ABSTRACT
Cancer is a worldwide problem that threatens human health. Radiotherapy plays an important role in a variety of cancer treatment methods. The administration of radiotherapy can alter the differentiation pathways and functions of T cells, which in turn improves the immune response of T cells. Radiotherapy can also induce up-regulation of PD-L1 expression, which means that it has great potential for enhancing the therapeutic effect of anti-PD-1/PD-L1 inhibitors and reducing the risk of drug resistance toward them. At present, the combination of radiotherapy and anti-PD-1/PD-L1 inhibitors has shown significant therapeutic effects in clinical tumor research. This review focuses on the mechanism of radiotherapy on T cells reported in recent years, as well as related research progress in the application of PD-1/PD-L1 blockers. It will provide a theoretical basis for the rational clinical application of radiotherapy combined with PD-1/PD-L1 inhibitors.
Introduction
According to the latest global cancer statistics from the International Agency for Research on Cancer, in 2018, 18.1 million newly diagnosed cancer cases were recorded worldwide, and the number of deaths reached 9.6 million.Citation1,Citation2 Radiotherapy is one of the most commonly used methods of cancer treatment.Citation3,Citation4 About 60% of newly diagnosed cancer patients, as well as many existing or recurrent cancer patients, choose RT as part of their treatment plan.Citation5 RT affects the differentiation pathways and functions of T cells in the tumor microenvironment. It then affects the anti-tumor immune response.Citation6 However, the application of RT is also accompanied by corresponding side-effects. In recent years, immunotherapy has become the focus of cancer treatment due to its limited side effects,Citation7 and its application has evolved from being an end-line treatment for some “hot tumors” to being the first-line. It has even entered the field of neoadjuvant therapy, where it has achieved amazing results.Citation8 Recently, it has been found that radiotherapy also plays an important role in immunotherapy.Citation9,Citation10 It can not only enhance the anti-tumor immune response, but can also prevent tumors cells from becoming resistant to the drug. These results are a key reason for the impact of radiotherapy on T cells in the tumor microenvironment. This article summarizes the recent scientific findings on this issue.
1. T-cell functional classification and transformation relationship in tumor microenvironment
Tumor microenvironment (TME) is the internal environment in which tumor cells are produced and survived, has complex regulatory mechanisms. In 1880, PagetCitation11 first proposed the renown “seed and soil” theory, which proposed the notion of TME as a kind of soil that provides the basis for the occurrence, development, invasion, and metastasis of tumors.
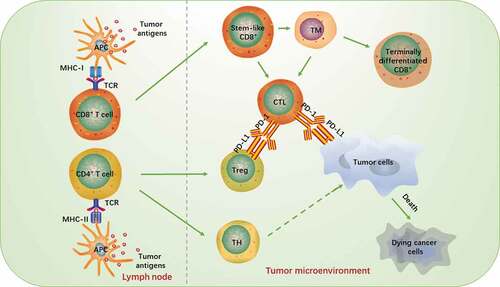
Recently, more and more researches have focused on the subsets of T cell. As an important component of TME, these subsets of T cell interact and restrict each other to form a complex network structure, and thereby maintaining a dynamic balance. In TME, antigen-presenting cells (APCs) are mostly needed for the process using T cells to killing tumors. Their surface includes a high variety of costimulatory factors, which can induce T cell activation by binding to corresponding antibodies on the surface of T cells. This specific process is shown in .
1.1 Classification of T cells
The intensity of the immune response in TME is regulated by various T cell types.Citation12 Functionally, T cells in TME, it can be classified into cytotoxic T cells, regulatory T cells, auxiliary T cells and memory T cells.
1.1.1 Cytotoxic T cells
Cytotoxic T cell (CTL): This type of T cell can identify the infected cells by recognizing the pathogenic antigens, and subsequently kill them. CTLs are the main force behind cellular immunity, and their main surface marker is CD8. It can mediate the selective apoptosis of tumor cells by releasing perforin, granzyme, Fas or tumor necrosis factor (TNF).Citation13 It has been confirmed that the number, location, and quality of CD8 + T cells positively correlate with the prognosis of many malignant tumors.Citation14
1.1.2 Regulatory T cells
Regulatory T cells (Treg): These cells are responsible for up- and downregulation of the immune response of the body. They are an essential force that maintains immune tolerance and prevents autoimmune reactions.Citation15,Citation16 Tregs have many phenotypes, among which are the CD4+ T cell, which can inhibit the immune response against autologous tumor cells. This inhibition is considered to be the main reason for the failure of immunotherapy.Citation17,Citation18
1.1.3 Helper T cells
Helper T cell (TH): These cells play an intermediate role in the immune response. They can activate other types of immune cells through amplification of their own numbers, causing them to directly participate in the immune response. The main surface marker is CD4. Studies have found that the expression of TH17 is associated with poor prognosis in patients with resectable colorectal cancer (CRC),Citation19 while the presence of TH1 is correlated with longer disease-free survival.
1.1.4 Memory T cells
Memory T cells (TM): These cells play an immune role when they are re-exposured to known antigens. However, no specific surface markers have been found. A large number of research data have shown that the long-term anti-tumor function of the body largely depends on the cell differentiation state during TM metastasis.Citation20 T memory stem cells (TSCM) and central memory (TCM) T cells, which have lower differentiation, longer life spanCitation21 and higher recombination ability.Citation22,Citation23 They are potentially effective in mediating anti-tumor immunity.
1.2 Transformation between CD4-CD8 lineages
Mature T cells mainly differentiate into two lineages, CD4+ and CD8+ T cells, which are different in MHC limitation and function.Citation24,Citation25 CD4+ T cells are usually restricted by MHC-II, while CD8+ T cells require MHC-I to exert cytotoxicity. Both CD4+ and CD8+ T cells can participate in cellular immunity, and there is a transformational relationship between them.
T cells are produced by hematopoietic progenitor cells and they mature in the thymus. They transform from CD4− CD8–double negative (DN) stageCitation26 into CD4+ CD8+-double positive (DP) thymocytes, producing CD4+CD8lo intermediate cells. When they receive TCR-mediated differentiation signals, negative or positive selection will occur. Only positive cells could receive TCR signals and further differentiate into CD4+- or CD8+- single positive (SP) T cells. However, the time, intensity, and duration of TCR signals influence the lineage stereotypes. For example, CD4 differentiation requires a longer stimulation time of TCR signals.Citation27
In addition to the stimulation of TCR signals, the finalization of the differentiation into CD4 or CD8 also requires the participation of many transformation factors. The differentiation into CD4 requires zinc finger proteins Th-POK and GATA3,Citation28,Citation29 as well as the trans-acting T cell factor 1 (TCF-1) and lymphoid enhancer factor 1 (LEF-1)Citation30 while the differentiation into CD8 is inseparable from the regulation of RUNX factor.Citation31 It has been found that Th-POK-RUNX is a key adjustment axis in the determining whether T cells differentiate into the CD4 or CD8 lineage, it plays a regulatory role through a negative feedback mechanism.Citation32,Citation33 When the presence of key transforming factors is absent or decreased in the course of the T cells maturation, the intermediate cells of CD4+CD8lo that should have developed into CD4+ SP, may instead develop into CD8+ SP, and vice versa. Therefore, we may be able to enhance the immune response of T cells in TME by re-engineer the differentiation of T cells into either CD4 or CD8 lineage.
