ABSTRACT
Human immunodeficiency virus (HIV)-exposed infants may be at increased risk of vaccine-preventable disease. This study was conducted as a post-licensure commitment in this population to evaluate the primary series, antibody persistence, and booster response to a licensed fully liquid hexavalent vaccine containing diphtheria (D), tetanus (T), acellular pertussis (aP), inactivated poliovirus (IPV), hepatitis B (HB), and Haemophilus influenzae type b antigens (PRP~T). This was a Phase III, open-label, randomized study conducted at a single center in the Republic of South Africa. The DTaP-IPV-HB-PRP~T vaccine was administered to HIV-exposed infected (Group A: N = 14) and HIV-exposed uninfected (Group B: N = 50) infants as a 6, 10, 14 week primary series with a toddler booster at 15–18 months of age. Immunogenicity of each antigen was measured using validated assays and vaccine reactogenicity was recorded using diary cards. The low number of HIV-exposed infected participants, due to widespread pre- and peri-natal retroviral treatment, meant that between-group comparisons should be treated with caution. In each group, primary series and booster immune seroprotection rates were strong, and pre-booster antibody persistence was good, although anti-HBs ≥10 mIU/mL in Group A was 78.6% post-primary series, 58.3% pre-booster, and 75.0% post-booster. There were no safety concerns. In conclusion, primary series and booster vaccination of the DTaP-IPV-HB-PRP~T vaccine were immunogenic and safe in HIV-exposed infected and uninfected infants. These results were comparable to historical data in healthy infants and toddlers.
Introduction
Pediatric combination vaccines allow the delivery of multiple antigens in a single vaccination and high vaccine coverage rates are crucial in maintaining the low prevalence of childhood diseases including diphtheria (D), tetanus (T), pertussis, polio, hepatitis B (HB), and Haemophilus influenzae type b (Hib).Citation1 Human immunodeficiency virus (HIV)-exposed infants, both infected and uninfected, have been shown to be at increased risk of vaccine-preventable diseases and perhaps more at risk of under-immunization.Citation2,Citation3
Hexaxim is a fully liquid hexavalent vaccine containing D, T, acellular pertussis (aP), inactivated poliovirus, HB, and Hib polysaccharide conjugated to tetanus protein (PRP~T) antigens (DTaP-IPV-HB-PRP~T) that was first licensed in 2012 after demonstrating strong immunogenicity and good safety during a thorough clinical development program in a wide range of schedules, on four continents, with or without a birth dose of HB, alone and in co-administration with other common pediatric vaccines.Citation4 Over 100 million doses of this DTaP-IPV-HB-PRP~T vaccine have been distributed in more than 100 countries worldwide and the vaccine is pre-qualified by the World Health Organization.Citation5 This vaccine was the first to be evaluated via the European Medicines Agency Article 58 procedure.Citation6 Its approval included a post-licensure commitment of the manufacturer to evaluate the vaccine’s immunogenicity and safety in immunocompromised subjects.
One of the most frequent sources of immunosuppression in infants from birth to 2 years of age is exposure to vertical transmission of HIV from infected mothers .Citation7–10 This population of infants, with ante-natal exposure to HIV and increased susceptibility to vaccine-preventable diseases as well as increased likelihood of reduced vaccination coverage, was therefore chosen for this study. HIV-exposed but uninfected infants, as well as HIV-exposed and infected infants, were included since they may be expected to experience lower immune responses due to indirect immunological consequences of ante-natal HIV exposure.Citation11
The study was conducted in the Republic of South Africa (RSA) where the prevalence of HIV infection in pregnant women is high (approximately 30%Citation12) and where the DTaP-IPV-HB-PRP~T vaccine is licensed and has been extensively evaluated in healthy infants and toddlers.Citation13–15 In the region of the study site, the prevalence of HIV in pregnant women is approximately 29% and about 60–65% of deliveries are by the vaginal route. Primary series immunogenicity, antibody persistence, and the response to a booster vaccination were assessed as primary study objectives, and the evaluation of primary and booster vaccine safety was included as a secondary objective.
