ABSTRACT
No proven remedy is identified for COVID-19 yet. SARS-CoV-2, the viral agent, is recognized by some endosomal and cytosolic receptors following cell entry, entailing innate and adaptive immunity stimulation, notably through interferon induction. Impairment in immunity activation in some patients, mostly elderlies, leads to high mortalities; thus, promoting immune responses may help. BCG vaccine is under investigation to prevent COVID-19 due to its non-specific effects on the immune system. However, other complementary immune-induction methods at early stages of the disease may be needed. Here, the potentially preventive immunologic effects of BCG and influenza vaccination are compared with the immune response defects caused by aging and COVID-19. BCG co-administration with interferon-α/-β, or influenza vaccine is suggested to overcome its shortcomings in interferon signaling against COVID-19. However, further studies are highly recommended to assess the outcomes of such interventions considering their probable adverse effects especially augmented innate immune responses and overproduction of proinflammatory mediators.
1. Introduction
The novel coronavirus disease 2019 (COVID-19) was announced as a pandemic by the World Health Organization (WHO) shortly after its first report in Dec. 2019 in Wuhan city, China.Citation1 While worldwide enormous efforts are ongoing to find preventive and/or therapeutic approaches for COVID-19, its huge burden on countries and societiesCitation2 has prompted politicians to look for solutions as well. Donald Trump asked a while ago whether the flu vaccine combats the novel coronavirus, which was answered with a direct NO! Although this naïve question was raised because of a lack of knowledge about vaccination basics, it seems that the answer to his question could probably be yes!
Recently, it was suggested that the booster BCG (Bacillus Calmette–Guérin) vaccine, could have beneficial effects on preventing COVID-19 infectionCitation3 and reducing the incidence and severity of COVID-19 in previously BCG-vaccinated groups through its non-specific effects (NSEs). It was also proposed that the differences in COVID-19 severity amongst countries could be, to some extent, explained by various national policies on BCG children vaccination.Citation4,Citation5 However, this claim was questioned by some other studies.Citation6,Citation7
In this context, an open-label two-group phase III randomized controlled trial was first begun in up to 4170 healthcare workers in Australia. It is currently ongoing and aims to reveal the possible preventive effects of BCG vaccination against COVID-19.Citation8 Presently, one observational and seven interventional clinical trials are also being conducted on this subject, which are briefed in .Citation9
Table 1. Clinical trials on BCG vaccine usage for prevention of COVID-19, Available from: https://clinicaltrials.gov/at 2020 September 1
The observed more efficient and improved immune responses against reinfections in the plants and invertebrate that lack adaptive immunity, and surprisingly in some mammals, encouraged researchers to investigate whether this process could also occur in humans.Citation10 Thus, several epidemiological studies and clinical trials were performed to explore the post-effects of vaccination with live-attenuated vaccines (LAVs), which mimic a natural infection. Though, the capability of LAVs in generating a sort of cross-protection against some other non-related pathogens was established in several studies.Citation11 In this regard, the NSEs of several vaccines (BCG, DTP, and measles vaccines) have been investigated.Citation12 One primary mechanism behind this phenomenon was found to be the induction of long-lasting epigenetic changes in the innate immune cells, a process called “trained immunity”.Citation11 Besides, heterologous lymphocyte responses may partly account for some NSEs of vaccines.Citation13 On this point, antigen cross-reactivity and bystander activation of unrelated T and B cells are considered as possible mechanisms. The bystander lymphocytes could exert protective roles against unrelated heterologous pathogens through antigen-specific bystander responses or antigen-non-specific innate mechanisms, such as interferon (IFN)-γ production, which activates macrophages.Citation11,Citation13
The higher mortality rates in elderlies is a main concern in the COVID-19 pandemic, which is probably related to immunosenescence, or age-associated deterioration of immune functionality,Citation14 leading to suboptimal immune responses. In view of this, boosting the immune responses against SARS-CoV-2 could potentially reduce the disease incidence and severity in this population as well.
Herein, a model on the immune responses against COVID-19 is proposed first. Then, the Yin and Yang of the NSEs of LAVs are described with a focus on BCG and influenza vaccines. Finally, the probable supportive roles of these vaccines on the protection against COVID-19 would be discussed.