1.3 Stem-like and terminally differentiated CD8+ T cells
In recent years, the team of Professor Haydn Kissick has found that tumor-infiltrating CD8+ T cells can be divided into two categories: stem-like CD8+ T cells and terminally differentiated CD8+ T cells.Citation34 Between them, the stem-like CD8+ T cells express low checkpoint molecules, high co-stimulatory molecules CD28, and transcription factor TCF-1, whereas the terminally differentiate type highly expresses immune checkpoint molecules TIM3, PD-1, CTLA4, and TIGIT. Stem-like and terminally differentiated CD8+ T cells accumulate in areas where antigen-presenting cells are abundant. Their expression levels are closely related to the efficacy of patients’ anti-tumor therapy, so they can be used as biomarkers for predicting treatment efficacy.Citation35
Differentiated lineage of CD8+ T cells has been found in patients with chronic lymphocytic choriomeningitis virus (LCMV) infection.Citation36 The general direction is that TCF-1+ stem-like CD8+ T cells first differentiate into CD101-Tim-3+ transient cells, and then transformed into CD101+ Tim-3+ terminally differentiated cells. The differentiation process of CD8+ T cells in tumors is similar.Citation37,Citation38,Citation38,Citation39 It can be seen that TCF-1 is a key transcription factor for stem-like CD8+ T cells,Citation39,Citation40,Citation42 which is essential for T cell proliferation and activation.Citation39,Citation41 TCF-1/TCF-7+ CD8+ T cells have been detected in human tumors, and their increased expression is associated with positive responses to immune checkpoint blockade therapy.Citation37,Citation42–44 Professor Zhang Zemin’s research teamCitation45 developed the STARTRAC method and found that there was a significant TCR overlap between the stem-like type and the terminally differentiated type of CD8+ T cells, which may determine whether tumor-infiltrating effector memory CD8+ T cells differentiate into effector T cells or “exhausted “ CD8+ T cells. This discovery also explained the source of the terminally differentiate type T cells in TME. Other studies have also found that the response of T cells in tumors depends on the ability of CD8 stem-like cells to produce terminally differentiated cells.Citation34,Citation39,Citation46–49 In conclusion, reversing the status of CD8 + T cells may change the efficacy of tumor immunotherapy.
2. Mechanism and effect of radiotherapy on T cells in TME
Radiotherapy plays an important role in the treatment of many tumors, such as neoadjuvant radiochemotherapyCitation50 and neoadjuvant radiotherapyCitation51 in patients with stage II and III rectal cancer, as well as concurrent postoperative radiotherapy and chemotherapy.Citation52,Citation53 RT can play an anti-tumor role by increasing the release of cytokines, recruiting antigen-presenting cells and their surface epitopesCitation54and enhancing the expression of antigens or receptors on the membrane of tumors or immune cells.Citation55–57 RT can not only control local lesions, but also produce distant effects,Citation58 which may be related to biological characteristics of tumor cells and factors such as TME remodeling caused by RT.Citation59 Previous studies have shown that RT can regulate the innate and adaptive immune systems,Citation60,Citation61 which mean that they have potential immunomodulatory effects. Therefore, it is evident that RT can affect T cells in TME. The following sections will list the various mechanisms of how RT regulates T cells in TME:
RT can enhance the binding of T cells onto antigens. RT can directly kill or induce tumor cell apoptosis by inducing DNA double-strand breaks,Citation62 up-regulating and releasing tumor-associated antigens (TAAs),Citation63–65 including adenosine triphosphate (ATP) and HMGB1 proteinCitation66,Citation67 released by stress cells, and increasing the expression level of damage-related model molecules (DAMPs)Citation68–70 to induce DC maturation. Studies have also confirmed that RT can induce the diversity of TCR sequencesCitation71 and increase the expression of MHC-I,Citation72 which enhances the directional selection of CD8+ T cells. Furthermore, RT can also increase the effectiveness of antigen presentation, thereby kickstarting the initial differentiation of T cells, and finally activating the memory of adaptive immunity of the body.
RT induces the production and release of cytokines. This is done by activating the cGAS-STING signaling pathway and increasing the sensitivity of tumor cells to RT.Citation73–78 The activation of this pathway increases the release of interferon regulatory factors (IRFs) and NF-κB, and the high expression of the latter can induce the release of type I interferons (INF-α and INF-β),Citation79 which is used in responding to the activation of pattern recognition receptors. The expression of NF-κB is also correlated with the release of type II interferon (IFN-γ). IFN-γ is mainly produced by T cells when recognizing homologous antigens. RT can up-regulate the expression of IFN-γCitation80 thereby activating anti-tumor immune response. Other studies have shown that RT promotes the secretion of CXCL16 in tumor cells. This cytokine can be combined with TH1 cells and CXCR6 on activated CD8 + T cellsCitation80 to promote the infiltration of local immune cellsCitation81 and induce the anti-tumor response of the body.Citation73,Citation82,Citation83
RT can regulate the ratio of CD4/CD8 T cells in TME by increasing the number of Tregs cells in TME.Citation84 Treg cells can convert ATP into adenosine, and they participate in immunosuppression by increasing the production of CTLA4, TGF β, IL-10, CD39, and CD73.Citation85,Citation86 According to various reports, RT can increase the number of Foxp3+ CD4+ Tregs in tumors, while the ratio of CD8+ T/Tregs decreases,Citation87 exerting a negative anti-tumor immunomodulatory effect.Citation88,Citation89.
RT changes the physical properties of TME, which in turn improves the recruitment of T cells. For example, the intercellular adhesion of molecule-1 (ICAM-1) on the cell surface is enhanced through the regulation of the extracellular matrix (ECM) interstitial fluid pressure,Citation90 and tissue stiffness,Citation91 as well as the oxygen content and pH value of tumor nourishment vessels. The expression of ICAM-1 in endothelial cells promotes the translocation of leukocytes to the endothelial cells, and induces the occurrence of inflammatory TME,Citation92 so as to increase the infiltration of T cells into tumor tissues.