Materials and methods
Study design and participants
This was a Phase III, open-label, randomized study conducted at a single center in RSA (WHO Universal Trial Number: U1111-1161-2610; ClinicalTrials.gov Identifier: NCT02817451; EU clinical register number: 2018–004708-21). The study protocol and three amendments were approved by the institutional ethics committee and the conduct of the study was consistent with the Declaration of Helsinki and compliant with the International Council for Harmonization guidelines for Good Clinical Practice as well as with all local and national regulations. An informed consent form was signed by each participant’s parents or legally acceptable representatives before enrollment into the study. The study was conducted between June 2016 and March 2019.
All infants included in the study were HIV-exposed, born to HIV-infected mothers who were identified through screening of ante-natal records. The parent was requested to provide consent for HIV testing of their infant, which is a standard of care in RSA. The study population consisted of HIV-exposed infected (Group A) and HIV-exposed uninfected (Group B) infants, as confirmed per polymerase chain reaction (PCR) testing. Participants in Group A were receiving anti-retroviral therapy according to the national recommendations at the time of the study.Citation16,Citation17 Participants had a birthweight of ≥2 kg and were aged 35–56 days (5–8 weeks) at the time of inclusion. All participants were to receive the hexavalent DTaP-IPV-HB-PRP~T vaccine administered as a 3-dose primary series at 6, 10, and 14 weeks of age and a booster vaccination in the second year of life (15–18 months of age). Although not recorded or evaluated in this study, participants were also to receive oral poliovirus vaccine and Bacillus Calmette-Guérin (BCG) vaccine at birth, pneumococcal vaccine at 6 weeks, 14 weeks, and 9 months of age, and rotavirus vaccine at 6 and 13 weeks of age according to the national recommendations.Citation18 The oral poliovirus, BCG, pneumococcal, and rotavirus vaccines would not be expected to have any effect on the safety or immunogenicity of the DTaP-IPV-HB-PRP~T vaccine, and coadministration with pneumococcal and rotavirus vaccines has been evaluated during the clinical development program .Citation4
The main exclusion criteria were the prior receipt of any vaccine containing D, T, P, poliovirus (except for a birth dose of oral poliovirus vaccine, which is routinely recommended in RSA), HB (except for a birth dose of standalone HB vaccine, which is recommended for infants born to HB surface antigen positive mothers in RSA), or Hib antigens, or history of infection with any of these diseases; previous (in the 4 weeks before enrollment) or planned (during the study period) participation in another clinical study; any chronic condition (except HIV infection in Group A); receipt of blood or blood-derived products; thrombocytopenia or bleeding disorder; history of seizures or uncontrolled neurological disorder; known hypersensitivity or contraindication to any vaccine component; acute illness or febrile illness (axillary temperature ≥38.0°C) on the day of vaccination. Additional contraindications to subsequent vaccination included receipt of immunosuppressive therapy, systemic corticosteroids (for more than 2 consecutive weeks), immunoglobulins, blood or blood-derived products, or any non-study vaccine containing D, T, P, IPV, HB, or Hib antigens since the preceding visit, any acute or severe chronic illness that could have interfered with the conduct of the study or any known contraindication to pertussis vaccination since the preceding visit.
The DTaP-IPV-HB-PRP~T vaccine was administered by intramuscular injection into the anterolateral area of the right thigh in both groups.
Study vaccine
The hexavalent DTaP-IPV-HB-PRP~T vaccine (Hexaxim, batch number M0179-F01 [expiry 30 November 2017] and N1E59F01 [expiry 30 April 2019]) was manufactured by Sanofi Pasteur, France and supplied as a sterile suspension for injection in single-dose, pre-filled syringes. Each 0.5 mL dose contained ≥20 IU D-toxoid, ≥40 IU T-toxoid, 25 µg PT, 25 µg FHA, 40, 8 and 32 D antigen units of poliovirus type 1, 2 and 3, respectively, 10 µg HBsAg, 12 µg Hib polysaccharide conjugated to 22–36 µg tetanus protein (PRP~T), and 0.6 mg aluminum hydroxide.
Serology
Blood samples were collected at four time points: pre-first primary series vaccination, 1 month post-third primary series vaccination, pre-booster vaccination, and 1 month post-booster vaccination. Pre-primary series evaluation included anti-D, anti-T, anti-PT, and anti-FHA antibodies. Post-primary series and pre- and post-booster evaluation included additional anti-HB, anti-polio 1, anti-polio 2, and anti-polio 3, and anti-PRP antibodies.