2. Interaction of SARS-CoV-2 with the immune system
A brief overview of immune responses to SARS-CoV-2 is represented in . SARS-CoV-2, similar to SARS-CoV, enters the cells through angiotensin-converting enzyme II (ACE2) receptor. It also uses transmembrane protease serine 2 (TMPRSS2) receptor to prime its spike (S) protein in target cells.Citation15,Citation16 TMPRSS2 plays a role in influenza virus pathogenicity as well by cleaving hemagglutinin.Citation17 Other yet-unknown cellular entry modes might also be involved in SARS-CoV-2 infection, especially considering that only minimal percentages of leukocytes express ACE2.Citation18–20
Figure 1. The schematic representation of immune responses against SARS-CoV-2. a. Optimal innate and adaptive immunity responses: The virus binds to ACE2 to infect cells. The engagement of pattern recognition receptors (PRRs) results in the production of interferons I and other proinflammatory mediators, which induces dendritic cells (DCs) to process antigens and present them to naïve circulating T cells, leading to T cell activation. The activated T cells migrate to the site of infection and secrete effector cytokines, such as IFN-γ. The APCs also induce B cells leading to optimal NAb production. b. Early and sub-optimal NAb activity results in antibody-dependent enhancement, leading macrophages to be exploited for virus replication. This process also causes elevated cytokine production and subsequent immunopathological overreactions
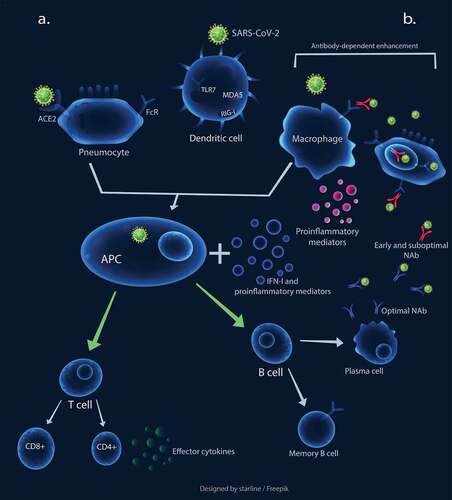
Host cells possibly recognize SARS-CoV-2, same as other RNA viruses, first by endosomal pattern recognition receptors, such as Toll-like receptor (TLR)3 and TLR7, as well as cytosolic RNA receptors, such as retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated protein (MDA) 5. IFN-α or IFN-β (hereafter referred as IFN-I) and other proinflammatory mediators are induced following recognition of the virus,Citation21 playing a crucial role in controlling CoV infections. Type-I IFNs could enhance the function of immune cells, including antigen-presenting cells (APCs), natural killer (NK) cells, T cells, and B cells.Citation22,Citation23
Lung resident respiratory dendritic cells (DCs) seem to process the acquired viral particles or antigens from the SARS-CoV-2-infected cells, then present it to the naïve circulating T cells in draining lymph nodes.Citation21,Citation24 The activated T cells, migrate to the infection site and secrete effector cytokines such as IFN-γ, which directly inhibits viral infection. Th1 type immune response seems to play a crucial role in the effective control of SARS-CoV infection. Higher rates of mortality was detected in patients with more serum Th2 cytokines.Citation21 The exposure of naïve T cells to IFN-I, IFN-γ, and IL-12 is vital for Th1 cell polarization.Citation25 Since neutralizing antibodies (NAbs) limit the viral infections, delayed and weak antibody production was also associated with poor clinical outcome in SARS.Citation20 Moreover, the timing of IFN-I induction also seems crucial for the fine-tuning of B cell activation and consequent NAb production.Citation22
3. Dysregulation of immune responses in COVID-19
Coronaviruses can circumvent the immune responses, particularly IFN-mediated antiviral responses, and replicate to reach a high peak load.Citation26 Similar to SARS and MERS, substantial dysregulations of immunological responses occur in SARS-CoV-2 infection.Citation20 Significant T cell lymphopenia, in particular CD4 + T cells, an elevated exhaustion level of T cells with reduced functionality, lower percentages of monocytes, eosinophils, and basophils, as well as higher neutrophil-lymphocyte-ratio (NLR), were observed in COVID-19 patients in different studies.Citation27–29 The elevated levels of IL-1B, IFN-γ, IFN-γ-inducible protein 10 kDa (CXCL10; IP10), monocyte chemoattractant protein (MCP)1, as well as IL-4 and IL-10, which are related to Th1 and Th2 responses, respectively, were also identified.
The enhanced proinflammatory cytokines, to some extent, could contribute to the increased infiltration and activation of leukocytes in the lungs and subsequent lethal pneumonia.Citation30,Citation31 Additionally, the elevated levels of IL-6 is considered as an early biomarker of the aggravation of COVID-19 clinical course, which is associated with cytokine storm.Citation32
Fu et al.Citation20 categorized SARS-CoV-2-mediated inflammatory responses into two different stages. The primary response, which occurs due to viral replication and consequently mounts the host antiviral responses and viral-induced ACE2 downregulation and shedding. The primary inflammatory response is almost tolerated by patients and has a protective role against the infection through viral load reduction or viral clearance.
