ABSTRACT
Human papillomavirus (HPV) infection is common in women and also the main cause of cervical cancer. Based on a dynamic compartmental model, we aimed to evaluate the population impact and cost-effectiveness of strategies that combined cervical cancer screening and HPV schoolgirl vaccination for Chinese women. The effectiveness of interventions was assessed by comparing modeled scenarios to the status quo, where a 3-y cervical cancer screening program remained at a 20% coverage and without a universal HPV vaccination program. Our study demonstrated that increasing screening coverage from 20% to 50% would reduce the high-risk HPV (HR-HPV) prevalence to 5.4%, whereas a universal schoolgirl vaccination program using the quadrivalent vaccine (qHPV) with a coverage of 50% would reduce the prevalence to 2.9% by 2069. Scaling-up the cervical screening coverage to 50% will prevent 16,012 (95% CI: 8,791 to 25,913) Disability-Adjusted Life-Years (DALYs) per year, with an incremental cost-effectiveness ratio (ICER) of US$ 10,958 (95% CI: $169 to $26,973)/DALY prevented. At the current qHPV price, vaccinating 50% of school girls will prevent 13,854 (95% CI: 8,355 to 20,776) DALYs/year, but the corresponding incremental cost-effectiveness ratio (ICER, US$ 83,043, 95% CI: $52,234 to $138,025) exceeds cost-effectiveness threshold (i.e., 3 times GDP per-capita of China: $30,792). The qHPV vaccine requires at least a 50% price reduction to be cost-effective. Vaccinating schoolgirls will result in a large population health benefit in the long term, but such a universal HPV vaccination program can only be cost-effective with a substantial price reduction.
Background
Human Papillomaviruses (HPV) include high-risk HPV (HR-HPV) and low-risk HPV (LR-HPV) subtypes.Citation1 HR-HPV infection can cause cervical cancer in infected women,Citation2 while LR-HPV types can cause genital warts. Approximately 12.0% of women are living with HPV of any genotype globally;Citation3 whereas this rate is around 15% in China.Citation4 In 2014, the age-adjusted mortality rate of cervical cancer was reported to be 3.21/100,000 in China, higher than the global average (2.98/100,000Citation5,Citation6). In the past decade, the development of HPV vaccines (bivalent [16/18, bHPV], quadrivalent [6/11/16/18, qHPV] and nonavalent [6/11/16/18/31/33/45/52/58, nHPV]) has significantly improved HPV control and prevention.Citation7–10 Large randomized controlled trials reported that the efficacy of qHPV against HPV-16 and −18 related cervical cancer and cancer precursors can reach 93.3–100%, and the efficacy against HPV-6/11/16/18 related genital warts is 95.4–99.9%.Citation11
By 2017, 80 countries have included vaccination against HPV as a part of their national vaccination schedule.Citation12 Australia initiated its universal HPV vaccination program for schoolgirls aged between 12 and 13 in 2007 and this was later expanded to include schoolboys in 2013. In 2018, an Australian national surveillance program reported that in the state of Victoria, the vaccine-targeted HPV genotypes were detected in only 1.7% of women aged between 18 and 25.Citation13 Genital warts have also become very rare in young Australian women and heterosexual men in the post-vaccination era.Citation14 Australia provides strong evidence that high coverage of HPV vaccine (women: 80%, men: 76% in 2019) can significantly reduce the prevalence of HPV in a population.Citation15 Indeed, Australia may become the first country to eliminate HPV.Citation16 In China, the high cost of HPV vaccines limits their usage. Although both bHPV and qHPV have been approved by the China Food and Drug Administration in 2017, and nHPV in 2018, the prices of the vaccines (bHPV: US$ 260; qHPV: US$ 450; nHPV: US$ 576) are not affordable for an average Chinese family.Citation17 A recent national survey reported that less than 6% of the Chinese women were willing to purchase the HPV vaccine at a price of higher than US$ 300 for themselves or their daughters.Citation18
In the absence of a universal vaccination program, HPV DNA-based cervical cancer screening is currently the only option recommended by the government for the early prevention of cervical cancer. The latest Chinese cervical cancer prevention guideline indicates that cervical cancer screening is recommended to be performed every 3 y for women aged 25–65.Citation19 Starting in 2012, China expanded the state-sponsored cervical cancer screening program to 1,140 counties, covering 30 million rural women.Citation20 As a result, a study conducted during 2013–2014 reported that around 20% of Chinese women over the age of 21 had received at least one cervical cancer screening in over the last 3 y.Citation21 However, scaling-up of cervical cancer screening (with HPV detection and follow-up treatments) is not an efficient approach for HPV prevention. It needs to combine with effective HPV vaccination to significantly reduce the burden of HPV infection.Citation22,Citation23 With the approval of the first domestic bivalent HPV vaccine Cecolin in December 2019, the Chinese government is facing an unprecedented opportunity to establish a universal schoolgirl vaccination program along with scaling-up the cervical cancer screening in adult women to achieve the goal of eliminating HPVCitation24-27.
Mathematical models have been widely used for HPV epidemic trend forecasting and economic evaluation.Citation28–33 Based on a compartmental model, we aimed to evaluate the population impact and cost-effectiveness of intervention strategies that combined cervical cancer screening with HPV schoolgirl vaccination and also identified the key factors that impact on the cost-effectiveness for women in a Chinese setting.
Methods
Data source
Epidemiological data were obtained from our previous systematic review on HPV infection for China.Citation4 To calibrate the primary model outputs, we synthesized the yearly prevalence of HPV infection and cervical intraepithelial neoplasia (CIN) for Chinese women over 2000–2017. An additional literature search was conducted to collect data on cervical cancer incidence and genital wart prevalence.Citation34–39 The epidemiology, behavior, intervention, and monetary parameters were collected for cervical cancer, precancerous lesions of CIN1-3, and genital warts (Table S1.1). If multiple values were reported for the same parameter, we pooled them using a weighted average (Table S1.2).