Anti-D (IU/mL), anti-T (IU/mL), anti-PT (EU/mL) and anti-FHA (EU/mL) antibody concentrations were measured using a multiplexed electrochemiluminescence immunoassay (ECL).Citation19 The ECL assay used in this study was fully validated. Anti-polio 1, 2, 3 antibody titers (1/dil) were measured by neutralization assay on Vero cells using wild type strains as target virus, anti-HB antibody concentrations (mIU/mL) were evaluated by a commercially available chemiluminescence assay (VITROS ECi/ECiQ), and anti-PRP antibody concentrations (µg/mL) were measured by a Farr-type radioimmunoassay.
All assays were performed at the Sponsor’s Global Clinical Immunology (GCI) laboratory (Swiftwater, PA, USA).
Reactogenicity and safety
Participants were observed at the study site for 30 minutes after each primary series and booster vaccination to assess immediate unsolicited adverse events (AEs). Subsequently, parent(s)/legal representative(s) used diary cards for 7 days to record the duration and intensity of solicited injection site reactions (tenderness, erythema, swelling, extensive swelling of the vaccinated limb [assessed for booster vaccination only]) and solicited systemic reactions (fever, vomiting, crying abnormal, drowsiness, appetite lost, irritability). All solicited reactions were automatically considered to be related to the vaccination and severity was assessed according to standard scales (Grade 1 [mild], 2 [moderate], or 3 [severe]). For temperature measurement, the axillary route was preferred. Unsolicited AEs were recorded using diary cards for 28 days after vaccination. Unsolicited injection site AEs were considered to be related to the vaccination and the Investigator assessed unsolicited systemic AEs for severity and causality. Extensive limb swelling, hypotonic hyporesponsive episodes, anaphylactic reactions, severe neurological conditions were considered to be of special interest and were to be classed as serious adverse events (SAEs). These and other SAEs, including deaths, were collected throughout the study and the Investigator was responsible for assessing causality.
Statistical analyses
No statistical hypotheses were tested and all evaluations were descriptive.
For the immunogenicity evaluation, seroprotection (SP) was defined as anti-D antibody ≥0.01 IU/mL, anti-T ≥ 0.01 IU/mL, anti-polio 1, 2, and 3 titers ≥8 1/dil, anti-HB ≥10 mIU/mL, and anti-PRP ≥0.15 µg/mL. For anti-PT and anti-FHA, seroconversion was defined as ≥fourfold increase in concentration from pre-primary series to post-primary series or post-booster; vaccine response (VR) was defined for the primary series as post-primary series concentration ≥4x lower limit of quantitation of the assay (LLOQ: 2 EU/mL) if pre-primary series concentration <4x LLOQ, or post-primary series concentration ≥pre-primary series concentration if pre-primary series concentration ≥4x LLOQ, and for the booster as post-booster concentration ≥fourfold increase from pre-primary series if pre-primary series concentration <4x LLOQ, or post-booster vaccination ≥twofold increase from pre-primary series concentration if pre-primary series concentration ≥4x LLOQ. Additionally, booster response for anti-PT and anti-FHA was defined as post-booster concentration ≥fourfold increase from pre-booster if pre-booster vaccination concentration <4x LLOQ, or post-booster vaccination ≥twofold increase from pre-booster vaccination concentration if pre-booster concentration ≥4x LLOQ.
Data are also presented for the following thresholds: anti-D ≥0.1 and ≥1.0 IU/mL, anti-T ≥0.1 and 1.0 IU/mL, anti-HB ≥100 mIU/mL, anti-PRP ≥1.0 µg/mL. Additionally, geometric mean concentrations (GMCs: anti-D, anti-T, anti-PT, anti-FHA, anti-PRP,) geometric mean titers (GMTs: anti-polio 1, 2, and 3), and the ratio of post/pre-vaccination (anti-PT and anti-FHA) are presented.
Data are presented with their 95% confidence intervals (CIs), calculated using the exact binomial method (Clopper-Pearson method)Citation20 for proportions and the normal approximation of the log10 concentrations and titers, followed by a back transformation, for GMCs and GMTs.