These possible mechanisms, in turn, cause elevated levels of proinflammatory cytokines/chemokine and cellular damage due to apoptosis and pyroptosis,Citation33 shaping the second stage of immune responses. This stage is initiated with the generation of adaptive immunity and NAb secretion.Citation23 The produced NAbs could trigger Fc receptor-mediated antibody-dependent enhancement (ADE) (), which possibly occurs due to early and sub-optimal antibody activity. This process could cause persistent viral replication, skewing of the macrophage responses, and further exuberant inflammatory responses, and subsequent cellular damage and lung injury.Citation31 Noteworthy, the possible role of ADE in COVID-19 severity was also suggested by other researchers recently.Citation32
Besides, given the crucial role of T cells, in particular CD4+ and CD8 + T cells, in modulating the over-activated inflammatory responses, some studies suggested that suboptimal and decreased T cell numbers generated by SARS-CoV-2 infection may also result in weak cellular immunity and undesired inflammatory responses.Citation27,Citation34
3.1. The possible role of IFNs in dysregulated immune responses
The relative timing of the IFN-I response and maximal viral replication was shown to contribute to the disease severity in SARS and MERS.Citation30,Citation35 Notably, IFN therapy before the virus titer peak decreased inflammatory cytokines production and generated protective roles in MERS-CoV infected mice. In contrast, at the later stages of the disease (two or 4-days post-infection), enhanced proinflammatory cytokines, increased infiltration, and the higher total number of highly activated monocytes, macrophages, and neutrophils may result in fatal pneumonia.Citation35
The key role of dysregulated IFN-mediated immune responses in SARS-CoV-2 infection was uncovered as well. Despite heavier virus replication than SARS-CoV, SARS-CoV-2 does not significantly induce type-I, -II, or -III IFNs.Citation36 Consistent with this finding, it was revealed that IFN-I deficiency could be considered as a hallmark of severe COVID-19.Citation37,Citation38 Moreover, impaired IFN-mediated immune responses, with a reduced dynamic range, in response to direct pattern recognition receptor (PRR) stimulation and viral infection were uncovered in older individuals.Citation39 The impaired IFN antiviral responses could, to some extent, explain the poor clinical outcomes in elderlies.Citation32 In congruence with these studies, Hadjadj et.al suggested that IFN administration could be used to overcome the IFN-I deficiency issue. On the other hand, applying anti-inflammatory agents that target IL-6 or TNFα, could, to some extent, dampen the inflammation and subsequent immunopathogenic over-reactions.Citation38
4. Taking advantage of available vaccines against COVID-19
As mentioned before, using LAVs might help in reducing the risks of other infections through NSEs.Citation11 In the following, the possible heterologous effects of BCG and influenza vaccines will be discussed. The impact of BCG, influenza vaccine, and IFN-I on the immune system in comparison with the immunity dysregulation caused by COVID-19 or aging are presented in .
Table 2. The impact of BCG, influenza vaccine, and interferon (IFN)-I on the immune system compared with the dysregulation of immunity as a result of COVID-19 or aging
4.1. BCG vaccine
BCG vaccine is a LAV used in many countries early after birth against tuberculosis (TB) disease. It has been injected to over four billion people so far.Citation66 BCG vaccination is also recommended to reduce the mortalities of pandemics.Citation67
Numerous studies have indicated the non-specific protective effects of BCG vaccination against viral infections caused by both RNA and DNA viruses. For instance, BCG vaccination could reduce the risk of respiratory tract infections in elderlies and adolescents and substantially enhance the responsiveness to influenza A and hepatitis B vaccines.Citation67
The heterologous effects of BCG vaccination against viral infections may be exerted through several mechanisms. One fundamental mechanism is trained immunity induction. BCG vaccination could stimulate immunological memory in NK cells, monocytes, and macrophages.Citation46,Citation68 It also led to enhanced IFN-γ and monocyte-derived cytokines ‘four- to seven-fold’ and ‘two-fold’, respectively, in healthy volunteers.Citation69
The importance of rapid and robust innate immune responses, as the primary SARS-CoV-2-mediated inflammatory response, in reducing viral load or even viral clearance is well-established.Citation20 However, the raised production of proinflammatory cytokines after BCG vaccination and subsequent cellular damage due to apoptosis or proptosis should be considered.Citation20,Citation33
On the other hand, BCG vaccination could also induce non-specific lymphocyte responses through both cross-reactivity and bystander activation.Citation11,Citation13 For instance, BCG vaccination prior to pathogen insult or vaccination boosted the antibody-mediated responses.Citation46 Moreover, it could enhance the responsiveness of Th1 to non-specific secondary infections as well as Th17 corresponding cytokine induction, such as IFN-γ, up to 1 year.Citation46
The beneficial impacts of BCG vaccine on adaptive immunity could reasonably dampen the secondary inflammatory responses to SARS-CoV-2 and the consequent multi-organ failure.Citation13,Citation70
While there are multiple publications on BCG-induced NSEs for viral infections, there is a concern for its use in COVID-19 prophylaxis; as it induces the suppressor of cytokine signaling 1 (SOCS1), a crucial negative regulator in the JAK-STAT signaling pathway,Citation54 which can lead to IFN signaling suppression. To minimize this obstacle, Mizuno et al.Citation54 demonstrated that the administration of SOCS1 antagonist-expressing recombinant BCG enhanced the immune responses in a mouse model. Interestingly, BCG plus IFN-α, as a vaccine adjuvant,Citation71 could promote Th1 type cytokines secretion and consequent effective immune responses.Citation72 Moreover, it has been revealed that pretreatment of DCs with IFN-β results in the production of larger amount of IL-12p70 and IL-12, which could improve DC function and causes consequent enhancement in Th1 responses. The authors conclude that IFN-β could be used as an adjuvant and enhance the BCG immunogenicity.Citation73,Citation74 Another study revealed that the treatment of cells with IFN-β or IFN-γ created epigenetic memory resulting in faster and higher IFN-stimulated gene (ISG) induction after re-stimulation.Citation75 Therefore, the administration of BCG plus IFN might rationally antagonize the adverse regulatory effects of SOCS1 and augment BCG-induced antiviral immune responses to some extent.
However, BCG vaccine-related pulmonary complications, such as hypersensitivity reactions and mycobacterial pneumonia, have to be considered.Citation76 Thus, cautious studies should be performed to investigate the potential synergistic effects and safety profile of the proposed approach.