Model description
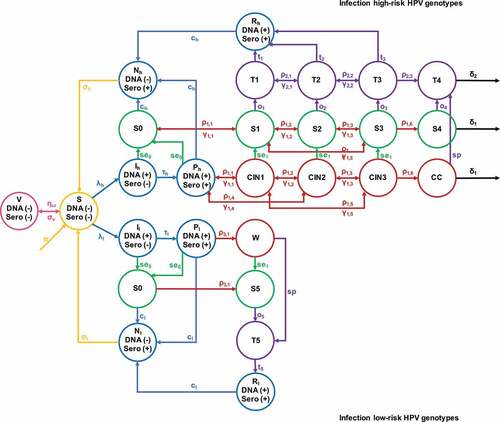
We first constructed a compartmental model, simulating the natural progression of HPV infection in Chinese women over the 2020–2069 period. By comparing the status quo trajectories to hypothetical scenarios of new interventions, we estimated the changes in prevalence, including HR (high risk) HPV, LR (low risk) HPV, CIN, cervical cancer, and genital wart (). 'Similar model framework has been published for HPV and other sexually transmitted infections.Citation1-3
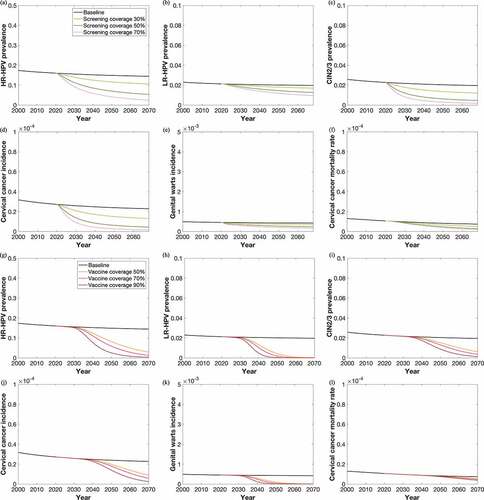
Figure 2. Temporal projection of the HPV epidemic among Chinese women aged >25, with (a–f) qHPV schoolgirls vaccination program only; (g–l) scale-up cervical cancer screening only, 2020–2069
1. Zhang, L., et al., Neisseria gonorrhoeae Transmission Among Men Who Have Sex With Men: An Anatomical Site-Specific Mathematical Model Evaluating the Potential Preventive Impact of Mouthwash. Sex Transm Dis, 2017. 44(10): p. 586–592.
2. Xianglong Xu, Eric P.F. Chow, Jason J. Ong, Christian JPA Hoebe, Deborah Williamson, Mingwang Shen, F.Y.S. Kong, Jane S Hocking, Christopher K. Fairley, Zhang L. Modelling the contribution that different sexual practices involving the oropharynx and saliva have on Neisseria gonorrhoeae infections at multiple anatomical sites in men who have sex with men. Sexually Transmitted Infections. DOI: 10.2139/ssrn.3578740. (accepted).
3. Xianglong Xu, Eric P.F Chow, Jason J Ong, Christian JPA Hoebe, Zhuoru Zou, Jane S Hocking, Christopher K Fairley, Zhang L. Chlamydia trachomatis transmission between the oropharynx, urethra and anorectum in men who have sex with men: A mathematical model. BMC Medicine; (accepted).
4. Zhang, L., et al., Targeted human papillomavirus vaccination for young men who have sex with men in Australia yields significant population benefits and is cost-effective. Vaccine, 2017. 35(37): p. 4923–4929.
We primarily modeled the schoolgirl vaccination program with qHPV, which prevents the infection of HPV 6, 11, 16, and 18. We did not consider the cross-protective effects for other HPV genotypes. The impact of other STIs over HPV progression was not considered. We simulated eight natural disease progression stages for HR-HPV infections and five for LR-HPV infections. Women with HR and LR-HPV infections were conceptually stratified into four conditions following their diagnosis and treatment status, including undiagnosed/natural disease progression, screened and diagnosed, receiving treatment, and recovering from treatment (). Notably, the suggested cervical cancer screening approaches differentiate in women at different ages. In this model, we assessed the following tests according to the Chinese HPV prevention guidelines: women aged 25–30 received cytology-based cervical cancer screening (liquid-based cytology, pap smear, or visual inspection with acetic acid (VIA)), whereas women aged >30 received combined cytological tests and HPV genotyping.Citation40,Citation41 Our model stipulated that individuals with a positive HPV screening test or showing cytological abnormalities would then accept colposcopy or biopsy examinations for confirmation.Citation40,Citation41 All women diagnosed with a condition of genital wart, CIN2+, or cervical cancer would accept subsequent treatments (loop electrosurgical excision procedure, cold knife conization, or hysterectomy). However, in practice, about half of the women (45.7%) diagnosed with CIN1Citation42 would choose to wait and observe, rather than accepting treatment for CIN1 at once. HPV positive women without clinical manifestation would also avoid any therapeutic interventions. We assumed, prior to treatment, cervical lesions progressed at the same rate in both diagnosed women and undiagnosed women; and the post-screening treatment usually occurred 2–3 months after diagnosis. Additionally, 90% of women who noticed their symptoms of cervical cancer and 60% of women with genital wartsCitation42 sought treatment on their own. The detailed description of the model compartmental structure and the system of differential equations with parameter explanation were included in the appendix.
