV).Citation28 Syncope was also reported to VAERS in 2010–2015 following vaccination with MenACWY-CRM.Citation18 There were 141 (7%) syncope reports in 11–18 year olds and 5 serious reports of vasovagal syncope. One additional study-based assessed vaccine-associated syncope in adolescents 13–17 years of age.Citation29 They found only 1 chart-confirmed case of syncope following 28,495 MenACWY doses. However, syncopal rates after adolescent vaccination were lower than what was anecdotally reported by clinic staff, suggesting an underestimation of events.
In 2012, Rowhani-Rahbar et al. published a case-centered analysis of Bell’s palsy after any vaccination among children ≤18 years old.Citation30 In this analysis, 8 Bell’s palsy cases were identified among individuals with history of MenACWY-D receipt in the prior year. In a consolidated group analysis of “any vaccine,” no association with Bell’s palsy was found during any of the risk intervals within 1–56 days following vaccination. Later in 2017, Tseng et al. observed a temporal association between occurrence of Bell’s palsy and receipt of MenACWY-CRM concomitantly administered with other vaccines.Citation27 In that study, 8 cases of Bell’s palsy occurred in the risk window, primarily between 5 and 10 weeks after vaccination. The authors suggested the need for further investigation of the association between MenACWY-CRM and Bell’s palsy, one reason being that the increased risk was only found in those who received other vaccines concomitantly and the association with concomitant vaccines could not be ruled out. All 8 Bell’s palsy cases resolved completely. In a cohort study of MenACWY-D and in a report of MenACWY-CRM based on VAERS data, no significantly increased risk of Bell’s palsy or facial nerve palsy was detected.Citation18,Citation19
Other safety outcomes following MenACWY vaccines have been reported. Jackson et al. found that the risk of local reactions was low and comparable to previous studies, occurring in 1.8 out of 10,000 MenACWY-D vaccinations.Citation31 Signals from a tree-temporal scan study among 11–18 year olds included diseases of the skin and subcutaneous tissue, fever, and urticaria after vaccination with MenACWY-D, all of which were already documented in pre-licensure studies.Citation33 However, this study found a signal for pleurisy 21–32 days post-vaccination. A post-hoc exploration found that 60% of the cases had an International Classification of Diseases-9th edition diagnosis code for conditions that could cause pleurisy (i.e., trauma, pneumonia, asthma, injury, surgery, etc.), suggesting that it could have been a false signal, although further investigation is warranted.Citation33
Not many studies have looked at safety events among an older vaccine recipient population, except when included in a safety evaluation of a larger age group.Citation16,Citation18,Citation19,Citation26,Citation28 However, Cheng et al evaluated the safety of MenACWY-D after hematopoietic cell transplantation (HCT) in a study population (n = 67) with a median age of 58 years. HCT, also known as bone marrow or stem cell transplantation, is a treatment for cancer and other conditions.Citation32 In this study, no adverse events were documented within 60 days of vaccine administration.Citation34
Discussion
This systematic review of the real-world evidence of the safety of MenACWY vaccines in the U.S. provides an updated safety assessment of these vaccines, particularly among high-risk infants, toddlers, and children 2–10 years. One additional safety study of MenACWY-CRM in pregnant women has been added to the literature since the last review in 2017. While early reports detected safety concerns regarding GBS and syncope in adolescent and young adult vaccine recipients, only Bell’s palsy was subsequently detected as a potential safety concern when MenACWY-CRM was received concomitantly with other vaccines. However, two later studies (one on MenACWY-D and one on MenACWY-CRM) did not find increased risks of Bell’s palsy following MenACWY vaccination regardless of co-administration with other vaccines.