RT increases the expression of PD-L1 in immune checkpoint. This is achieved through activating the cGAS-STING pathway, which influences the production of interferon through IRFs and NF-κB,Citation79 and promotes the expression of PD-L1 on the surface of tumor cell membranes,Citation93 thus affecting the response of immune checkpoint inhibitors.Citation94,Citation95 RT has the potential to trigger the anti-tumor immune responses by improving the efficacy of immune checkpoint blockade therapy (ICBs).Citation96,Citation97
RT may alter the differentiation between CD4-CD8 categories. In the study of Jacek et al., GATA-3 expression was significantly increased after stereotactic ablation and radiotherapy, which was the main regulator of humoral immune responses.Citation98 GATA3, was the first transcription factor identified in CD4 differentiation. In the absence of GATA3 expression, MHC-II restricted thymocytes can be “redirected” to differentiate into CD8+ T cells.Citation99 At present, there is no research on the relationship between radiotherapy and the Th-POK-RUNX axis.
3. Limitations of the application of PD-1/PD-L1 inhibitors
In recent years, PD-1/PD-L1 immune checkpoint inhibitors have become the focus of tumor treatment,Citation100,Citation101 and their expression level has been found to be related to the pathological type, clinical pathological stage, drug resistance,Citation102 and the prognosis of some tumors.Citation103–105 The expression level of PD-1/PD-L1 is significantly different in different types of tumors or different subtypes of the same tumor.Citation78 While there are some tumor patients with positive PD-L1 who do not respond to the treatment, there are also some patients with negative PD-L1 who can benefit from it.Citation106,Citation107 Therefore, the efficacy of ICBs may be related to the MSI classification and drug resistance of tumors.
3.1 The effect of MSI classification on efficacy
The results of clinical trials of anti-PD-1/PD-L1 drugs suggest that, compared with MSS tumors, patients with MSI tumors can achieve better clinical remission and longer survival time.Citation108,Citation109 These advantages may be attributed to the expression of multiple immune molecules in TME of patients with MSI type. At present, solid tumors with high incidence of MSI-H are known to include endometrial cancer (20% ~ 30%), gastric cancer (15% ~ 20%) and colorectal cancer (12 ~ 15%, of which stage IV colorectal cancer is 4% ~ 5%).Citation110 It has been pointed out that MSI-H is the key factor for the benefits of ICBs, which has nothing to do with specific type of cancer.Citation59 It can be seen that MSI classification has an important impact on the application of ICBs, while MSS tumors account for a large proportion. The quest to reverse the sensitivity of MSS to ICBs, so that more cancer patients can benefit from it, will be a long-term and difficult one in anti-tumor treatment.
3.2 The effect of drug resistance on efficacy
The difference in efficacy of PD-1/PD-L1 blockers may be attributed to tumors that become drug-resistant.Citation111 The emergence of drug resistance is not only related to the heterogeneity of tumor,Citation112 but also caused by the changes in the composition and function of T cells in TME.Citation113 At present, the mechanism of resistance to PD-1/PD-L1 is generally divided into two types: primary resistance and acquired resistance.
3.2.1 Primary resistance
Primary drug resistance means that the patient’s display drug resistance upon his/her first use of ICBs, also known as “congenital drug resistance”Citation80 The primary resistance toward PD-1/PD-L1 blocking therapy is mainly caused by the lack of PD-L1 expression and the production of inhibitory cytokines by some immune cells in TME. These cytokines inhibit the activation and function of T cells by influencing the activation of corresponding pathways and inducing adaptive immune response. The specific mechanisms include:
3.2.1.1 Activation of MAPK pathway
The MAPK pathway plays a vital role in cell proliferation, differentiation, migration, apoptosis, and survival.Citation114 It can promote the production of immunomodulatory cytokines,Citation115 and promote the activation and infiltration of CD8+ T cells.
3.2.1.2 Loss of PTEN expression
The absence of PTEN increases the expression of immunosuppressive cytokines,Citation116 and decreases the cytotoxicity CD8+ T cells and the killing effect of NK cellsCitation117 in TME. These lead to an increase of resistance in tumor cells and inhibition of their apoptosis, which in turn leads to poor prognosis.Citation118
3.2.1.3 Enhancement of PI3K/Akt signaling pathway
The abnormal activation of the PI3K/AKT pathway can be observed in many human tumors.Citation119,Citation120 The activation of this pathway leads to an increase in the expression of PD-L1, mediates the immune escape of tumor cells, and promotes the occurrence and progress of tumors.Citation80,Citation121
3.2.1.4 Wnt-β-catenin signaling pathway is continuously activated
The typical WNT-β-catenin signal transduction pathway is activated due to the binding of WNT family proteins to cell surface receptors, which leads to the nuclear translocation and transcriptional activation of β-catenin,Citation122 reduction in the production of immunosuppressive cytokines, and inhibition of T cell recruitments,Citation80,Citation123 eventually allowing for the occurrence of immune tolerance.Citation124
3.2.1.5 Loss of IFN-γ signaling pathway
IFN-γ can up-regulate the expression of MHC and PD-L1, thereby increasing the positive response to PD-1 therapy.Citation125 Therefore, the absence of IFN-γ and its receptor may cause the occurrence of or maintain drug resistance in tumor cells,Citation126,Citation127 resulting in no response to ICBs.Citation128,Citation129
3.2.1.6 Epigenetic modification changes
Epigenetic modification of cells, including DNA methylation, histone methylation/acetylation and microRNA regulation, can regulate not only the processing and presentation of antigens, but also the expression of PD-1Citation130/PD-L1.Citation131 Therefore, altering or regulating the process of epigenetic modification of cells may exacerbate the drug resistance of tumors.
3.2.1.7 Overexpression of CDK4/CDK6
The activation and overexpression of the cell division cycle (CDK) genes and defects in the CDK functional inhibitors (CDKIs) are found in most tumor cells.Citation132 Among them, CDK4/CDK6 are related to the tumorigenesis.Citation133–135 When combined with Cyclin D, they can cause cells to transition from the G1 phase to the S phase in the cell cycle,Citation122 which in turn drives tumor resistance.Citation136
3.2.2 Acquired resistance
Acquired drug resistance means that patients initially respond to ICBs treatment, but as the disease progress or recur during treatment (rather than after discontinuation), reapplying immune checkpoint blockers becomes ineffective. The mechanisms of acquired drug resistance mostly involve some changes the activity of T cells,Citation137 which are triggered by a mutation of β2 M gene in MHC-I molecules, the up-regulation of other immune checkpoints, and the activation of JAK/STAT/IFN-γ pathway.
3.2.2.1 β2 M gene mutation
Immune editing can cause mutations in the β2 M gene of MHC-I, resulting in the loss of HLA and reduce diversity,Citation43,Citation125 altering the presentation of antigens,Citation59,Citation138,Citation139 thereby inhibiting the activation and function of T cells.Citation140 Therefore, the mutation of β2 M gene is the key to the development of resistance toward PD-1/PD-L1 therapy.Citation125,Citation128,Citation141,Citation142
3.2.2.2 Up-regulation of other immune checkpoints
A study has found that CLTA-4, TIM-3, LAG-3, B and T lymphocyte attenuators (BTLA), NKG2A, VISTA, and TIGIT are involved in the regulation of PD-1/PD-L1 in TME.Citation143–145 If PD-1/PD-L1 checkpoint is blocked, the expression of other molecular immune monitoring points of tumor cells will be up-regulated,Citation130 enabling those cells to evade the anti-tumor immune response of the body.