Population in simulation
The universal vaccination program in our model was implemented for schoolgirls aged 9–16, and the scale-up cervical cancer screening intervention was for women aged 25–65. A catch-up vaccination rate of 5%, corresponding to the voluntary vaccination rate, was implemented for girls aged 17–25. Given that women aged ≤25 are seldomly afflicted by cervical concerns in ChinaCitation43–45 and prevalence calibration data were only available for >25 women, only women aged >25 were simulated in the intervention projection module (: high-risk and low-risk two branches). The population size of women aged >25 was estimated from the census statistics released by the National Bureau of Statistics of China.Citation46 In 2015, approximately 632 million Chinese women were registered in census surveys, and 69.1% of whom aged >25. Prospectively, the population size will shrink over the next few decades, the size of women aged >25 is estimated to be around 350 million by 2069.Citation47 We anticipated that the population structure in China would change over the next 50 y. Population growth rate matched with current mortality rate were both set as constants, to couple with prospective population size estimation. Since the group of vaccinated schoolgirls were not included in the intervening projection compartment (: compartment V), we calculated the vaccinated female population size who would enter their 25 and inputted this number to the intervening projection compartment on a yearly basis to realize the vaccination intervention (: high-risk and low-risk branches).
Intervention strategy
The current coverage of the 3-y cervical cancer screening program was around 20% in women,Citation21 but no population-level study has yet reported any HPV vaccination coverage for China. Therefore, for intervention projection, we allowed qHPV vaccination coverage to vary between 0% and 90%, and the 3-y cervical cancer screening coverage to vary between 20% and 70% (each by 1% increment). In each intervening scenario, the median with a 95% confidence intervals (95% CIs) of the total number of CIN2/3 cases, cervical cancer cases, cervical cancer deaths, genital wart cases, and cervical cancer DALYs prevented was calculated. For each intervening scenario, the number of more cases reverted beyond which in the baseline scenario (20% screening) was also recorded.
Population impact and cost-effectiveness analysis (CEA)
Cost-effectiveness ratios were calculated for eleven intervening combinations (three screening-only, two vaccination-only, and six combined interventions) in a 50-y time window. We used the ‘Disability Adjusted Life Years (DALYs) prevented’ as the indicator to assess the intervention effectiveness.Citation48 Cervical cancer screening cost, vaccination cost, and treatment cost (genital wart/CIN1/CIN2/CIN3/cervical cancer) are three major components for medical expenditure calculation (Table S4). We used the currency exchange rate in 2020Citation49 and the yearly price discounting rate of 3% to estimate the cost in US Dollar (US$). The World Health Organization (WHO) recommended 1 and 3 times the GDP per capita (US$ 10,264 in 2020, appendix) as thresholds of ‘very cost-effective’ and ‘cost-effective’ for public health interventions, and the horizontal threshold equals to zero as cost-saving.Citation50 Incremental Cost-Effectiveness Ratio (ICER) was calculated as ‘Incremental cost per DALY prevented’ in this study. The accumulated cases of cervical cancers, genital warts, cervical cancer deaths, and DALYs, as well as the overall number of cervical cancers, genital warts, death cases, and DALYs prevented, with their 95% CIs, were reported in each intervention scenario. We also varied the qHPV price, by discounting the current price at a rate varying from 10% to 90% (by 10% increment).
Budget analysis for cervical cancer prevention
Based on the official report from the Chinese government, the current annual budget for cervical cancer prevention is estimated around ~US$ 47.8 million, which is the amount of funding to maintain a 20% cervical cancer screening coverage every 3 y.Citation51 In high-income countries, such as the USA, cervical cancer screening coverage is maintained at 60–70% among adult women.Citation52 With a fixed yearly fund of US$ 47.8 million, we searched potential strategy combinations (at step length of 1% for the coverage of each intervention) of cervical cancer screening scale-up and universal schoolgirl vaccination to identify the ‘optimal strategy’. The ‘optimal strategy’ was defined as the strategy that would result in the most DALYs prevented given the fixed amount of funding (US$ 47.8 m).
Uncertainty and sensitivity analysis
A probability sensitivity analysis was also performed on cost fluctuation and uncertainties around prevalence variations. A Latin Hypercubic sampling method was adopted to randomly extract values from price ranges, and epidemiological parameter ranges to construct parametric matrices for simulation. One-thousand random scenarios were generated to visualize the uncertainty, as shown in . Another univariate sensitivity analysis with delayed HPV vaccination (initiated in 2025 and 2030, Figure S5-6) were conducted to investigate the impact of delayed initiation on HPV epidemics. In addition to a 50-y cost-effective analysis, we also performed an analysis in a 20-y time window (Table S5.1). We projected the alternative vaccination intervention at the same strenth by replacing qHPV to bHPV, to contrast the effectiveness between vaccines. bHPV derived results were included in the Appendix (Table S5.2). Our intervention projection did not include nHPV due to its high commercial price and limited availability in mainland China in 2020. The simulation of HPV natural disease progression and subsequent intervention projection were both conducted in MATLAB R2019a.
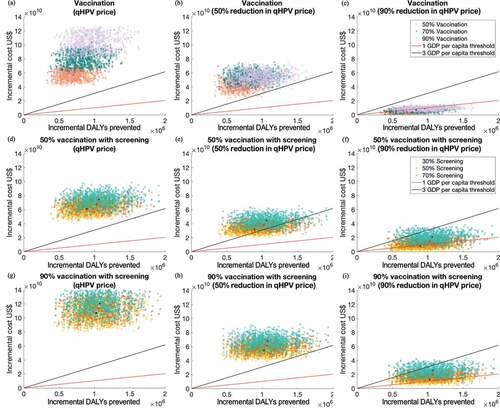
Figure 3. Two-dimensional cost-effectiveness plane demonstrating the distribution of 1,000 simulations for incremental cost and DALYs prevented under intervening strategies with different vaccine prices. (a–c): single intervention with qHPV at different price levels; (d)-(f) 50% vaccination with scale-up screening strategies; (g–i): 90% vaccination with scale-up screening strategies
Results
Projected HPV infection in the status quo
Our model indicated that at status quo (3-y screening coverage of 20%), the prevalence of HR-HPV in Chinese women would reduce from 16.0% in 2020 to 12.0% in 2069; cervical cancer incidence would decrease from 2.7/100,000 to 2.3/100,000, and the mortality rate would decrease from 1.0/100,000 to 0.7/100,000. In contrast, the genital wart incidence would only decline slightly from 0.046% to 0.042% during this period ().