The real-world post-licensure safety assessment of MenACWY in infants, toddlers, and children 2–10 years at high-risk of contracting meningococcal disease is important. While pre-licensure clinical trials were conducted in healthy individuals, MenACWY has been recommended in high-risk children post-licensure. The three published post-licensure studies including these high-risk populations found no new safety concerns potentially related to MenACWY vaccination, although the outcomes were often sporadic. However, gathering a large sample size of high-risk-vaccinated individuals was a challenge considering the indicated conditions for vaccination (medical or high risk of exposure) can be rare. For example, only 116 and 138 infant/toddlers over a course of 3 years met inclusion criteria for the two separate studies conducted at Kaiser Permanente Northern California (KPNC) and Kaiser Permanente Southern California (KPSC), respectively.Citation20,Citation21 In addition, 1,421 and 327 children 2–10 years of age, respectively, were included in the two childhood vaccination safety assessments, although “healthy” children receiving an early dose of a routine MenACWY dose typically given at age 11–12 years per ACIP recommendations were also included.Citation21,Citation22 Nevertheless, these studies conducted in large-integrated health care settings had unique strengths. Safety assessments of MenACWY-CRM, one in infant/toddlers (2–23 months) and the other in children (2–10 years) included detailed chart reviews to improve accuracy in assessing eligibility and identifying incident safety outcome information, which was feasible with smaller sample sizes.Citation20,Citation22 The study design of the MenACWY-D safety assessment in children 9 months to 10 years of age gathered comparison data allowing for a direct comparison of outcomes and calculations of association estimates.Citation21
Although MenACWY vaccines are not approved for use in pregnant women, four studies in total have published findings on this population, two studies of VAERS data and two cohort studies. Findings were not suggestive of an increased risk of pregnancy- or birth-related adverse events. Inadvertent exposure can occur in pregnant young women, often in the early gestational period when the status of pregnancy may be unknown. All the evaluations of MenACWY exposure during pregnancy were descriptive in nature with no comparison group. However, a unique feature of the study of 92 pregnant women included the systematic identification of MenACWY exposure and pregnancy and birth outcomes that were chart reviewed in detail.Citation17 This is in contrast to the two studies based on VAERS data that relied on passive reporting of inadvertent exposure and/or adverse events with limited access to medical records for detailed review.Citation16,Citation18 Detailed review of outcomes was also limited in the evaluation of 25 exposed pregnancies with outcome data only available for 18 women.
Real-world safety studies are necessary and the only way to evaluate vaccines in the public after they have been licensed. MenACWY vaccine safety has been mainly evaluated via VAERS reports or in health care settings. Four studies have been conducted using the VAERS system, 4 have been exclusively conducted at Kaiser Permanente Southern California (MenACWY-CRM), 4 exclusively at Kaiser Permanente Northern California (MenACWY-D), a large Vaccine Safety Datalink (VSD) data mining study involving 1.25 million MenACWY-D doses, and a combined analysis of over 2 million MenACWY-D doses from the VSD and 5 U.S. health plans to assess the association between MenACWY-D and GBS. The study settings complement each other. For example, VAERS studies have the highest potential for selection bias since they are reliant on passive reporting, without clear population denominators for the calculation of rates. However, VAERS is valuable for signal detection that triggers larger follow-up studies in large health care systems, such as in the case of GBS and syncope after MenACWY-D vaccination.Citation23,Citation28 Systematic evaluation of vaccine safety is needed especially among high-risk groups for which MenACWY is recommended. Although small sample size or lack of an adequate comparison group has been an issue for studies of high-risk populations, these studies still provide valuable real-world evidence of MenACWY, not otherwise available pre-licensure or systematically in VAERS.
Post-licensure studies have generated reassuring real-world evidence of the safety of MenACWY vaccines. This review included post-licensure safety studies for MenACWY-D and MenACWY-CRM, which have been used in the U.S. for 15 and 10 years, respectively. Because of the potential limitation that the sample size of high-risk populations and pregnant women receiving these vaccines is still small, constant monitoring of the safety of these vaccines in this population is warranted. Consideration for future research includes the post-licensure safety evaluation of MenACWY-TT, a newly licensed MenACWY vaccine for which safety has been evaluated in 5 double-blind, randomized clinical trials.Citation11
Disclosure of potential conflicts of interest
This work was supported by Kaiser Permanente Southern California.
TBC, LSS, ZS and HFT received research funding from GSK for meningococcal vaccine studies and other unrelated vaccine studies. LSS, HFT, and ZS received research support from Novavax for studies unrelated to this paper. HFS and LSS received research support from Seqirus for studies unrelated to this paper. LSS and ZS received research support from Dynavax for studies unrelated to this paper. HFT received research support from Genentech for studies unrelated to this paper. ZS received research support from Gilead for studies unrelated to this paper.
Additional information
Funding
References
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. 13 ed. Washington, D.C.: Public Health Foundation; 2015.
- Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, McIntyre P, Ramsay ME, Sáfadi MAP. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30(Suppl 2):B26–36. doi:10.1016/j.vaccine.2011.12.032.
- MacNeil JR, Blain AE, Wang X, Cohn AC. Current Epidemiology and Trends in Meningococcal Disease—United States, 1996–2015. Clin Infec Dis. 2018;66(8):1276–81. doi:10.1093/cid/cix993.