4.2. Influenza vaccines
Different types of influenza vaccines, including live-attenuated influenza vaccine (LAIVs) and inactivated influenza vaccines (IIVs), are marketed containing the antigens from certain viral strains. Some formulations also include adjuvants.Citation77 Such diverse constituents cause variety in cytokine induction capabilities of influenza vaccines.Citation78 For instance, TIV (trivalent subunit inactivated influenza vaccine) showed a stronger early induction of type-I and -III IFNs and higher amounts of activated DCs and proinflammatory cytokines, such as TNF, IL-6, −10, and −1β, than LAIV in an in vitro research on unadjuvanted influenza vaccines. Moreover, TIVs have shown some adverse events in children.Citation79
On the other hand, LAIVs were found to protect against respiratory infectionsCitation80 and induce innate immunity through various methods,Citation56 while also triggering a broader spectrum of immunity against different serotypes of influenza virus than TIVs.Citation81
Both TIV and LAIV induced the expression of IFN genes, which was observed on day one and seven after vaccination for TIV and LAIV, respectively.Citation55 It was suggested that the trained immunity induced by influenza vaccine is, to some extent, related to induction of the pro-inflammatory mediators and IFN production.Citation62,Citation80
It is hypothesized that a respiratory virus infection confers immunity against the same and other respiratory viruses for a short time, perhaps a few weeks. Such protection is because of activation of the innate immune response mediated by the release of IFN-I and other cytokines that have broad protective effects against a range of viruses. This phenomenon is called viral interference.Citation82 To inspect this concept, the short-term non-specific protection of cold-adapted, live attenuated influenza vaccine (CAIV) against subsequent RSV infection was investigated in a mouse infection modelCitation80 The author hypothesized that since influenza vaccine and RSV share common features in term of pathogenesis, influenza vaccine could attenuate the severity of RSV infection. Noteworthy, the protective effects of influenza vaccine were significantly diminished in TLR3-/-TLR7-/-mice, which suggests the importance of TLR3/7 signaling pathways in the beneficial protective effects of influenza vaccine. The author proposed that the heterologous effects of influenza vaccine against MERS infection need further investigations.Citation80 An epidemiological article also validated this effect on RSV infection.Citation82
The immune responses to CoV and influenza infections seem comparable as they share some common features, including stimulation of TLRs and RIG-I as well as subsequent antiviral-mediated immune responses.Citation21 Therefore, the potential role of influenza vaccine in generating a non-specific, short term antiviral effect against SARS-CoV-2 has been suggested.Citation80 In this regard, a few recent reports have displayed a hypothetical beneficial role of influenza vaccines against COVID-19 infection in high-risk groups.Citation81,Citation83 While Salem et al. described a flu-induced bystander effect of the generated immune responses as a probable protective mechanism, Kiseleva et al. opined that the LAIV might be more suitable than IIV because of its broad-spectrum potency.
On the other hand, there are concerns about the dampening effect of influenza vaccines on the immune responses to SARS-CoV-2, as well. Because influenza infection can result in TLR desensitization for several months, suppressing one of the main defense mechanisms of the innate immunity against COVID-19.Citation84 Furthermore, influenza disease has shown some bizarre phenomena such as “vaccine-associated viral interference”, which implies that a viral infection would generate a non-specific short-time protection against other viruses normally,Citation85 which could be impeded when a vaccine is administered.Citation86 Another concern is “original antigenic sin” phenomenon, which denotes that in encountering with a similar or close pathogen, the immune system may fail to develop an effective immune response against the newer pathogen and would depend on the memory of the previous pathogen.Citation87 Therefore, a successful vaccination should include all the subtypes of a pathogen.Citation88 This phenomenon is highly important in vaccine design, expressing the importance of extensive clinical trials to validate the safety and side effects of vaccines.Citation89 However, original antigenic sin has been rarely reported in a cross-disease manner and is mostly important for expected mutations in the same pathogen family. A similar phenomenon is “heterosubtypic non-specific temporary immunity”, in which the previous infection with a pathogen temporarily reduces the risk of future infections with another subtype. The phenomenon has been observed for influenza in the unvaccinated populations who had previous seasonal influenza infections and were less susceptible to get infected with pandemic influenza A (H1N1) 2009.Citation90,Citation91 It is also rarely observed among different families of pathogens.Citation92,Citation93 However, a substantial negative association between influenza vaccination and higher COVID-19 incidence or other infections has not been reported.Citation94,Citation95
All in all, the positive and negative sides of the influenza vaccines should be further investigated to evaluate their impact on COVID-19. On the other hand, BCG vaccination has been suggested as an adjuvant for influenza vaccination to increase its efficacy, especially in elderlies.Citation96 It could be conferred from that influenza vaccine could effectively induce IFN mediated immune responses. Hence, the co-administration of BCG and influenza vaccine might harness the beneficial heterologous effects of these vaccines and be a potentially effective approach to combat COVID-19. However, it needs to be validated through experiments.
5. Conclusion and future directions
Given the role of immune system overreactions in COVID-19-induced cytokine storm and the resultant serious harm to body organs and even death, the interventions that could reverse such hyperactivated immunological responses could be of high value. In this regard, LAVs, especially BCG vaccine, could hinder viral replication and the subsequent pathological inflammatory responses in COVID-19 via the arousal of innate and adaptive immunity, especially in the aged and immunocompromised groups. However, the probable adverse effects of BCG vaccination should be considered, including the induction of SOCS1 expression, which may cause the suppression of IFN signaling. In view of the importance of IFN signaling pathway in reducing viral replication early after the infection, the use of a SOCSI-antagonist expressing recombinant BCG or a combination of BCG vaccine with IFN-I might help to partly reverse this detrimental effect and boost the immune system successfully against SARS-CoV-2. These approaches deserve more investigation and experience to be validated in respect to protocol efficacy and safety.