Projected HPV infection and CEA in the scaled-up cervical cancer screening scenario
Increasing cervical cancer screening coverage to 30% would reduce the prevalence of HR-HPV to 10.6% in 2069, preventing 427 (95% CI: 129 to 1,145) cervical cancer cases and 360 (95% CI: 113 to 943) deaths annually in 2020–2069. Increasing screening coverage to 50% or 70% would further reduce the prevalence to 5.4% and 2.5%, preventing 856 (95% CI: 265 to 2,273) and 1,028 (95% CI: 323 to 2,721) cervical cancer cases, 771 (95% CI: 240–2,003) and 983 (95% CI: 311 to 2,550) deaths annually. The screening scale-up revealed a minimal impact on genital wart in these scenarios (, ).
Table 1. Cost-effective analysis of qHPV vaccination and screening strategies with a 50-year forecast, 2020 – 2069
Scaling-up cervical cancer screening alone would be cost-effective and even cost-saving (ICER of US$ 5,429 [95% CI: −9,104 to 18,676] per DALY prevented, ). A screening with 30% coverage would require maximumly an extra US$ 8 billion over the next five decades, but expect to reduce 8,500 (95% CI: 4,497 to 13,906) DALYs each year, corresponding to an ICER of US$5,470 (95% CI: −9,116 to 18,663) per DALY prevented. In contrast, increasing the screening coverage to 50% or 70% would result in ICERs of US$ 10,958 (95% CI: 169 to 26,973) and 15,673 (95% CI: 3,212 to 37,021) for each DALY prevented, respectively (). Probabilistic sensitivity analyses indicated that 99.6–100.0% of these scenarios (70–30% coverage) were cost-effective, and 50.3–88.3% were very cost-effective (, Figure S7.1).
Projected HPV infection and CEA in where HPV vaccination implemented at the baseline level of cervical cancer screening
With the current cervical cancer screening coverage, vaccinating 50% schoolgirls would reduce the prevalence of HR-HPV from 16.0% to 2.9% by 2069, preventing 363 (95% CI: 115 to 861) cervical cancer cases and 148 (95% CI: 50 to 342) deaths annually from 2020 to 2069 due to the lag effect of vaccination. Vaccinating 70% or 90% schoolgirls would reduce HR-HPV prevalence to 1.4% and 0.3%, and would prevent 468 (95% CI: 147 to 1,125) and 631 (95% CI: 194 to 1,539) cervical cancer cases, 194 (95% CI: 65 to 445) and 281 (95% CI: 92 to 674) deaths annually, respectively. LR-HPV could be nearly eradicated (<0.1%) in four decades with qHPV vaccination at a 50% coverage level ().
Schoolgirl vaccination programs could hardly be cost-effective under the current high price of the qHPV vaccine. Vaccinating 50% of schoolgirls would require an extra US$ 57 (45 to 68) billions over the next five decades. This strategy would reduce 13,854 (95% CI: 8,355 to 20,776) DALYs in each year, corresponding to an ICER of US$ 83,043 (95% CI: 52,234 to 138,025) for each prevented DALY. Vaccinating 70% and 90% schoolgirls would reduce 16,427 (95% CI: 9,594 to 24,858) and 18,992 (95% CI: 10,692 to 29,032) DALYs annually, corresponding to an ICER of US$ 98,100 (95% CI: 61,800 to 168,200) and 110,500 (95% CI: 69,000 to 192,700) for each DALY (). Whereas, in the vaccination-only scenarios where the qHPV cost was 90% discounted, the ICERs decreased to US$ 6,541 (95% CI: 512 to 13,204), 8,190 (95% CI: 2,796 to 15,421) and 9,558 (95% CI: 4,241 to 17,179) for 50%, 70%, and 90% coverage levels, respectively (), ).
Combining the HPV vaccination program with scaled-up cervical cancer screening
We assessed multiple combined vaccination and screening strategies. In particular, vaccinating 50% schoolgirls and screening 50% women for cervical cancer would prevent 963 (95% CI: 302 to 2,528) cervical cancer cases, 820 (95% CI: 258 to 2,128) deaths, 8,390 (95% CI: 5,401 to 11,026) genital warts and avert 20,138 (95% CI: 11,468 to 31,090) DALYs in each year from 2020 to 2069. Genital wart incidence would decline significantly with the increase of vaccination coverage; cervical cancer cases and cervical cancer deaths are more sensitive to variations in cervical cancer screening coverage (Figure S4).
Budget analysis of the combined interventions for an optimal strategy
The optimal intervention strategy varied with vaccine price discounting. If the current annual budget for HPV interventions would increase to 3 times of the current level (that is, US$ 143.4 million/year), and the vaccine price remains unchanged, our model indicated that all investments should be directed to cervical cancer screening. This would allow 67% women to receive cervical cancer screening in a 3-y interval, and 950,000 DALYs would be prevented in five decades. In comparison, with a 90% vaccine price deduction, the strategy of 40% screening coverage and 65% vaccination coverage would result in the largest DALY reduction of 1,017,000 units over the next 50 y (, Figure S4).
Discussion
Our analysis indicates that the current cervical cancer screening program targeting adult women will moderately reduce HPV infections and cervical cancer cases over the next five decades. The addition of a universal schoolgirl vaccination program using qHPV is effective in reducing more than half of cervical cancer incidence by 2069. However, given the high price of the vaccine, HPV prevention of cervical cancer should focus on cervical cancer screening to maximize its attainable coverage for now. A schoolgirl vaccination program can approach the cost-effective threshold with a 50% vaccine price deduction and could be very cost-effective with a 90% vaccine price reduction. With an anticipated three times increase in government investment for HPV prevention, we found that the optimal strategy in the context of a 90% deduction in qHPV price, was to have a 65% vaccination coverage and 40% screening coverage. This scenario would result in the aversion of an extra 67,000 DALYs than the current optimal strategy (i.e., 67% screening coverage) with no reduction in vaccine price (950,000 DALYs). An HPV vaccination alone without cervical cancer screening for adult women will not be very effective for cancer prevention.