- Kaplan SL, Schutze GE, Leake JA, Barson WJ, Halasa NB, Byington CL, Tan TQ, Hoffman JA, Wald ER, et al. Multicenter surveillance of invasive meningococcal infections in children. Pediatrics. 2006;118(4):e979–984. doi:10.1542/peds.2006-0281.
- Madelaine T, Cour M, Bohé J, Floccard, Duperret S, Hernu R, Argaud L. Invasive meningococcal disease-induced myocarditis in critically ill adult patients: initial presentation and long-term outcome. Intensive Care Med. 2017;43(2):279–81. doi:10.1007/s00134-016-4623-x.
- Kirsch EA, Barton RP, Kitchen L, Giroir BP. Pathophysiology, treatment and outcome of meningococcemia: a review and recent experience. Pediatr Infect Dis J. 1996;15(11):967–79. quiz 979. doi:10.1097/00006454-199611000-00009.
- Edwards MS, Baker CJ. Complications and sequelae of meningococcalinfections in children. J Pediatr. 1981;99(4):540–45. doi:10.1016/S0022-3476(81)80250-8.
- Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner CH, Baker CJ, Messonnier NE. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend Rep. 2013;62(RR–2):1–28.
- Sanofi Pasteur Inc. Menactra package insert: highlights of prescribing information; 2018. [accessed date 2020 Jun 25]. https://www.fda.gov/files/vaccines,%20blood%20&%20biologics/published/Package-Insert—Menactra.pdf
- GlaxoSmithKline Biologicals SA. MENVEO package insert: highlights of prescribing information; 2017. [accessed date 2020 Jun 25]. https://www.fda.gov/media/78514/download
- Sanofi Pasteur Inc. MenQuadfi, Meningococcal (Groups A, C, Y, W) conjugate vaccine; 2020. [accessed date 2020 Jun 29]. https://www.fda.gov/media/137306/download.
- MacNeil JR, Rubin L, McNamara L, Briere EC, Clark TA, Cohn AC. Use of MenACWY-CRM vaccine in children aged 2 through 23 months at increased risk for meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2013. MMWR Morb Mortal Wkly Rep. 2014;63:527–30.
- Keshavan P, Pellegrini M, Vadivelu-Pechai K, Nissen M. An update of clinical experience with the quadrivalent meningococcal ACWY-CRM conjugate vaccine. Expert Rev Vaccines. 2018;17(10):865–80. doi:10.1080/14760584.2018.1521280.
- Myers TR, McNeil MM. Current safety issues with quadrivalent meningococcal conjugate vaccines. Hum Vaccin Immunother. 2018;14(5):1175–78. doi:10.1080/21645515.2017.1366393.
- U.S. Food & Drug Administration. Framework for FDA’s real-world evidence program; 2018. [accessed date 2020 Jun 04]. https://www.fda.gov/media/120060/download.
- Zheteyeva Y, Moro PL, Yue X, Broder K. Safety of meningococcal polysaccharide-protein conjugate vaccine in pregnancy: a review of the vaccine adverse event reporting system. Am J Obstet Gynecol. 2013;208(6):478.e471-476. doi:10.1016/j.ajog.2013.02.027.
- Becerra-Culqui TA, Sy LS, Ackerson BK, Chen LH, Fischetti CA, Solano Z, Schmidt JE, Malvisi L, Curina C, Pellegrini M, et al. Safety of MenACWY-CRM vaccine exposure during pregnancy. Vaccine. 2020;38(12):2683–90. doi:10.1016/j.vaccine.2020.02.008.
- Myers TR, McNeil MM, Ng CS, Li R, Lewis PW, Cano MV. Adverse events following quadrivalent meningococcal CRM-conjugate vaccine (Menveo®) reported to the Vaccine Adverse Event Reporting system (VAERS), 2010-2015. Vaccine. 2017;35(14):1758–63. doi:10.1016/j.vaccine.2017.02.030.
- Hansen J, Zhang L, Klein NP, Robertson CA, luo MD, Greenberg DP, Bassily E, Baxter R. Post-licensure safety surveillance study of routine use of quadrivalent meningococcal diphtheria toxoid conjugate vaccine. Vaccine. 2017;35(49):6879–84. doi:10.1016/j.vaccine.2017.09.032.
- Becerra-Culqui TA, Sy LS, Ackerson BK, Slezak JM, Luo Y, Fischetti CA, Ohadike YU, Curina C, Pellegrini M, Solano Z, et al. Safety of quadrivalent meningococcal conjugate vaccine in infants and toddlers 2 to 23-months old. Vaccine. 2020;38(2):228–34.