3.2.2.3 Activation of JAK/STAT/IFN-γ pathway
JAK1 and JAK2 are the key signals of IFN.Citation80,Citation125 The activation of IFN-γ signaling pathway can be found in drug-resistant tumor cells, and it prompts high transcription of genes such as JAK1 and STAT1, as well as the expression of Apelin receptor, which encodes interferon signal regulators. They can increase the sensitivity of tumor cells to IFN-γCitation122 The JAK2 gene is on the same chromosome as PD-L1 and PD-L2, which can be further enhanced by co-amplification.Citation80 Therefore, mutations in JAK1/2 play an important role in anti-PD-1/PD-L1 therapy.Citation146
4. Application of radiotherapy combined with PD-1/PD-L1 blocker
Previous studies have found that the response rate of PD-1/PD-L1 blockade therapy is extremely low,Citation45,Citation147 indicating that there are limitations to using this treatment alone. At present, RT combined with an anti-PD-1/PD-L1 treatment is more effective than only using radiotherapy Citation148 or anti-PD-1/PD-L1 antibody treatment alone.Citation149,Citation150Many clinical trials have confirmed that RT, when combined with anti-PD-1/PD-L1 therapy, has a synergistic sensitization effect.Citation151,Citation152 Therefore, combination therapy strategies can provide more drug targets and improve efficacy.
4.1 The mechanisms of synergy between RT and PD-1/PD-L1 therapies
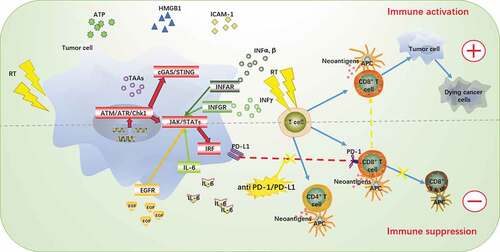
As described above in detail, RT can induce DNA double-strand breaks in tumor cells, up-regulate the expression of TAAsCitation70 and DAMPs,Citation75 and then promote the maturation of DCs. Mature DCs can recruit and activate naive T cells in TME. The effect of RT on the differentiation of CD4-CD8 lineages can also increase the infiltration of CD8 T cells in TME,Citation99 which leads to the increase of the PD-1 expression. In addition to its positive immunomodulatory effect, RT can also induce an immunosuppressive microenvironment.Citation153 Recent preclinical studies have shown that RT can up-regulate the expression of PD-L1 through the use of three mechanisms. However, they are ultimately achieved through STAT/IRF pathway.Citation154 The details are as follows: 1) DNA damage response activated the ATM/ATR/Chk1 kinase and cGAS/STING pathway.Citation155,Citation156 2) Increased secretion of IFN,Citation157 especially IFN – γCitation158 can continuously stimulate the JAK-STAT-IRF pathway, thereby promoting the production of PD-L1 for a long time.Citation129,Citation153 3) After irradiation, the secretion of epidermal growth factor (EGF) and IL-6 increased, which enhanced the EGFR signal and activated the IL-6/JAK/STAT3 pathway. The combination of the PD-1 of T cells with the PD-L1 of tumor cells will make T cells lose their ability to kill tumor cells, and lead to immune escape and resistance. Therefore, if PD-1/PD-L1 blockers are administered in the early stage of RT, the activity of “exhausted” CD8+ T cells can be restored and an effective anti-tumor immune response can be produced.Citation159 The detailed process is shown in .
4.2 Influencing factors of combination therapy
Evidently, if one hopes to achieve the synergistic effect of combination therapy,Citation55,Citation93,Citation160,Citation161 they first need to formulate a reasonable treatment plan and analyze possible influential factors. The combined application can regulate and amplify the remote effect of RT,Citation54 increase the mutation load,Citation162 and improve the immune function and anti-tumor capabilities.Citation163,Citation164 With further research, we have learned that the therapeutic effect and risk of toxicity of radiotherapy combined with anti-PD-1/PD-L1 depend on the sequence of its application,Citation165 as well as the grade and dose of radiation therapy.Citation166
Dove-di et al.Citation167 compared three different combined treatment regimens using the mouse model. The results showed that in order to achieve long-term tumor control, anti-PD-L1 therapy should be administered simultaneously with radiotherapy. The experiments of Chen et al.Citation168 also showed that the simultaneous application of radiotherapy and PD-1/PD-L1 monoclonal antibody treatment regimen was significantly better than sequential therapy and radiotherapy alone, in terms of survival period and 1-year progression-free survival rate. Recently, the PFS of patients with locally advanced unresectable stage III non-small-cell lung cancer (NSCLC), who were treated with pembrolizumab and concurrent chemoradiotherapy, was 69.7% after 12 months, and had a high toxicity tolerance.Citation169 However, the PEMBRO-RT trial showed that the administration of anti-PD-1/PD-L1, if administered within one week following radiotherapy, significantly prolonged OS and PFS in PD-L1 negative NSCLC patients, thus supporting the feasibility of sequential therapy.Citation152 The initial publication of the KEYNOTE-001 and PACIFIC trials demonstrated the feasibility and effectiveness of sequential radiotherapy combined with PD-1/PD-L1 inhibitors.Citation149,Citation170 Additionally, the clinical results of stereotactic radiotherapy and anti-PD-1 therapy for the treatment of melanoma brain metastases showed no significant difference in OS and local control.Citation171 Komori T et al. discovered that the toxicity risk demonstrated no impact from the different treatment sequences.Citation172 At present, there is no worldwide consensus on the optimal dosage and mode of combination therapy. For optimal dose fractions, one report found that a single high-dose irradiation (20 Gy in a single fraction) can lead to reduced immunogenicity.Citation74 However, in another clinical trial, where patients were divided into three radiotherapy regimens (15–24 Gy in 1 fraction, 18–24 Gy in 3 fractions, or 25 Gy in 5 fractions), the results showed that there was no difference in OS and PFS.Citation168 Therefore, in order to improve the efficiency of the treatment and achieve long-term control of the disease, more clinical trials are needed to verify the sequence, dose, and grading of the combined treatment.
4.3 Screening of benefit groups in combination therapy
In recent years, many preclinical and clinical studies have shown that,Citation79,Citation173–175 in advanced or metastatic melanoma, colorectal cancer, Hodgkin’s lymphoma, breast cancer, non-small cell lung cancer, renal cell carcinoma and esophageal cancer, radiotherapy combined with PD-1/PD-L1 inhibitors can improve the chances of long-term survival and prevent tumor recurrence.Citation176–179 So, figuring out how to select the beneficiaries of this treatment more accurately and effectively has become an urgent task.