The co-administration of BCG and influenza vaccine might also be potentially a candidate approach to combat COVID-19 and could probably exploit the positive impacts of these vaccines. However, the probability of undesired immunopathological overreactions and cumulative immune aberrations generated by the two vaccines should be taken seriously. In this respect, the risk of augmented innate immune responses, in particular, overproduction of proinflammatory mediators and overstimulation of immune responses, should also be regarded.
In a nutshell, if any vaccine is to be used to support against COVID-19, while compensation for the virus-induced attenuated immune response is the objective, maintaining a balance during both innate and adaptive immune responses should be considered as well. Further studies are highly recommended to assess the outcomes of such interventions.
Disclosure of potential conflicts of interest
The authors declare no conflict of interests.
Additional information
Funding
References
- Negahdaripour M. The battle against covid-19: where do we stand now? Iran J Med Sci. 2020;45:81–82.
- Negahdaripour M. A world of changes: the inheritance of COVID-19. Iran J Med Sci. 2020;45:155–56.
- Amirlak I, Haddad R, Hardy JD, Khaled NS, Chung MH, Amirlak B Effectiveness of booster BCG vaccination in preventing Covid-19 infection. medRxiv 2020; 2020.08.10.20172288. doi: 10.1101/2020.08.10.20172288.
- Dayal D, Gupta S. Connecting BCG vaccination and COVID-19: additional data. medRxiv 2020; 2020.04.07.20053272. doi: 10.1101/2020.04.07.2005327.2
- Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc Natl Acad Sci U S A. 2020;117:17720–26. doi:10.1073/pnas.2008410117.
- Hamiel U, Kozer E, Youngster I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA - J Am Med Assoc. 2020;323:2340–41. doi:10.1001/jama.2020.8189.
- Riccò M, Gualerzi G, Ranzieri S, Luigi Bragazzi N. Stop playing with data: there is no sound evidence that bacille calmette-guérin may avoid SARS-CoV-2 infection for now. Acta Biomed. 2020;91:207–13.
- BCG vaccination to protect healthcare workers against COVID-19 - ClinicalTrials.gov [Internet]. [ cited 2020 Sep 1]. Available from: https://clinicaltrials.gov/ct2/show/NCT04327206
- ClinicalTrials.gov [Internet]. [ cited 2020 Sep 1]. Available from: https://clinicaltrials.gov/.
- Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, O’Neill LAJ, Xavier RJ. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284):427. doi:10.1126/science.aaf1098.
- Goodridge HS, Ahmed SS, Curtis N, Kollmann TR, Levy O, Netea MG, Pollard AJ, Van Crevel R, Wilson CB. Harnessing the beneficial heterologous effects of vaccination. Nat Rev Immunol. 2016;16(6):392–400. doi:10.1038/nri.2016.43.
- Higgins J, Reingold A. Systematic review of the non-specific effects of BCG, DTP and measles containing vaccines. Wkly Epidemiol Rec. 2014;89:1–34.
- Messina NL, Zimmermann P, Curtis N. The impact of vaccines on heterologous adaptive immunity. Clin Microbiol Infect. 2019;25(12):1484–93. doi:10.1016/j.cmi.2019.02.016.
- To KKW, Tsang OTY, Leung WS, Tam AR, Wu TC, Lung DC, Yip CCY, Cai JP, Chan JMC, Chik TSH, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–74. doi:10.1016/S1473-3099(20)30196-1.
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80. doi:10.1016/j.cell.2020.02.052.
- Owji H, Negahdaripour M, Hajighahramani N. Immunotherapeutic approaches to curtail COVID-19. Int Immunopharmacol. 2020;88:106924. doi:10.1016/j.intimp.2020.106924.
- Antalis TM, Bugge TH, Wu Q. Membrane-anchored serine proteases in health and disease. Prog Mol Biol Transl Sci. 2011;99:1–50.
- Yongwen C, Feng Z, Diao B, Wang R, Wang G, Wang C, Tan Y, Liu L, Wang C, Liu Y, et al. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv 2020; 2:2020.03.27.20045427; doi: 10.1101/2020.03.27.20045427
- Park MD. Macrophages: a Trojan horse in COVID-19? Nat Rev Immunol. 2020;20(6):351. doi:10.1038/s41577-020-0317-2.
- Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35:266–71. doi:10.1007/s12250-020-00207-4.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1–9.
- Crouse J, Kalinke U, Oxenius A. Regulation of antiviral T cell responses by type i interferons. Nat Rev Immunol. 2015;15:231–42. doi:10.1038/nri3806.
- McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87–103.
- Braciale TJ, Hahn YS. Immunity to viruses. Immunol Rev. 2013;255:5–12. doi:10.1111/imr.12109.
- Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4 + T cells in immunity to viruses. Nat Rev Immunol. 2012;12:136–48. doi:10.1038/nri3152.
- Kindler E, Thiel V, Weber F. Interaction of SARS and MERS coronaviruses with the antiviral interferon response. Adv Virus Res. 2016;96:219–43.
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. SSRN Electron J. 2020. doi:10.2139/ssrn.3541136.
- Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Liu Y, Wang G, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020. doi:10.1016/fimmu.2020.00827.
- Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, Dong XQ, Zheng YT. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17:541–43. doi:10.1038/s41423-020-0401-3.
- Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–39. doi:10.1007/s00281-017-0629-x.
- Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–70. doi:10.1038/s41577-020-0308-3.
- Matricardi PM, Dal Negro RW, Nisini R. The first, holistic immunological model of COVID-19: implications for prevention, diagnosis, and public health measures. Pediatr Allergy Immunol. 2020;31:454–70. doi:10.1111/pai.13271.
- Yang M. Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV infection. SSRN Electron J. 2020. doi:10.2139/ssrn.3527420.
- Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–93. doi:10.1016/j.chom.2016.01.007.
- Channappanavar R, Fehr AR, Zheng J, Wohlford-Lenane C, Abrahante JE, Mack M, Sompallae R, McCray PB, Meyerholz DK, Perlman S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest. 2019;129:3625–39. doi:10.1172/JCI126363.
- Chu H, Chan JFW, Wang Y, Yuen TTT, Chai Y, Hou Y, Shuai H, Yang D, Hu B, Huang X, et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis. 2020;71:1400–09.
- Wei L, Ming S, Zou B, Wu Y, Hong Z, Li Z, Zheng X, Huang M, Luo L, Liang J, et al. Viral invasion and type i interferon response characterize the immunophenotypes during COVID-19 infection. SSRN Electron J. 2020. doi:10.2139/ssrn.3555695.
- Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Pere H, Charbit B, Bondet V, Chenevier-Gobeaux C, Breillat P, et al. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients. Science. 2020;369(6504):718–72461059. doi:10.1126/science.abc6027.
- Molony RD, Malawista A, Montgomery RR. Reduced dynamic range of antiviral innate immune responses in aging. Exp Gerontol. 2018;107:130–35. doi:10.1016/j.exger.2017.08.019.
- Angelidou A, Diray-Arce J, Conti MG, Smolen KK, van Haren SD, Dowling DJ, Husson RN, Levy O. BCG as a case study for precision vaccine development: lessons from vaccine heterogeneity, trained immunity, and immune ontogeny. Front Microbiol. 2020;11:332. doi:10.3389/fmicb.2020.00332.
- Covián C, Fernández-Fierro A, Retamal-Díaz A, Díaz FE, Vasquez AE, Lay MK, Riedel CA, González PA, Bueno SM, Kalergis AM. BCG-induced cross-protection and development of trained immunity: implication for vaccine design. Front Immunol. 2019;10:2806. doi:10.3389/fimmu.2019.02806.
- Pulendran B, Maddur MS. Innate immune sensing and response to influenza. Curr Top Microbiol Immunol. 2015;386:23–71.
- Fischer WA, Chason KD, Brighton M, Jaspers I. Live attenuated influenza vaccine strains elicit a greater innate immune response than antigenically-matched seasonal influenza viruses during infection of human nasal epithelial cell cultures. Vaccine. 2014;32:1761–67. doi:10.1016/j.vaccine.2013.12.069.
- Sirén J, Pirhonen J, Julkunen I, Matikainen S. IFN-α regulates TLR-dependent gene expression of IFN-α, IFN-β, IL-28, and IL-29. J Immunol. 2005;174:1932–37. doi:10.4049/jimmunol.174.4.1932.
- Oh SJ, Lee JK, Shin OS. Aging and the immune system: the impact of immunosenescence on viral infection, immunity and vaccine immunogenicity. Immune Netw. 2019;19(6):e37. doi:10.4110/in.2019.19.e37.
- Uthayakumar D, Paris S, Chapat L, Freyburger L, Poulet H, De Luca K. Non-specific effects of vaccines illustrated through the BCG example: from observations to demonstrations. Front Immunol. 2018;9:2869. doi:10.3389/fimmu.2018.02869.
- Bessler H, Djaldetti M. Research article global vaccines and immunology glob vaccines immunol. Glob Vaccines Immunol. 2016;2:1–4.
- Valensi JP, Carlson JR, Van Nest GA. Systemic cytokine profiles in BALB/c mice immunized with trivalent influenza vaccine containing MF59 oil emulsion and other advanced adjuvants. J Immunol. 1994;153:4029–39.
- Tregoning JS, Russell RF, Kinnear E. Adjuvanted influenza vaccines. Hum Vaccines Immunother. 2018;14:550–64. doi:10.1080/21645515.2017.1415684.
- Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, Melero I. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res. 2011;17:2619–27. doi:10.1158/1078-0432.CCR-10-1114.
- Minciullo PL, Catalano A, Mandraffino G, Casciaro M, Crucitti A, Maltese G, Morabito N, Lasco A, Gangemi S, Basile G. Inflammaging and anti-inflammaging: the role of cytokines in extreme longevity. Arch Immunol Ther Exp (Warsz). 2016;64:111–26. doi:10.1007/s00005-015-0377-3.
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–90. doi:10.1038/s41574-018-0059-4.
- Kandasamy R, Voysey M, McQuaid F, De Nie K, Ryan R, Orr O, Uhlig U, Sande C, O’Connor D, Pollard AJ. Non-specific immunological effects of selected routine childhood immunisations: systematic review. BMJ. 2016;355:i5225. doi:10.1136/bmj.i5225.