The full analysis set (FAS) was used for the immunogenicity analyses (participants who received at least one vaccination [for the primary series analysis] or all participants who received the booster vaccination [for the booster analysis]) and the safety analysis set (SS) was used for all safety analyses (for each vaccination, participants who received the vaccination).
The planned sample size was 50 participants per group, in order to have 30 participants included in the analyses per group assuming a 40% attrition rate. The statistical analyses were done under the responsibility of Sanofi Pasteur’s biostatistics group using SAS® software, Version 9.4 (SAS Institute, Cary, NC, USA).
Results
Participants studied
Despite screening more than 5000 HIV-exposed infants over an extended period of 18 months, only 14 infants were identified to have acquired HIV and recruited into Group A. In Group B, 50 HIV-exposed uninfected infants were included as planned. Of these, 14 (Group A) and 47 (Group B) participants completed the primary vaccination series and 12 (Group A) and 41 (Group B) participants received the booster. No participant received a birth dose of HB vaccine. One participant in Group B received the third vaccination from a commercial batch of Hexaxim rather than the study batch and was excluded from all analysis sets but continued in the study as planned. Participant disposition is presented in . Due to the limited number of participants in Group A, the results should be interpreted with caution.
Figure 1. Disposition of study participants
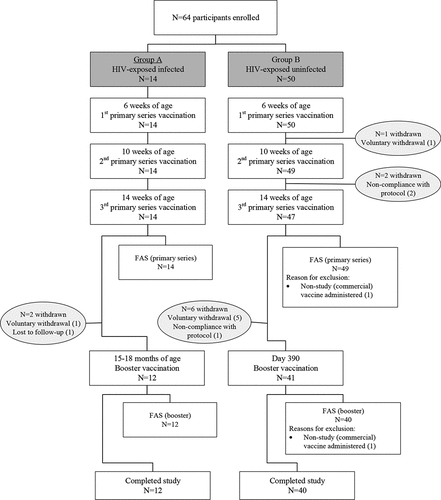
There was a similar number of male and female participants in each group (64.3% and 59.2% female in Group A and B, respectively), and at the time of the first primary series vaccination mean±SD age was similar in Group A (6.07 ± 0.267 weeks) and Group B (5.78 ± 0.422 weeks).
Immunogenicity
Primary series
Prior to the first vaccination, in Group A and Group B, respectively, 7.1% and 26.5% of participants (anti-D), and 92.9% and 98.0% of participants (anti-T) had antibodies ≥0.01 IU/mL; 35.7% and 55.1% of participants (anti-PT) and 71.4% and 89.8% of participants (anti-FHA) had antibodies ≥LLOQ. At 1 month post-third primary series vaccination, SP rate (and VR for anti-PT and anti-FHA) was 100% for each antigen in Group A and Group B, except for anti-HB ≥10 mIU/mL (78.6% in Group A) and anti-PRP ≥0.15 µg/mL (92.9% [Group A] and 97.7% [Group B]). For the remaining antibody thresholds, post-primary immune responses were high in each group (). For anti-D, anti-T, anti-polio 2, anti-PT, anti-FHA, and anti-PRP, GMCs and GMTs were higher post-primary series in Group A than Group B whereas for anti-HB, anti-polio 1 and 3 GMCs and GMTs were higher post-primary series in Group B than Group A, although 95% CI were overlapping ().
Table 1. Seroprotection rates, seroconversion rates, and vaccine response rates pre- and post-primary series and booster vaccination (FAS)
Table 2. Geometric mean concentrations and titers pre- and post-primary series and booster vaccination (FAS)
Antibody persistence
Prior to the booster vaccination, 100% of participants in both groups had antibody persistence for anti-D (≥0.01 IU/mL), anti-T (≥0.01 IU/mL), and anti-FHA (≥LLOQ). For the remaining antigens, antibody persistence was high in both groups and slightly lower in Group A than Group B (anti-HB ≥10 mIU/mL: 58.3% and 85.0%; anti-polio 1 ≥8 1/dil: 91.7% and 100%; anti-polio 2 ≥8 1/dil: 75.0% and 100%; anti-polio 3 ≥8 1/dil: 75.0% and 100%; anti-PT ≥LLOQ: 91.7% and 100%; and anti-PRP ≥0.15 µg/mL: 58.3% and 76.9%) (). Pre-booster GMCs and GMTs were higher in Group A than Group B for anti-T, anti-PT, and anti-FHA, and higher in Group B than Group A for anti-HB, anti-D, and anti-polio 1, 2, and 3, although 95% CIs were overlapping ().