A variety of biomarkers have been observed, including the expression level of CTL,Citation35,Citation180 PD-1/PD-L1 in TME,Citation181 the apparent diffusion coefficient,Citation182,Citation183 mismatch repair (MMR), microsatellite instability,Citation184,Citation185 and some neutrophil-lymphocyte ratio,Citation186which can predict the efficacy of RT combined with PD-1/PD-L1 inhibitors to some extent. Although no precise predictive biomarkers have been found, previous studies have found that patients with a history of RT have higher PFS, OS, and response rates than those who do not receive anti-PD-1/PD-L1 antibodies.Citation187,Citation188 The OS difference was only found in the PD-L1 negative subgroup when combined therapy was used.Citation152 These results suggest that radiotherapy may be an important factor in reversing PD-L1 expression. Therefore, the accuracy of predictions can be improved by combining multiple prediction indicators.
5. Summary
In recent years, with the continuous development of treatment methods, health-care technologies, and patients’ growing demands for a better quality of life, the treatment plan of tumor patients has gradually changed from standardization to precision and individualization. Radiotherapy can not only reshape T cells in TME, but also provide a basis for immune checkpoint-blocked therapy. It also shows great potential in reversing the immune resistance of tumor cells. One study found that the combination of radiotherapy and immunotherapy can benefit patients with giant lung metastatic melanoma.Citation189 It is imperative that, when combining radiotherapy with anti-PD-1/PD-L1 antibody treatment, medical professionals pay attention to its adverse reactions, such as the increase in the incidence of radiation pneumonia.Citation149 At present, more clinical studies are needed to confirm whether (1) radiotherapy can facilitate the conversion between T cell subsets and their specific transformation mechanism; (2) the combined treatment of radiotherapy with PD-1/PD-L1 inhibitors can be used as a first-line treatment program for more tumor patients; (3) the combined application can reverse the immune tolerance of MSS patients and the mechanism of action. It is believed that in the field of tumor therapy, the combined treatment of radiotherapy and immunotherapy will have a broader application prospects in the near future.
Author contributions
Liu Yanlong and Chen Chen made substantial contributions to the conception and design of this study. Chen Chen wrote the first draft of the manuscript. All authors made substantial contributions to the acquisition or analysis of data used in this article. Cui Binbin revised the manuscript for the purpose of important intellectual content.
Ethical approval and ethical standards
The study was performed in accordance with the Declaration of Helsinki. This study does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent is not required for this study.
Disclosure of potential conlficts of interest
The authors declare no potential conflict of interest. All authors approve of this manuscript.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492.
- Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–53.
- Muroyama Y, Nirschl TR, Kochel CM, Lopez-Bujanda Z, Theodros D, Mao W, Carrera-Haro MA, Ghasemzadeh A, Marciscano AE, Velarde E, et al. Stereotactic radiotherapy increases functionally suppressive regulatory T cells in the tumor microenvironment. Cancer Immunol Res. 2017;5(11):992–1004. doi:10.1158/2326-6066.CIR-17-0040.
- Darragh LB, Oweida AJ, Karam SD. Overcoming resistance to combination radiation-immunotherapy: a focus on contributing pathways within the tumor microenvironment. Front Immunol. 2018;9:3154. doi:10.3389/fimmu.2018.03154.
- Jaffray DA. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol. 2012;9(12):688–99. doi:10.1038/nrclinonc.2012.194.
- Janssen LME, Ramsay EE, Logsdon CD, Overwijk WW. The immune system in cancer metastasis: friend or foe? J Immunother Cancer. 2017;5:79. doi:10.1186/s40425-017-0283-9.
- Gato-Canas M, Arasanz H, Blanco-Luquin I, Glaria E, Arteta-Sanchez V, Kochan G, Escors D. Novel immunotherapies for the treatment of melanoma. Immunotherapy. 2016;8:613–32. doi:10.2217/imt-2015-0024.
- Chen G. Current situation and progress of immunotherapy for colorectal cancer. J Precis Med. 2019;34:1–5.
- Bockel S, Durand B, Deutsch E. Combining radiation therapy and cancer immune therapies: from preclinical findings to clinical applications. Cancer Radiother. 2018;22:567–80. doi:10.1016/j.canrad.2018.07.136.
- De Ruysscher D. Combination of radiotherapy and immune treatment: first clinical data. Cancer Radiother. 2018;22:564–66. doi:10.1016/j.canrad.2018.07.128.
- Paget S. The distribution of secondary growths in cancer of the breast 1889. Cancer Metastasis Rev. 1989;8:98–101.
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30. doi:10.1038/nature21349.
- Fousek K, Ahmed N. The evolution of T-cell therapies for solid malignancies. Clin Cancer Res. 2015;21(15):3384–92. doi:10.1158/1078-0432.CCR-14-2675.
- Ganesan AP, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, Samaniego-Castruita D, Singh D, Seumois G, Alzetani A, Woo E, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol. 2017;18:940–50. doi:10.1038/ni.3775.
- Panduro M, Benoist C, Mathis D. Tissue tregs. Annu Rev Immunol. 2016;34(1):609–33. doi:10.1146/annurev-immunol-032712-095948.
- Ramsdell F, Ziegler SF. FOXP3 and scurfy: how it all began. Nat Rev Immunol. 2014;14(5):343–49. doi:10.1038/nri3650.
- Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, Zhao L, Vatan L, Shao I, Szeliga W, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18(12):1332–41. doi:10.1038/ni.3868.
- Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, Meinhof K, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. 2017;169:750–65 e17. doi:10.1016/j.cell.2017.04.014.
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman W-H, Pagès F, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71(4):1263–71. doi:10.1158/0008-5472.CAN-10-2907.
- Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;43:2797–809. doi:10.1002/eji.201343751.
- Biasco L, Scala S, Basso Ricci L, Dionisio F, Baricordi C, Calabria A, Giannelli S, Cieri N, Barzaghi F, Pajno R, et al. In vivo tracking of T cells in humans unveils decade-long survival and activity of genetically modified T memory stem cells. Sci Transl Med. 2015;7(273):273ra13. doi:10.1126/scitranslmed.3010314.
- Cieri N, Oliveira G, Greco R, Forcato M, Taccioli C, Cianciotti B, Valtolina V, Noviello M, Vago L, Bondanza A, et al. Generation of human memory stem T cells after haploidentical T-replete hematopoietic stem cell transplantation. Blood. 2015;125(18):2865–74. doi:10.1182/blood-2014-11-608539.