The consensus from the published literature is that adding HPV vaccination to existing cervical cancer screening program is the most effective strategy for reducing new cancer cases and cervical cancer mortality.Citation29–33,Citation53 Our study adds to the cumulating evidence that combined interventions of schoolgirl vaccination and the scale-up of cervical cancer screening programs will result in a significant reduction in both new infections and cervical cancer mortality.Citation29,Citation30,Citation32 The optimal price of HPV vaccines for Chinese women remains a matter of debate. Some modeling studiesCitation30,Citation31 suggest that HPV vaccines at the current price have significant population impact, while others argue that the vaccine price needs to be at least 50–90% lower to be financially feasible.Citation32,Citation33,Citation53,Citation54 Our study finding seems to be consistent with the latter.
A substantial reduction in HPV vaccine price is necessary to guarantee the feasibility of a universal vaccination program. However, such a reduction is unlikely if all vaccines are imported. The commercial quadrivalent HPV vaccines have been very popular among urban women since they entered the Chinese market, but they are under-supply in mainland China. Women often have to wait up to 12 months to schedule their first injection.Citation55 Confined by these circumstances, the implementation of a universal schoolgirl HPV vaccination program seems not to be possible. It is likely that the domestically developed bivalent vaccines Cecolin will dramatically change this situation. First, according to the manufacturing of Gardasil it costs around US$2.5 per dose,Citation56 which is only 0.6% of the current market price of qHPV in China (US$450), demonstrating room for a substantial reduction. The expiration of the patent for Gardasil qHPV production in 2023 also serves as another good stimulus for local manufacturer production.Citation57 The domestic vaccine Cecolin became available in community hospitals in multiple provinces for purchase and injection in May 2020.Citation58 The market price for CecolinCitation59 (about US$ 46.5/dose) is much lower than the comparable imported Cervarix vaccine. State procurement of a large amount of the vaccine for a vaccination program will likely further reduce the price. Second, as the Chinese government stipulates, the production of mandatory vaccines for children should be under the regulation of the Chinese National Vaccination Scheme.Citation60 Domestic vaccines will face less resistance in implementation at the regulation and operation levels.
A high HPV vaccination coverage is necessary to achieve the goal of HPV elimination in China. Our study indicates that at a 90% vaccine price reduction, a 65% vaccination and 40% screening coverage may result in the best economic outcome. Consistent with previous studies,Citation26,Citation61 our finding indicates that although increasing cervical cancer screening could be cost-effective without a universal HPV vaccination program, screening alone cannot eliminate cervical cancer. First, cervical cancer screening only detects HPV infection after the infection occurs and thus cannot prevent the initial acquisition of HPV. Once infected, women are facing the risk of persistent HPV infections without instant and effective management.Citation62 In contrast, HPV vaccination reduces HPV acquisition risk in schoolgirls before their sexual debut, lowering the risk of cervical cancer in their later lives.Citation63 Second, cervical cancer screening coverages vary substantially across populations with various socioeconomic backgrounds.Citation64 People from resource-limited settings are less likely to accept regular screening.Citation65 Hence, a state-funded universal vaccination program can provide relatively fair access to HPV prevention to disadvantaged groups and complement the access inequality in cervical cancer screening.
Our studies have several limitations. First, data for calibration were obtained from a secondary source, given that access to first-hand surveillance data for HPV is restricted in China. Our modeling estimation may not adequately reflect the HPV prevalence trend in recent years. Also, as data were extracted from peer-reviewed articles, most cervical cancer screening test results were only available in women age >25 and thus not very representative for the entire population. Second, we did not include the cross-protective effect of the vaccine over other untargeted genotypes when simulating the consequences of HPV persistence; therefore, we may underestimate the vaccine efficacy against HPV infection. HPV prevalence differs by region of residence.Citation66 Although the HPV prevalence was higher in rural women than in urban women,Citation4 that difference was not statistically significant in our previous analysis. Therefore, we did not stratify the population by their residence in this model. Third, cervical cancer incidence increases with age. China has a rapidly aging population, and the birth control policy is expected to change in the future, yet we assumed a fixed age structure over time in this model. Fourth, we assumed a homogenous cervical cancer screening sensitivity in the simulated population. However, the adoption of screening test varies by graphical regions, and the variation was not captured in this model. Fifth, we only assessed the preventive effects of a single type of HPV vaccine (bHPV or qHPV) in each scenario but did not consider the mixed usage, which is close to the real situation.
In conclusion, our study indicates that scaling-up cervical cancer screening in adult women is the most affordable strategy to maximize health benefits. Vaccinating schoolgirls will result in a large population benefit in the long term, but a universal HPV vaccination program can only be cost-effective with a substantial price reduction. Domestic vaccines may potentially be the game-changer.
Disclosure of potential conflicts of interest
Listed authors had no conflict of interest to report.
Supplemental Material
Download MS Word (86.9 MB)Supplementary material
Supplemental data for this article can be accessed online at http://dx.doi.org/10.1080/21645515.2020.1832835.
Additional information
Funding
References
- Centers for Disease Control and Prevention. Basic Information about HPV and Cancer. 2018 Aug 22. [accessed 2020 Jun 11]. https://www.cdc.gov/cancer/hpv/basic_info/index.htm.
- Senkomago V. Human papillomavirus–attributable cancers—United States, 2012–2016. MMWR Morb Mortal Wkly Rep. 2019 Aug 23;68(33):724–28. doi:10.15585/mmwr.mm6833a3. PMID: 31437140.
- Laprise C, Trottier H, Monnier P, Coutlée F, Mayrand MH. Prevalence of human papillomaviruses in semen: a systematic review and meta-analysis. Hum Reprod. 2013 Dec 22;29(4):640–51. doi:10.1093/humrep/det453. PMID: 24365799.