Booster response
After the booster vaccination, 100% of participants in Group A had antibody levels above each threshold except for anti-HB ≥10 mIU/mL (75.0%), anti-HB ≥100 mIU/mL (66.7%), anti-T ≥1.0 IU/mL (91.7%), anti-PT seroconversion (91.7%), anti-FHA booster response (85.7%), and anti-PRP ≥1 µg/mL (91.7%). In Group B, the booster response was also high for each antigen, being 100% for each threshold except anti-HB ≥100 mIU/mL (90.0%), anti-D ≥ 1.0 IU/mL (97.5%), anti-T ≥1.0 IU/mL (95.0%), anti-PT seroconversion (92.5%), anti-FHA VR (95.0%), seroconversion (80.0%) and booster response (85.0%), and anti-PRP ≥1 µg/mL (97.5%) (). The comparison of GMCs and GMTs reflected that for pre-booster, and was particularly marked for anti-HB (GMCs of 306 in Group A and 1713 in Group B) ().
Safety and tolerability
There were no immediate adverse reactions (i.e., within 30 minutes post-vaccination) for any primary series or booster vaccination.
Solicited injection site reactions were less commonly reported in Group A than Group B for the primary series (28.6% and 56.3%, respectively) but not for the booster (41.7% and 37.5%, respectively). For solicited systemic reactions, the incidence was similar in each group for the primary series (71.4% and 72.9%) and booster (50.0% and 40.0%) and less commonly reported for the booster vaccination. In each group, most solicited reactions were Grade 1 or Grade 2 in severity and resolved spontaneously. The most common solicited injection site reaction was tenderness and the most common solicited systemic reaction was abnormal crying ().
Table 3. Immediate, solicited, unsolicited, and serious adverse events during the study (SS)
The incidence of unsolicited AEs was similar in Group A and Group B for the primary series (50.0% and 59.2%); for the booster, no unsolicited AEs were reported in Group A, and for 25.0% of participants in Group B.
Up to 1 month post-primary series, six SAEs were reported by four participants in Group A and one participant reported an SAE in Group B. Between the primary series and booster vaccinations, a further seven SAEs were reported by five participants in Group A and three SAEs by three participants in Group B. No SAEs were reported following the booster vaccination, and overall no SAE was considered by the Investigator to be related to the study vaccine. No AEs led to any discontinuation from the study and there were no deaths.
Discussion
This study provides the first immunogenicity and safety data following the administration of the DTaP-IPV-HB-PRP~T vaccine to HIV-exposed infected and HIV-exposed uninfected infants and addresses a post-licensure commitment in an immunocompromised population.
The study was conducted in RSA to maximize the chance of enrolling 50 participants in each group, i.e. HIV-exposed infected and uninfected, due to the high prevalence of HIV infection in pregnant women in that country. Nevertheless, it was not possible to recruit the planned number of HIV-exposed infected participants (Group A) due to the widespread and effective use of pre- and peri-natal anti-HIV treatment in RSA that has led to a marked reduction in mother-to-child transmission (prevention of mother-to-child transmission [PMTCT] program) of HIV in RSA in recent years, Citation21 with most HIV-exposed infants being uninfected. The effectiveness of the PMTCT program was higher than had been expected, and led to difficulty in recruiting infected infants in this study. Over 5000 HIV-exposed infants were screened for study participation over an 18 month period, with only 14 infected infants being identified and enrolled. The decision to stop screening was taken for practical reasons after 18 months and the study continued with a reduced population of infected infants.
Post-primary series and booster immune responses were strong for all antigens, and antibody persistence pre-booster was high for each antigen, although some small differences between groups were noted, e.g. in Group A anti-HB seroprotection rates were lower than Group B on each occasion and anti-polio seroprotection rates were lower in Group A than Group B before the booster vaccination. However, it should be noted that the small number of infected participants (Group A) precludes a robust group comparison and overall there were not considered to be any marked differences of clinical significance in immunogenicity between HIV-exposed infected and uninfected participants.