- Roberto A, Castagna L, Zanon V, Bramanti S, Crocchiolo R, McLaren JE, Gandolfi S, Tentorio P, Sarina B, Timofeeva I, Santoro A, et al. Role of naive-derived T memory stem cells in T-cell reconstitution following allogeneic transplantation. Blood. 2015;125:2855–64.
- Xiong Y, Bosselut R. CD4-CD8 differentiation in the thymus: connecting circuits and building memories. Curr Opin Immunol. 2012;24:139–45. doi:10.1016/j.coi.2012.02.002.
- Robins E, Zheng M, Ni Q, Liu S, Liang C, Zhang B, Guo J, Zhuang Y, He Y-W, Zhu P, et al. Conversion of effector CD4+ T cells to a CD8+ MHC II-recognizing lineage. Cell Mol Immunol. 2020. doi:10.1038/s41423-019-0347-5.
- Hashimoto M, Kamphorst AO, Im SJ, Kissick HT, Pillai RN, Ramalingam SS, Araki K, Ahmed R. CD8 T Cell Exhaustion in Chronic Infection and Cancer: opportunities for Interventions. Annu Rev Med. 2018;69:301–18. doi:10.1146/annurev-med-012017-043208.
- Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801.
- Rothenberg EV. The chromatin landscape and transcription factors in T cell programming. Trends Immunol. 2014;35(5):195–204. doi:10.1016/j.it.2014.03.001.
- Ciucci T, Vacchio MS, Bosselut R. A STAT3-dependent transcriptional circuitry inhibits cytotoxic gene expression in T cells. Proc Natl Acad Sci U S A. 2017;114(50):13236–41. doi:10.1073/pnas.1711160114.
- Steinke FC, Yu S, Zhou X, He B, Yang W, Zhou B, Kawamoto H, Zhu J, Tan K, Xue -H-H, et al. TCF-1 and LEF-1 act upstream of Th-POK to promote the CD4+ T cell fate and interact with Runx3 to silence Cd4 in CD8+ T cells. Nat Immunol. 2014;15(7):646–56. doi:10.1038/ni.2897.
- Xing S, Shao P, Li F, Zhao X, Seo W, Wheat JC, Ramasamy S, Wang J, Li X, Peng W, et al. Tle corepressors are differentially partitioned to instruct CD8 + T cell lineage choice and identity. J Exp Med. 2018;215(8):2211–26. doi:10.1084/jem.20171514.
- Vacchio MS, Bosselut R. What happens in the thymus does not stay in the thymus: how T cells recycle the CD4+–CD8+ lineage commitment transcriptional circuitry to control their function. J Immunol. 2016;196(12):4848–56. doi:10.4049/jimmunol.1600415.
- Shan Q, Zeng Z, Xing S, Li F, Hartwig SM, Gullicksrud JA, Kurup SP, Van Braeckel-Budimir N, Su Y, Martin MD, Varga SM, et al. The transcription factor Runx3 guards cytotoxic CD8(+) effector T cells against deviation towards follicular helper T cell lineage. Nat Immunol. 2017;18:931–39. doi:10.1038/ni.3773.
- Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, Cardenas M, Wilkinson S, Lake R, Sowalsky AG, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019;576(7787):465–70. doi:10.1038/s41586-019-1836-5.
- Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133–50.
- Hudson WH, Gensheimer J, Hashimoto M, Wieland A, Valanparambil RM, Li P, Lin JX, Konieczny BT, Im SJ, Freeman GJ, Leonard WJ, et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1(+) stem-like CD8(+) T cells during chronic infection. Immunity. 2019;51:1043–58 e4. doi:10.1016/j.immuni.2019.11.002.
- Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, Carmona SJ, Scarpellino L, Gfeller D, Pradervand S, Luther SA, et al. Intratumoral Tcf1(+)PD-1(+)CD8(+) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 2019;50:195–211 e10. doi:10.1016/j.immuni.2018.12.021.
- Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, Kiialainen, A., Hanhart, J., Schill, C., Hess, C., et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24:994–1004. doi:10.1038/s41591-018-0057-z.
- Utzschneider DT, Charmoy M, Chennupati V, Pousse L, Ferreira DP, Calderon-Copete S, Danilo, M., Alfei, F., Hofmann, M., Wieland, D., et al. T cell factor 1-expressing memory-like CD8(+) T cells sustain the immune response to chronic viral infections. Immunity. 2016;45:415–27. doi:10.1016/j.immuni.2016.07.021.
- Zhang J, Zheng X, Zhang Q. EglN2 positively regulates mitochondrial function in breast cancer. Mol Cell Oncol. 2016;3:e1120845. doi:10.1080/23723556.2015.1120845.
- Lee J, Ahn E, Kissick HT, Ahmed R. Reinvigorating exhausted T cells by blockade of the PD-1 pathway. For Immunopathol Dis Therap. 2015;6:7–17.
- Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20(3):326–36. doi:10.1038/s41590-019-0312-6.
- Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane JP, Bjorgaard, S L., Hammond, M R., Vitzthum, H., Blackmon, S M., et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun. 2017;8:1136. doi:10.1038/s41467-017-01062-w.
- Kurtulus S, Madi A, Escobar G, Klapholz M, Nyman J, Christian E, Pawlak, M., Dionne, D., Xia, J., Rozenblatt-Rosen, O., et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1(-)CD8(+) tumor-infiltrating T cells. Immunity. 2019;50:181–94 e6. doi:10.1016/j.immuni.2018.11.014.
- Zhang L, Yu X, Zheng L, Zhang Y, Li Y, Fang Q, Gao, R., Kang, B., Zhang, Q., Huang, J Y., et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature. 2018;564:268–72.
- Brummelman J, Mazza EMC, Alvisi G, Colombo FS, Grilli A, Mikulak J, Mavilio D, Alloisio M, Ferrari F, Lopci E, et al. High-dimensional single cell analysis identifies stem-like cytotoxic CD8+ T cells infiltrating human tumors. J Exp Med. 2018;215(10):2520–35. doi:10.1084/jem.20180684.
- Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–21. doi:10.1038/nature19330.
- Wieland D, Kemming J, Schuch A, Emmerich F, Knolle P, Neumann-Haefelin C, Held W, Zehn D, Hofmann M, Thimme R, et al. TCF1+ hepatitis C virus-specific CD8+ T cells are maintained after cessation of chronic antigen stimulation. Nat Commun. 2017;8(1):15050. doi:10.1038/ncomms15050.
- Gullicksrud JA, Li F, Xing S, Zeng Z, Peng W, Badovinac VP, Harty, J T., Xue, H-H Differential requirements for Tcf1 long isoforms in CD8(+) and CD4(+) T cell responses to acute viral infection. J Immunol. 2017;199:911–19. doi:10.4049/jimmunol.1700595.
- McNichol L, Lund C, Rosen T, Gray M. Medical adhesives and patient safety: state of the science: consensus statements for the assessment, prevention, and treatment of adhesive-related skin injuries. Orthop Nurs. 2013;32(5):267–81. doi:10.1097/NOR.0b013e3182a39caf.