- Ma X, Wang Q, Ong JJ, Fairley CK, Su S, Peng P, Jing J, Wang L, Soe NN, Cheng F, et al. Prevalence of human papillomavirus by geographical regions, sexual orientation and HIV status in China: a systematic review and meta-analysis. Sex Transm Infect. 2018 Sep;94(6):434–42. doi:10.1136/sextrans-2017-053412. PMID: 29794242.
- Gu XY, Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen WQ, He J. Incidence and mortality of cervical cancer in China, 2014. Zhonghua Zhong Liu Za Zhi [Chin J Oncol]. 2018 Apr;40(4):241–46. doi:10.3760/cma.j..0253-3766.2018.04.001. PMID: 297309088.
- Yanofsky VR, Patel RV, Goldenberg G. Genital warts: a comprehensive review. J Clin Aesthet Dermatol. 2012 Jun;5(6):25–36. PMID: 22768354.
- Cutts FT, Franceschi S, Goldie S, Castellsague X, de Sanjose S, Garnett G, Edmunds WJ, Claeys P, Goldenthal KL, Harper DM, et al. Human papillomavirus and HPV vaccines: a review. World Health Organization. 2007 Sep 1. [accessed 2020 Mar 23]. https://www.who.int/bulletin/volumes/85/9/06-038414/en/.
- Castellsague X, Munoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, Luna J, Myers E, Mallary S, Bautista OM, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br J Cancer. 2011 Jun 28;105(1):28–37. doi:10.1038/bjc.2011.185. Epub 2011 May 31. PMID: 21629249.
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED Jr, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015 Feb 19;372(8):711–23. doi:10.1056/NEJMoa1405044. PMID: 25693011.
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled trial. Lancet. 2004 Nov 13–19;364(9447):1757–65. doi:10.1016/S0140-6736(04)17398-4. PMID: 15541448.
- Gellin B, Modlin JF, Barr E, Tamms G. Quadrivalent human papillomavirus vaccine. Clin Infect Dis. 2007 Sep 1;45(5):609–7. doi:10.1086/520654. PMID: 17682997.
- World Health Organization. Immunization, vaccines and biologicals. 2019 Nov 19. [accessed 2018 Oct 18]. http://www.who.int/immunization/global_vaccine_action_plan/en/.
- Garland SM, Cornall AM, Brotherton JM, Wark JD, Malloy MJ, Tabrizi SN. VACCINE study group. Final analysis of a study assessing genital human papillomavirus genoprevalence in young Australian women, following eight years of a national vaccination program. Vaccine. 2018 May 31;36(23):3221–30. doi:10.1016/j.vaccine.2018.04.080. PMID: 29724506.
- Chow EP, Read TR, Wigan R, Donovan B, Chen MY, Bradshaw CS, Fairley CK. Ongoing decline in genital warts among young heterosexuals 7 years after the Australian human papillomavirus (HPV) vaccination programme. Sex Transm Infect. 2015 May;91(3):214–19. doi:10.1136/sextrans-2014-051813. PMID: 25305210.
- Dyda A, Shah Z, Surian D, Martin P, Coiera E, Dey A, Leask J, Dunn AG. HPV vaccine coverage in Australia and associations with HPV vaccine information exposure among Australian Twitter users. Hum Vaccin Immunother. 2019;15(7–8):1488–95. doi:10.1080/21645515.2019.1596712. PMID: 30978147.
- Albeck-Ripka L Australia could become first country to eradicate cervical cancer. New York Times. 2018 Oct 3 [accessed 2018 Oct 18]. https://www.nytimes.com/2018/10/03/world/australia/cervical-cancer-hpv-vaccine.html.
- Zhang Y, Wang Y, Liu L, Fan Y, Liu Z, Wang Y, Nie S. Awareness and knowledge about human papillomavirus vaccination and its acceptance in China: a meta-analysis of 58 observational studies. BMC Public Health. 2016 Mar 3;16:216. doi:10.1186/s12889-016-2873-8. PMID: 26936076.
- Zhao FH, Tiggelaar SM, Hu SY, Zhao N, Hong Y, Niyazi M, Gao XH, Ju LR, Zhang LQ, Feng XX, et al. A multi-center survey of HPV knowledge and attitudes toward HPV vaccination among women, government officials, and medical personnel in China. Asian Pac J Cancer Prev. 2012;13(5):2369–78. doi:10.7314/apjcp.2012.13.5.2369. PMID: 22901224.
- Wang B, He M, Chao A, Engelgau MM, Saraiya M, Wang L, Wang L. Cervical cancer screening among adult women in China, 2010. Oncologist. 2015 Jun;20(6):627–34. doi:10.1634/theoncologist.2014-0303. PMID: 25956407.
- Wang SM, Qiao YL. Implementation of cervical cancer screening and prevention in China—challenges and reality. Jpn J Clin Oncol. 2015 Jan;45(1):7–11. doi:10.1093/jjco/hyu188. PMID: 25398583.
- Bao H, Zhang L, Wang L, Zhang M, Zhao Z, Fang L, Cong S, Zhou M, Wang L. Significant variations in the cervical cancer screening rate in China by individual‐level and geographical measures of socioeconomic status: a multilevel model analysis of a nationally representative survey dataset. Cancer Med. 2018 May;7(5):2089–100. doi:10.1002/cam4.1321. PMID: 29573569.
- Sankaranarayanan R, Qiao YL, Keita N. The next steps in cervical screening. Womens Health (Lond). 2015 Mar;11(2):201–12. doi:10.2217/whe.14.70. PMID: 25776294.
- Hestbech MS, Lynge E, Kragstrup J, Siersma V, Baillet MV, Brodersen J. The impact of HPV vaccination on future cervical screening: a simulation study of two birth cohorts in Denmark. BMJ Open. 2015 Aug 14;5(8):e007921. doi:10.1136/bmjopen-2015-007921. PMID: 26275901.