- Mizuno S, Soma S, Inada H, Kanuma T, Matsuo K, Yasutomi Y. SOCS1 antagonist–expressing recombinant bacillus Calmette–Guérin enhances antituberculosis protection in a mouse model. J Immunol. 2019;203:188–97. doi:10.4049/jimmunol.1800694.
- Cao RG, Suarez NM, Obermoser G, Lopez SMC, Flano E, Mertz SE, Albrecht RA, García-Sastre A, Mejias A, Xu H, et al. Differences in antibody responses between trivalent inactivated influenza vaccine and live attenuated influenza vaccine correlate with the kinetics and magnitude of interferon signaling in children. J Infect Dis. 2014;210:224–33. doi:10.1093/infdis/jiu079.
- Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12:786–95. doi:10.1038/ni.2067.
- Lee AJ, Ashkar AA. The dual nature of type I and type II interferons. Front Immunol. 2018;9:2061.
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. doi:10.1016/S0140-6736(20)30211-7.
- Bernatowska E, Skomska-Pawliszak M, Wolska-Kuśnierz B, Pac M, Heropolitanska-Pliszka E, Pietrucha B, Bernat-Sitarz K, Dąbrowska-Leonik N, Bohynikova N, Piątosa B, et al. BCG moreau vaccine safety profile and NK cells—double protection against disseminated BCG infection in retrospective study of BCG vaccination in 52 polish children with severe combined immunodeficiency. J Clin Immunol. 2020;40:138–46. doi:10.1007/s10875-019-00709-1.
- Schapiro JM, Segev Y, Rannon L, Alkan M, Rager‐Zisman B. Natural killer (NK) cell response after vaccination of volunteers with killed influenza vaccine. J Med Virol. 1990;30:196–200. doi:10.1002/jmv.1890300310.
- Tai LH, Zhang J, Scott KJ, De Souza CT, Alkayyal AA, Ananth AA, Sahi S, Adair RA, Mahmoud AB, Sad S, et al. Perioperative influenza vaccination reduces postoperative metastatic disease by reversing surgery-induced dysfunction in natural killer cells. Clin Cancer Res. 2013;19:5104–15. doi:10.1158/1078-0432.CCR-13-0246.
- Brillantes M, Beaulieu AM. Memory and memory-like NK cell responses to microbial pathogens. Front Cell Infect Microbiol. 2020;10:102. doi:10.3389/fcimb.2020.00102.
- Iorio AM, Bistoni O, Galdiero M, Lepri E, Camilloni B, Russano AM, Neri M, Basileo M, Spinozzi F. Influenza viruses and cross-reactivity in healthy adults: humoral and cellular immunity induced by seasonal 2007/2008 influenza vaccination against vaccine antigens and 2009 A(H1N1) pandemic influenza virus. Vaccine. 2012;30:1617–23. doi:10.1016/j.vaccine.2011.12.107.
- Moris P, Van Der Most R, Leroux-Roels I, Clement F, Dramé M, Hanon E, Leroux-Roels GG, Van Mechelen M. H5N1 influenza vaccine formulated with AS03 A induces strong Cross-reactive and polyfunctional CD4 T-Cell responses. J Clin Immunol. 2011;31:443–54. doi:10.1007/s10875-010-9490-6.
- Moon C. Fighting COVID-19 exhausts T cells. Nat Rev Immunol. 2020;20:277. doi:10.1038/s41577-020-0304-7.
- Dara M, Acosta CD, Rusovich V, Zellweger JP, Centis R, Migliori GB. Bacille Calmette-Guérin vaccination: the current situation in Europe. Eur Respir J. 2014;43:24–35. doi:10.1183/09031936.00113413.
- Moorlag SJCFM, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25:1473–78. doi:10.1016/j.cmi.2019.04.020.
- Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–88.
- Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Ifrim DC, Saeed S, Jacobs C, Van Loenhout J, De Jong D, Hendrik S, et al. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109:17537–42. doi:10.1073/pnas.1202870109.
- Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–74. doi:10.1126/science.abb8925.
- Rizza P, Capone I, Moretti F, Proietti E, Belardelli F. IFN-α as a vaccine adjuvant: recent insights into the mechanisms and perspectives for its clinical use. Expert Rev Vaccines. 2011;10:487–98. doi:10.1586/erv.11.9.
- Rivas-Santiago CE, Guerrero GG. IFN-α boosting of mycobacterium bovis Bacillus Calmette Güerin-vaccine promoted Th1 type cellular response and protection against M. tuberculosis Infection . Biomed Res Int. 2017;2017:8796760.
- Giacomini E, Remoli ME, Gafa V, Pardini M, Fattorini L, Coccia EM. IFN-β improves BCG immunogenicity by acting on DC maturation. J Leukoc Biol. 2009;85(3):462–68. doi:10.1189/jlb.0908583.
- El-Sahrigy SAF, Rahman AMOA, Samaha DY, Mohamed NA, Saber SM, Talkhan HA, Ismail GA, Ibraheem EM, Riad EM. The influence of interferon-β supplemented human dendritic cells on BCG immunogenicity. J Immunol Methods. 2018;457:15–21. doi:10.1016/j.jim.2018.03.003.
- Kamada R, Yang W, Zhang Y, Patel MC, Yang Y, Ouda R, Dey A, Wakabayashi Y, Sakaguchi K, Fujita T, et al. Interferon stimulation creates chromatin marks and establishes transcriptional memory. Proc Natl Acad Sci U S A. 2018;115:E9162–71. doi:10.1073/pnas.1720930115.