- Wang X, Zheng B, Lu X, Bai R, Feng L, Wang Q, Zhao, Y., He, S., Preoperative short-course radiotherapy and long-course radiochemotherapy for locally advanced rectal cancer: meta-analysis with trial sequential analysis of long-term survival data. PLoS One. 2018;13:e0200142. doi:10.1371/journal.pone.0200142.
- Tang Y, Jin J, Zhu Y, Liu S, Yuan X, Wang W, Wang X, Zhang Z, Tian Y, Gao Y, et al. Consensus and contouring atlas for the delineation of clinical target volume in pre-/post-operative image-guided intensity mdulated radiotherapy for rectal cancer. J Clin Surg. 2018;27:227–234.
- Tao J, Wang G. Prognostic factors of pathological complete remission after neoadjuvant radiotherapy and chemotherapy for locally advanced rectal cancer. J Clin Surg. 2018;26:756–59.
- Marciscano AE, Walker JM, McGee HM, Kim MM, Kunos CA, Monjazeb AM, Shiao, S L., Tran, P T., Ahmed, M M. Incorporating radiation oncology into immunotherapy: proceedings from the ASTRO-SITC-NCI immunotherapy workshop. J Immunother Cancer. 2018;6:6. doi:10.1186/s40425-018-0317-y.
- Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14:365–79.
- Diegeler S, Hellweg CE. Intercellular communication of tumor cells and immune cells after exposure to different ionizing radiation qualities. Front Immunol. 2017;8:664. doi:10.3389/fimmu.2017.00664.
- Demaria S, Coleman CN, Formenti SC. Radiotherapy: changing the Game in immunotherapy. Trends in Cancer. 2016;2(6):286–94. doi:10.1016/j.trecan.2016.05.002.
- Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 2015;356(1):82–90. doi:10.1016/j.canlet.2013.09.018.
- Strom T, Harrison LB, Giuliano AR, Schell MJ, Eschrich SA, Berglund A, Fulp W, Thapa R, Coppola D, Kim S, et al. Tumour radiosensitivity is associated with immune activation in solid tumours. Eur J Cancer. 2017;84:304–14. doi:10.1016/j.ejca.2017.08.001.
- Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1:1325–32. doi:10.1001/jamaoncol.2015.2756.
- Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese, T L., Drake, C G. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3:345–55. doi:10.1158/2326-6066.CIR-14-0196.
- Ceccaldi R, Rondinelli B, D’Andrea AD. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 2016;26:52–64. doi:10.1016/j.tcb.2015.07.009.
- Chao Y, Xu L, Liang C, Feng L, Xu J, Dong Z, Tian, L., Yi, X., Yang, K., Liu, Z., et al. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat Biomed Eng. 2018;2:611–21. doi:10.1038/s41551-018-0262-6.
- Wu Q, Allouch A, Martins I, Brenner C, Modjtahedi N, Deutsch E, Perfettini, J-L. Modulating both tumor cell death and innate immunity is essential for improving radiation therapy effectiveness. Front Immunol. 2017;8:613. doi:10.3389/fimmu.2017.00613.
- Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM, Chai S, Herring LE, Zhang L, Zhang T, et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12(9):877–82. doi:10.1038/nnano.2017.113.
- Hu ZI, McArthur HL, Ho AY. The abscopal effect of radiation therapy: what is it and how can we use it in breast cancer? Curr Breast Cancer Rep. 2017;9(1):45–51. doi:10.1007/s12609-017-0234-y.
- Onishi M, Okonogi N, Oike T, Yoshimoto Y, Sato H, Suzuki Y, Kamada, T., Nakano, T High linear energy transfer carbon-ion irradiation increases the release of the immune mediator high mobility group box 1 from human cancer cells. J Radiat Res. 2018;59:541–46. doi:10.1093/jrr/rry049.
- Huang CY, Chiang SF, Ke TW, Chen TW, Lan YC, You YS, Shiau, A-C., Chen, W.T.L., Chao, K.S.C. Cytosolic high-mobility group box protein 1 (HMGB1) and/or PD-1+ TILs in the tumor microenvironment may be contributing prognostic biomarkers for patients with locally advanced rectal cancer who have undergone neoadjuvant chemoradiotherapy. Cancer Immunol Immunother. 2018;67:551–62. doi:10.1007/s00262-017-2109-5.
- Dosset M, Vargas TR, Lagrange A, Boidot R, Vegran F, Roussey A, Chalmin F, Dondaine L, Paul C, Marie-Joseph EL, et al. PD-1/PD-L1 pathway: an adaptive immune resistance mechanism to immunogenic chemotherapy in colorectal cancer. Oncoimmunology. 2018;7(6):e1433981. doi:10.1080/2162402X.2018.1433981.
- Liu P, Zhao L, Pol J, Levesque S, Petrazzuolo A, Pfirschke C, Engblom C, Rickelt S, Yamazaki T, Iribarren K, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun. 2019;10(1):1486. doi:10.1038/s41467-019-09415-3.
- Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–77. doi:10.1038/nature14292.
- Dillon MT, Bergerhoff KF, Pedersen M, Whittock H, Crespo-Rodriguez E, Patin EC, Pearson A, Smith HG, Paget JTE, Patel RR, et al. ATR inhibition potentiates the radiation-induced inflammatory tumor microenvironment. Clin Cancer Res. 2019;25(11):3392–403. doi:10.1158/1078-0432.CCR-18-1821.
- Sen T, Rodriguez BL, Chen L, Corte CMD, Morikawa N, Fujimoto J, Cristea S, Nguyen T, Diao L, Li L, et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019;9(5):646–61. doi:10.1158/2159-8290.CD-18-1020.
- Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8(1):15618. doi:10.1038/ncomms15618.
- Mardjuki RE, Carozza JA, Li L. Development of cGAMP-Luc, a sensitive and precise coupled enzyme assay to measure cGAMP in complex biological samples. J Biol Chem. 2020;295(15):4881–92. doi:10.1074/jbc.RA119.012170.
- Marcus A, Mao AJ, Lensink-Vasan M, Wang L, Vance RE, Raulet DH. Tumor-derived cGAMP triggers a STING-mediated interferon response in non-tumor cells to activate the NK cell response. Immunity. 2018;49(4):754–63 e4. doi:10.1016/j.immuni.2018.09.016.
- Diamond JM, Vanpouille-Box C, Spada S, Rudqvist NP, Chapman JR, Ueberheide BM, Pilones, K A., Sarfraz, Y., Formenti, S C., Demaria, S., et al. Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol Res. 2018;6:910–20. doi:10.1158/2326-6066.CIR-17-0581.