- Zou Z, Fairley CK, Ong JJ, Hocking J, Canfell K, Ma X, Chow EP, Xu X, Zhang L, Zhuang G. Domestic HPV vaccine price and economic returns for cervical cancer prevention in China: a cost-effectiveness analysis. The Lancet Global Health. 2020 Oct 1;8(10):e1335–44.
- Soe NN, Ong JJ, Ma X, Fairley CK, Latt PM, Jing J, Cheng F, Zhang L. Should human papillomavirus vaccination target women over age 26, heterosexual men and men who have sex with men? A targeted literature review of cost-effectiveness. Human vaccines & immunotherapeutics. 2018 Dec 2;14(12):3010–8.
- Wang Q, Ma Z, Zhang X, Ong JJ, Jing J, Zhang L, Wang LH. Human papillomavirus infection and associated factors for cervical intraepithelial neoplasia in women living with HIV in China: a cross-sectional study. Sexually transmitted infections. 2019 Mar 1;95(2):140–4.
- Fairley CK, Zou H, Zhang L, Chow EP. Human papillomavirus vaccination in men who have sex with men–what will be required by 2020 for the same dramatic changes seen in heterosexuals. Sexual Health. 2017 Feb 1;14(1):123–5.
- Van de Velde N, Brisson M, Boily MC. Understanding differences in predictions of HPV vaccine effectiveness: A comparative model-based analysis. 2010 Jul 26;28(33);5473–84. doi:10.1016/j.vaccine.2010.05.056. PMID: 20573580.
- Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007 Jan;13(1):28–41. doi:10.3201/eid1301.060438. PMID: 17370513.
- Mo X, Tobe RG, Wang L, Liu X, Wu B, Luo H, Nagata C, Mori R, Nakayama T. Cost-effectiveness analysis of different types of human papillomavirus vaccination combined with a cervical cancer screening program in mainland China. BMC Infect Dis. 2017 Jul 18;17(1):502. doi:10.1186/s12879-017-2592-5. PMID: 28720082.
- Choi HC, Jit M, Leung GM, Tsui KL, Wu JT. Simultaneously characterizing the comparative economics of routine female adolescent nonavalent human papillomavirus (HPV) vaccination and assortativity of sexual mixing in Hong Kong Chinese: a modelling analysis. BMC Med. 2018 Aug 17;16(1):127. doi:10.1186/s12916-018-1118-3. PMID: 30115065.
- Song XB, Zhao QJ, Zhou Z, Fang Y. Health economic evaluation of bivalent human papilloma virus vaccine in China: based on the dynamic model. Zhonghua Yu Fang Yi Xue Za Zhi [Chin J Preventive Med]. 2017 Sep 6;51(9):814–20. doi:10.3760/cma.j..0253-9624.2017.09.008. PMID: 28881547.
- Zhang Q, Liu YJ, Hu SY, Zhao FH. Estimating long-term clinical effectiveness and cost-effectiveness of HPV 16/18 vaccine in China. BMC Cancer. 2016 Nov 4;16(1):848. doi:10.1186/s12885-016-2893-x. PMID: 27814703.
- Cai J, Yang Y. The results analysis of uterine cervical cancer screening in 753310 cases. China Med Herald. 2012;13:134–36.
- Cao J, Han H, Li X. Analysis of the prevalence of women’s diseases in Hubei Province from 1998 to 2003. Maternal Child Health Care China. 2005;20:307–08.
- Wang L, Zhang Y, Wang C, Han L. Analysis of surveillance status of common sexually transmitted diseases among females in Beijing from 2005 to 2010. Chin J Gen Pract. 2011;25:2923–25.
- Geng Q, Sun Z, Lu Y, Wang Q, Li N. Study of the prevalence of RTI/STD and related factors among bearing-age married women in rural areas of Meixian and Huayin of Shaanxi Province. J Xi’an Jiaotong Univ (Med). 2007;28:220–24.
- Wu H. Analysis of reproductive tract infections and women’s diseases in 47,538 rural women in Jianshui County. Chin Commun Doctors. 2012;14:300–01.
- Wang F. Investigation and analysis of 8,529 cases of reproductive tract infections in married women of childbearing age in rural areas. J Henan Med Coll Staff Workers. 2012;24:34–36.
- Expert Committee of China Eugenics Association Colposcopy and Cervical Pathology Branch. Consensus on Cervical cancer screening abnormality regulation. Chin J Clin Obstet Gynecol. 2017 Mar;18(2):190–92. [accessed 2018 Jan 14]. http://www.cdgrlyy.org/common/attached/file/20180614/20180614172446_54232.pdf.
- American Cancer Society. Signs and symptoms of cervical cancer. 2020 Jan 3 [accessed 2020 Jul 18]. https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/signs-symptoms.html.
- Lv W Cervical Intraepithelial Neoplasias(CIN) Clinical Treatment Guideline (ASCCP). Zhejiang Province gynaecology Annual Conference; 2008; Zhejiang Province. [accessed 2018 Jan 14]. http://cpfd.cnki.com.cn/Article/CPFDTOTAL-ZJKX200811001007.htm.
- Li X, Zheng R, Li X, Shan H, Wu Q, Wang Y, Chen W. Trends of incidence rate and age at diagnosis for cervical cancer in China, from 2000 to 2014. Chin J Cancer Res. 2017 Dec;29(6):477–86. doi:10.21147/j..1000-9604.2017.06.02. PMID: 29353970.
- Li J, Kang LN, Qiao YL. Review of the cervical cancer disease burden in mainland China. Asian Pac J Cancer Prev. 2011 Jan 1;12(5):1149–53. PMID: 21875257.
- Wen X, Wen D, Yang Y, Chen Y, Akazawa K, Liu Y, Shan B. Urban-rural disparity in cervical cancer in China and feasible interventions for tackling the rural excess. Medicine (Baltimore). 2019 Jan;98(1):e13907. doi:10.1097/MD.0000000000013907.