- Leeson CE, Ismail A, Hashad MM, Elmansy H, Shahrour W, Prowse O, Kotb A. Systematic review: safety of intravesical therapy for bladder cancer in the era of COVID-19. SN Compr Clin Med. 2020;18:1–5.
- Fiore AE, Bridges CB, Katz JM, Cox NJ. Inactivated influenza vaccines. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. 6th Ed. Elsevier Saunders; 2012. p. 257–93.
- van Essen GA, Beran J, Devaster JM, Durand C, Duval X, Esen M, Falsey AR, Feldman G, Gervais P, Innis BL, et al. Influenza symptoms and their impact on elderly adults: randomised trial of AS03-adjuvanted or non-adjuvanted inactivated trivalent seasonal influenza vaccines. Influenza Other Respi Viruses. 2014;8:452–62. doi:10.1111/irv.12245.
- Cowling BJ, Fang VJ, Nishiura H, Chan KH, Ng S, Ip DKM, Chiu SS, Leung GM, Malik Peiris JS. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis. 2012;54:1778–83. doi:10.1093/cid/cis307.
- Lee YJ, Lee JY, Jang YH, Seo SU, Chang J, Seong BL. Non-specific effect of vaccines: immediate protection against respiratory syncytial virus infection by a live attenuated influenza vaccine. Front Microbiol. 2018;9:83. doi:10.3389/fmicb.2018.00083.
- Kiseleva I. New points of departure for more global influenza vaccine use. Vaccines. 2020;8:1–8. doi:10.3390/vaccines8030410.
- Ånestad G. Interference between outbreaks of respiratory syncytial virus and influenza virus infection. Lancet. 1982;319:502. doi:10.1016/S0140-6736(82)91466-0.
- Salem ML, El-Hennawy D. The possible beneficial adjuvant effect of influenza vaccine to minimize the severity of COVID-19. Med Hypotheses. 2020;140:109752. doi:10.1016/j.mehy.2020.109752.
- Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, Lawrence T, Van Rijt LS, Lambrecht BN, Sirard JC, et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med. 2008;205:323–29. doi:10.1084/jem.20070891.
- Linde A, Rotzén-Ostlund M, Zweygberg-Wirgart B, Rubinova S, Brytting M. Does viral interference affect spread of influenza? Euro Surveill. 2009;14:40.
- Dyer O. What did we learn from Tamiflu? BMJ. 2020;368:m626. doi:10.1136/bmj.m626.
- Vatti A, Monsalve DM, Pacheco Y, Chang C, Anaya JM, Gershwin ME. Original antigenic sin: A comprehensive review. J Autoimmun. 2017;83:12–21. doi:10.1016/j.jaut.2017.04.008.
- Midgley CM, Bajwa-Joseph M, Vasanawathana S, Limpitikul W, Wills B, Flanagan A, Waiyaiya E, Tran HB, Cowper AE, Chotiyarnwon P, et al. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol. 2011;85:410–21. doi:10.1128/JVI.01826-10.
- Suzuki M, Camacho A, Ariyoshi K. Potential effect of virus interference on influenza vaccine effectiveness estimates in test-negative designs. Epidemiol Infect. 2014;142:2642–46. doi:10.1017/S0950268814000107.
- Kelly H, Barry S, Laurie K, Mercer G. Seasonal influenza vaccination and the risk of infection with pandemic influenza: A possible illustration of nonspecific temporary immunity following infection. Eurosurveillance. 2010;15:47. doi:10.2807/ese.15.47.19722-en.
- Skowronski DM, de Serres G, Crowcroft NS, Janjua NZ, Boulianne N, Hottes TS, Rosella LC, Dickinson JA, Gilca R, Sethi P, et al. Association between the 2008-09 seasonal influenza vaccine and pandemic H1N1 illness during spring-summer 2009: four observational studies from Canada. PLoS Med. 2010;7:e1000258. doi:10.1371/journal.pmed.1000258.
- Wolff GG. Influenza vaccination and respiratory virus interference among department of defense personnel during the 2017–2018 influenza season. Vaccine. 2020;38:350–54. doi:10.1016/j.vaccine.2019.10.005.
- Rynda-Apple A, Robinson KM, Alcorn JF. Influenza and bacterial superinfection: illuminating the immunologic mechanisms of disease. Infect Immun. 2015;83:3764–70. doi:10.1128/IAI.00298-15.
- Rikin S, Jia H, Vargas CY, Castellanos de Belliard Y, Reed C, LaRussa P, Larson EL, Saiman L, Stockwell MS. Assessment of temporally-related acute respiratory illness following influenza vaccination. Vaccine. 2018;36:1958–64. doi:10.1016/j.vaccine.2018.02.105.
- CDC. Key facts about seasonal flu vaccine [Internet]. Program2008 [ cited 2020 Aug 17]; 1–3. Available from: http://www.cdc.gov/flu/protect/keyfacts.htm.
- Leentjens J, Kox M, Stokman R, Gerretsen J, Diavatopoulos DA, Van Crevel R, Rimmelzwaan GF, Pickkers P, Netea MG. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: A randomized, placebo-controlled pilot study. J Infect Dis. 2015;212:1930–38. doi:10.1093/infdis/jiv332.