The primary series and booster immunogenicity observed in this study is aligned with that shown in a cohort of healthy infants at the same study site who had previously received the same DTaP-IPV-HB-PRP~T vaccine in the same 6, 10, 14 weeks schedule with a booster in the second year of life.Citation13,Citation15 Furthermore, pre-school follow up of the healthy cohort showed strong antibody persistence at 4.5 years of ageCitation14 and based on the similarity of the primary series and booster immunogenicity prolonged persistence could be expected for each antigen following administration of the DTaP-IPV-HB-PRP~T vaccine to HIV-exposed infected and uninfected infants and toddlers.
The DTaP-IPV-HB-PRP~T vaccine showed a good safety profile in this study in both groups, which accords with the previous extensive clinical evaluation, particularly in the comparable cohort of healthy infants and toddlers in the previous studies in RSA .Citation13,Citation15 The incidence of AEs was generally lower following the booster vaccination than for the primary series (with the exception of solicited injection site reactions), which is expected based on similar findings in previous studies with the DTaP-IPV-HB-PRP~T vaccine in non-HIV infected infants.Citation4,Citation13,Citation15
The main limitation of the present study is the lower than expected recruitment of HIV-exposed infected infants in Group A, which precludes a robust interpretation of the study results. However, a strength of the study is to have evaluated the immunogenicity and safety of the DTaP-IPV-HB-PRP~T vaccine in at least a small group of these infants and toddlers, and to be able to make a comparison (albeit with caution, due to the small number of participants) not only to a group of HIV-exposed uninfected participants but also to historical data from a comparable cohort of healthy infants.
In conclusion, the DTaP-IPV-HB-PRP~T vaccine was highly immunogenic following 6, 10, 14 week primary series vaccination, showed good pre-booster antibody persistence and a strong post-booster immune response at 15–18 months of age in HIV-exposed infected and uninfected infants and toddlers, and had a good safety profile in both groups. Immunogenicity and safety data in this immunocompromised population were comparable to historical data in healthy infants and toddlers.Citation13,Citation15
Disclosure of potential conflicts of interest
Clinical investigators (AK and SM) received fees from Sanofi Pasteur through their institution for the conduct of these clinical studies, but did not receive any direct payment from Sanofi Pasteur in this regard. They may have received expenses for conference attendance for the presentation of data from these studies.
Acknowledgments
The authors thank the participants and their families for their generous contribution to advancing the knowledge of vaccination.
The authors would also like to acknowledge the study nurses and other staff at the study site, Sanofi Pasteur’s Global Clinical Immunology (GCI) laboratory (Swiftwater, PA, USA) for serological testing, and Fabrice Guitton (Sanofi Pasteur) for project management.
Dr Andrew Lane (Lane Medical Writing) provided medical writing assistance, funded by Sanofi Pasteur, in the preparation and development of the manuscript in accordance with the European Medical Writers Association guidelines and Good Publication Practice.
Additional information
Funding
References
- Decker M, Edwards K, Bogaerts H. Combination vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM editors. Vaccines. 7th. PA (USA): Elsevier; 2018. p. 198–227.
- Succi RC, Krauss MR, Harris DR, Machado DM, de Moraes-pinto MI, Mussi-Pinhata MM, Ruz NP, Pierre RB, Kolevic L, Joao E, et al. Undervaccination of perinatally HIV-infected and HIV-exposed uninfected children in Latin America and the Caribbean. Pediatr Infect Dis J. 2013;32(8):845–50.
- Ndirangu J, Barnighausen T, Tanser F, Tint K, Newell ML. Levels of childhood vaccination coverage and the impact of maternal HIV status on child vaccination status in rural KwaZulu-Natal, South Africa. Trop Med Int Health. 2009;14(11):1383–93. doi:10.1111/j.1365-3156.2009.02382.x.
- Syed YY. DTaP-IPV-HepB-Hib vaccine (Hexyon®): an updated review of its use in primary and booster vaccination. Paediatr Drugs. 2019;21(5):397–408. doi:10.1007/s40272-019-00353-7.
- World Health Organization. WHO prequalified vaccines. [Accessed 2020 September 17]. https://extranet.who.int/gavi/PQ_Web/
- Paterson L. The European medicine agency’s article 58 procedure: reflections on the first approval for a vaccine. Regulatory Rapporteur. 2013;10:19–23.
- Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. J Am Med Assoc. 2011;305(6):576–84. doi:10.1001/jama.2011.100.
- Madhi SA, Izu A, Violari A, Cotton MF, Panchia R, Dobbels E, Sewraj P, van Niekerk N, Jean-Philippe P, Adrian PV, et al. Immunogenicity following the first and second doses of 7-valent pneumococcal conjugate vaccine in HIV-infected and -uninfected infants. Vaccine. 2013;31(5):777–83. doi:10.1016/j.vaccine.2012.11.076.
- Simani OE, Izu A, Violari A, Cotton MF, van Niekerk N, Adrian PV, Madhi SA. Effect of HIV-1 exposure and antiretroviral treatment strategies in HIV-infected children on immunogenicity of vaccines during infancy. AIDS. 2014;28(4):531–41. doi:10.1097/QAD.0000000000000127.
- Thanee C, Pancharoen C, Likitnukul S, Luangwedchakarn V, Umrod P, Phasomsap C, Apornpong T, Chuanchareon T, Butterworth O, Puthanakit T, et al. The immunogenicity and safety of pneumococcal conjugate vaccine in human immunodeficiency virus-infected Thai children. Vaccine. 2011;29(35):5886–91. doi:10.1016/j.vaccine.2011.06.072.
- Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clin Exp Immunol. 2014;176(1):11–22. doi:10.1111/cei.12251.
- Woldesenbet SA, Kufa T, Lombard C, Manda S, Ayalew K, Cheyip M, Pure A. The 2017 national antenatal sentinel HIV survey, South Africa, national department of health. [Accessed 2020 September 17]. https://www.nicd.ac.za/wp-content/uploads/2019/07/Antenatal_survey-report_24July19.pdf
- Madhi SA, Mitha I, Cutland C, Groome M, Santos-Lima E. Immunogenicity and safety of an investigational fully liquid hexavalent combination vaccine versus licensed combination vaccines at 6, 10, and 14 weeks of age in healthy South African infants. Pediatr Infect Dis J. 2011;30(4):e68–74. doi:10.1097/INF.0b013e31820b93d2.
- Madhi SA, Lopez P, Zambrano B, Jordanov E, B’Chir S, Noriega F, Feroldi E. Antibody persistence in pre-school children after hexavalent vaccine infant primary and booster administration. Hum Vaccin Immunother. 2019;15(3):658–68.
- Madhi SA, Koen A, Cutland C, Groome M, Santos-Lima E. Antibody persistence and booster vaccination of a fully liquid hexavalent vaccine coadministered with measles/mumps/rubella and varicella vaccines at 15-18 months of age in healthy South African infants. Pediatr Infect Dis J. 2013;32:889–97.
- South Africa National Department of Health. 2019 ART clinical guidelines for the management of HIV in adults, pregnancy, adolescents, children, infants and neonates. [Accessed 2020 September 17]. https://www.knowledgehub.org.za/elibrary/2019-art-clinical-guidelines-management-hiv-adults-pregnancy-adolescents-children-infants
- South Africa National Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. 2015 [Accessed 2020 September 17]. https://www.scribd.com/doc/268965647/National-Consolidated-Guidelines-for-PMTCT-and-the-Management-of-HIV-in-Children-Adolescents-and-Adults
- Meyer JC, Khosa LA, Nhira S, Chiloane L, Sibanda M, Schönfeldt M, Burnett RJ. Childhood vaccination and the role of the pharmacist. South African Pharm J. 2018;85(4):26–39.
- Itell HL, McGuire EP, Muresan P, Cunningham CK, McFarland EJ, Borkowsky W, Permar SR, Fouda GG. Development and application of a multiplex assay for the simultaneous measurement of antibody responses elicited by common childhood vaccines. Vaccine. 2018;36(37):5600–08. doi:10.1016/j.vaccine.2018.07.048.
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857–72. doi:10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E.
- Barron P, Pillay Y, Doherty T, Sherman G, Jackson D, Bhardwaj S, Robinson P, Goga A. Eliminating mother-to-child HIV transmission in South Africa. Bull World Health Organ. 2013;91(1):70–74. doi:10.2471/BLT.12.106807.