- Census Office. Tabulation of the 2010 population census of the people republic of China. Stat.gov; 2010 [accessed 2018 Dec 3]. http://www.stats.gov.cn/tjsj/pcsj/rkpc/6rp/indexch.htm.
- Yi F, Su J. The fertility wishes and population policy-from the perspective of the practice of two children policy. China Dev Obs. 2018;12:58–74.
- Health statistics and information systems. Metrics: disability-Adjusted Life Year (DALY). World Health Organization. [accessed 2018 Oct 18]. http://www.who.int/healthinfo/global_burden_disease/metrics_daly/en/.
- CEIC Data. China GDP per capita. [accessed 2020 Feb 20]. https://www.ceicdata.com/en/indicator/china/gdp-per-capita.
- World Health Organization. Cost effectiveness and strategic planning (WHO-CHOICE). [accessed 2018 Oct 18]. http://www.who.int/choice/cost-effectiveness/en/.
- Investment on breast cancer and cervical cancer screening. Shanghai municipal commission of health and family Planning. [accessed 2018 Oct 18]. http://www.wsjsw.gov.cn/wsj/n429/n432/n1487/n1511/u1ai82600.html.
- Watson M, Benard V, Flagg EW. Assessment of trends in cervical cancer screening rates using healthcare claims data: United States, 2003–2014. Prev Med Rep. 2018 Feb 2;9:124–30. doi:10.1016/j.pmedr.2018.01.010. PMID: 29527465.
- Jiang Y, Ni W, Wu J. Cost-effectiveness and value-based prices of the 9-valent human papillomavirus vaccine for the prevention of cervical cancer in China: an economic modelling analysis. BMJ Open. 2019 Nov 24;9(11):e031186. doi:10.1136/bmjopen-2019-031186. PMID: 31767588.
- Levin CE, Sharma M, Olson Z, Verguet S, Shi JF, Wang SM, Qiao YL, Jamison DT, Kim JJ, Gelband H, et al. An extended cost-effectiveness analysis of publicly financed HPV vaccination to prevent cervical cancer in China. Vaccine. 2015 Jun 4;33(24):2830–41. doi:10.1596/978-1-4648-0349-9_ch18. PMID: 26913341.
- The market size of HPV vaccine is expected to exceed 30 billions. Xinhua Net; 2017 Dec 18 [accessed 2019 Jan 14]. http://www.xinhuanet.com//fortune/2017-12/18/c_1122124456.htm.
- Clendinen C, Zhang Y, Warburton RN, Light DW. Manufacturing costs of HPV vaccines for developing countries. Vaccine. 2016 Nov 21;34(48):5984–89. doi:10.1016/j.vaccine.2016.09.042. PMID: 27771183.
- Fair A Shot for vaccine affordability – understanding and addressing the effects of patents on access to newer vaccines. 2019 Jul [accessed 2019 Jan 14]. https://msfaccess.org/sites/default/files/VAC_report_A%20Fair%20Shot%20for%20Vaccine%20Affordability_ENG_2017.pdf.
- China focus: Chinese HPV vaccine to be available in May. Xinhua Net; 2020 Apr 26 [accessed 2020 Jun 11]. http://www.xinhuanet.com/english/2020-04/26/c_139009381.htm.
- Qiao YL, Wu T, Li RC, Hu YM, Wei LH, Li CG, Chen W, Huang SJ, Zhao FH, Li MQ, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine: an interim analysis of a randomized clinical trial. J Natl Cancer Inst. 2020 Feb 1;112(2):145–53. doi:10.1093/jnci/djz074. PMID: 31086947.
- HPV vaccine market will exceed 100 billions. SINA Finance and Economics; 2018 Jul 28 [accessed 2018 Oct 18]. https://finance.sina.cn/stock/ssgs/2018-07-28/detail-ihfxsxzf7904104.d.html?cre=tianyi&mod=wpage&loc=2&r=32&doct=0&rfunc=91&tj=none™32&cref=cj.
- Lew JB, Simms KT, Smith MA, Hall M, Kang YJ, Xu XM, Caruana M, Velentzis LS, Bessell T, Saville M, et al. Primary HPV testing versus cytology-based cervical screening in women in Australia vaccinated for HPV and unvaccinated: effectiveness and economic assessment for the National Cervical Screening Program. Lancet Public Health. 2017 Feb 2:(2):e96–e107. doi:10.1016/S2468-2667(17)30007-5. PMID: 29253402.
- Hoffman SR, Le T, Lockhart A, Sanusi A, Dal Santo L, Davis M, McKinney DA, Brown M, Poole C, Willame C, et al. Patterns of persistent HPV infection after treatment for cervical intraepithelial neoplasia (CIN): A systematic review. Int J Cancer. 2017 Jul 1;141(1):8–23. doi:10.1002/ijc.30623. PMID: 28124442.
- Canadian Task Force on Preventive Health Care. Recommendations on screening for cervical cancer. CMAJ. 2013 Jan 08;185(1):13–14. doi:10.1503/cmaj.121505.
- Johnson HC, Lafferty EI, Eggo RM, Louie K, Soldan K, Waller J, Edmunds WJ. Effect of HPV vaccination and cervical cancer screening in England by ethnicity: a modelling study. Lancet Public Health. 2018 Jan;3(1):e44–e51. doi:10.1016/S2468-2667(17)30238-4. PMID: 29307388.
- Llanos AA, Tsui J, Rotter D, Toler L, Stroup AM. Factors associated with high-risk human papillomavirus test utilization and infection: a population-based study of uninsured and underinsured women. BMC Womens Health. 2018 Oct 3;18(1):162. doi:10.1186/s12905-018-0656-3. PMID: 30285820.
- Sabeena S, Bhat PV, Kamath V, Bhat SK, Nair S. Community-based prevalence of genital human papillomavirus (HPV) infection: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2017 Jan 1;18(1):145–54. doi:10.22034/APJCP.2017.18.1.145. PMID: 28240509.