- Hansen J, Zhang L, Eaton A, Baxter R, Robertson CA, Decker MD, Greenberg DP, Bassily E, Klein NP. Post-licensure safety surveillance study of routine use of quadrivalent meningococcal diphtheria toxoid conjugate vaccine (MenACWY-D) in infants and children. Vaccine. 2018;36(16):2133–38. doi:10.1016/j.vaccine.2018.02.107.
- Tartof SY, Sy LS, Ackerson BK, Hechter RC, Haag M, Slezak JM, Fischetti CA, Takhar HS, et al. Safety of Quadrivalent Meningococcal Conjugate Vaccine in Children 2–10 Years. Pediatr Infect Dis J. 2017;36(11):1087–92. doi:10.1097/INF.0000000000001696.
- Centers for Disease Control and Prevention. Update: guillain-Barré syndrome among recipients of Menactra meningococcal conjugate vaccine–United States, June 2005-September 2006. MMWR Morb Mortal Wkly Rep. 2006;55(41):1120–24.
- Yih WK, Weintraub E, Kulldorff M. No risk of Guillain-Barré syndrome found after meningococcal conjugate vaccination in two large cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(12):1359–60. doi:10.1002/pds.3353.
- Velentgas P, Amato AA, Bohn RL, Arnold Chan K, Cochrane T, Funch, Dashevsky I, Duddy AL, Gladowski P, Greenberg SA, et al. Risk of Guillain-Barré syndrome after meningococcal conjugate vaccination. Pharmacoepidemiol Drug Saf. 2012;21(12):1350–58. doi:10.1002/pds.3321.
- Baxter R, Lewis N, Bakshi N, Vellozzi C, Klein NP. Recurrent Guillain-Barre syndrome following vaccination. Clin Infec Dis. 2012;54(6):800–04. doi:10.1093/cid/cir960.
- Tseng H-F, Sy LS, Ackerson BK, Hechter RC, Tartof SY, Haag M, Slezak JM, Luo Y, Fischetti CA, Takhar HS, et al. Safety of quadrivalent meningococcal conjugate vaccine in 11- to 21-Year-Olds. Pediatrics. 2017;139(1):e20162084. doi:10.1542/peds.2016-2084.
- Centers for Disease Control and Prevention. Syncope after vaccination–United States, January 2005-July 2007. MMWR Morb Mortal Wkly Rep. 2008;57(17):457–60.
- Narwaney KJ, Breslin K, Ross CA, Shoup JA, Wain KF, Weintraub ES, McNeil MM, Hambidge SJ. Vaccine adverse events in a safety net healthcare system and a managed care organization. Vaccine. 2017;35(9):1335–40. doi:10.1016/j.vaccine.2017.01.017.
- Rowhani-Rahbar A, Klein NP, Lewis N, Fireman B, Ray P, Rasgon B, Black S, Klein JO, Baxter R. Immunization and Bell’s Palsy in children: a case-centered analysis. Am J Epidemiol. 2012;175(9):878–85. doi:10.1093/aje/kws011.
- Jackson LA, Yu O, Nelson J, Belongia EA, Hambidge SJ, Baxter R, Naleway A, Nordin J, Baggs J, Iskander J, et al. Risk of medically attended local reactions following diphtheria toxoid containing vaccines in adolescents and young adults: a Vaccine Safety Datalink study. Vaccine. 2009;27(36):4912–16. doi:10.1016/j.vaccine.2009.06.038.
- Gyurkocza B, Rezvani A, Storb RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol. 2010;3(3):285–99. doi:10.1586/ehm.10.21.
- Li R, Weintraub E, McNeil MM, Kulldorff M, Lewis EM, Nelson, Xu S, Qian L, Klein NP, Destefano F, et al. Meningococcal conjugate vaccine safety surveillance in the Vaccine Safety Datalink using a tree-temporal scan data mining method. Pharmacoepidemiol Drug Saf. 2018;27(4):391–97. doi:10.1002/pds.4397.
- Cheng MP, Pandit A, Antin JH, Walsh SR, Huynh D, Ghobrial IM, Baden LR, Marty FM, Issa NC. Safety and immunogenicity of conjugate quadrivalent meningococcal vaccination after hematopoietic cell transplantation. Blood Advances. 2018;2(11):1272–76. doi:10.1182/bloodadvances.2018018911.