- Gullo I, Carvalho J, Martins D, Lemos D, Monteiro AR, Ferreira M, Das K, Tan P, Oliveira C, Carneiro F, Oliveira P., et al. The transcriptomic landscape of gastric cancer: insights into epstein-barr virus infected and microsatellite unstable tumors. Int J Mol Sci. 2018;19(7):2079.
- Chiang SF, Huang CY, Ke TW, Chen TW, Lan YC, You YS, Chen, W.T.L., Chao, K.S.C. Upregulation of tumor PD-L1 by neoadjuvant chemoradiotherapy (neoCRT) confers improved survival in patients with lymph node metastasis of locally advanced rectal cancers.Cancer Immunol Immunother. 2019;68:283–96. doi:10.1007/s00262-018-2275-0.
- Wang Q, Wu X. Primary and acquired resistance to PD-1/PD-L1 blockade in cancer treatment. Int Immunopharmacol. 2017;46:210–19. doi:10.1016/j.intimp.2017.03.015.
- Jiang W, Chan CK, Weissman IL, Kim BYS, Hahn SM. Immune priming of the tumor microenvironment by radiation. Trends in Cancer. 2016;2(11):638–45. doi:10.1016/j.trecan.2016.09.007.
- Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548(7668):466–70. doi:10.1038/nature23470.
- Wang Z, Chen J, Hu J, Zhang H, Xu F, He W, Wang, X., Li, M., Lu, W., Zeng, G., et al. cGAS/STING axis mediates a topoisomerase II inhibitor-induced tumor immunogenicity. J Clin Invest. 2019;130:4850–62. doi:10.1172/JCI127471.
- Billiard F, Buard V, Benderitter M, Linard C. Abdominal gamma-radiation induces an accumulation of function-impaired regulatory T cells in the small intestine. Int J Radiat Oncol Biol Phys. 2011;80:869–76. doi:10.1016/j.ijrobp.2010.12.041.
- Mondini M, Loyher PL, Hamon P, Gerbe de Thore M, Laviron M, Berthelot K, Clémenson, C., Salomon, B L., Combadière, C., Deutsch, E., et al. CCR2-dependent recruitment of tregs and monocytes following radiotherapy is associated with TNFalpha-mediated resistance. Cancer Immunol Res. 2019;7:376–87. doi:10.1158/2326-6066.CIR-18-0633.
- de Leve S, Wirsdorfer F, Jendrossek V. Targeting the immunomodulatory CD73/adenosine system to improve the therapeutic gain of radiotherapy. Front Immunol. 2019;10:698.
- Baird JR, Friedman D, Cottam B, Dubensky TW Jr., Kanne DB, Bambina S, Bahjat, K., Crittenden, M R., Gough, M J. Radiotherapy combined with novel STING-targeting oligonucleotides results in regression of established tumors. Cancer Res. 2016;76:50–61. doi:10.1158/0008-5472.CAN-14-3619.
- Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DSM, Pauken KE, Huang AC, et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell. 2016;167(6):1540–54 e12. doi:10.1016/j.cell.2016.11.022.
- Liu H, Golji J, Brodeur LK, Chung FS, Chen JT, deBeaumont RS, Bullock CP, Jones MD, Kerr G, Li L, et al. Tumor-derived IFN triggers chronic pathway agonism and sensitivity to ADAR loss. Nat Med. 2019;25(1):95–102. doi:10.1038/s41591-018-0302-5.
- Su C, Zhang B, Liu W, Zheng H, Sun L, Tong J, Wang T, Jiang X, Liang H, Xue L, et al. High extracellular pressure promotes gastric cancer cell adhesion, invasion, migration and suppresses gastric cancer cell differentiation. Oncol Rep. 2016;36(2):1048–54. doi:10.3892/or.2016.4841.
- Jerrell RJ, Parekh A. Matrix rigidity differentially regulates invadopodia activity through ROCK1 and ROCK2. Biomaterials. 2016;84:119–29. doi:10.1016/j.biomaterials.2016.01.028.
- Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64(12):4328–37. doi:10.1158/0008-5472.CAN-04-0073.
- Dovedi SJ, Cheadle EJ, Popple AL, Poon E, Morrow M, Stewart R, Yusko EC, Sanders CM, Vignali M, Emerson RO, et al. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res. 2017;23(18):5514–26. doi:10.1158/1078-0432.CCR-16-1673.
- Wang H, Hu S, Chen X, Shi H, Chen C, Sun L, Chen, Z J. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc Natl Acad Sci U S A. 2017;114:1637–42. doi:10.1073/pnas.1621363114.
- Eckert F, Gaipl US, Niedermann G, Hettich M, Schilbach K, Huber SM, Zips D. Beyond checkpoint inhibition - Immunotherapeutical strategies in combination with radiation. Clin Transl Radiat Oncol. 2017;2:29–35. doi:10.1016/j.ctro.2016.12.006.
- Wang L, Shureiqi I, Stroehlein JR, Wei D. Novel and emerging innate immune therapeutic targets for pancreatic cancer. Expert Opin Ther Targets. 2018;22:977–81. doi:10.1080/14728222.2018.1538361.
- Iurescia S, Fioretti D, Rinaldi M. Targeting cytosolic nucleic acid-sensing pathways for cancer immunotherapies. Front Immunol. 2018;9:711. doi:10.3389/fimmu.2018.00711.
- Rutkowski J, Slebioda T, Kmiec Z, Zaucha R. Changes in systemic immune response after stereotactic ablative radiotherapy. Preliminary results of a prospective study in patients with early lung cancer. Pol Arch Intern Med. 2017;127:245–53.
- Wang L, Wildt KF, Zhu J, Zhang X, Feigenbaum L, Tessarollo L, Paul, W E., Fowlkes, B J., Bosselut, R. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4(+) T cells. Nat Immunol. 2008;9:1122–30. doi:10.1038/ni.1647.
- Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015;27(1):39–46. doi:10.1093/intimm/dxu095.
- Ding Y, Jiang J. Antitumor immune mechanism of programmed death receptor-1/ligand 1 monoclonal antibody combined with radiotherapy. Chin J Exp Surg. 2018;35:2362–64.
- Teixido C, Gonzalez-Cao M, Karachaliou N, Rosell R. Predictive factors for immunotherapy in melanoma. Ann Transl Med. 2015;3:208.
- Boger C, Behrens HM, Mathiak M, Kruger S, Kalthoff H, Rocken C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget. 2016;7:24269–83.
- Gao Y, Li S, Xu D, Chen S, Cai Y, Jiang W, Zhang X, Sun J, Wang K, Chang B, et al. Prognostic value of programmed death-1, programmed death-ligand 1, programmed death-ligand 2 expression, and CD8(+) T cell density in primary tumors and metastatic lymph nodes from patients with stage T1-4N+M0 gastric adenocarcinoma. Chin J Cancer. 2017;36:61. doi:10.1186/s40880-017-0226-3.
- Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, Piperdi, B., Mehnert, J M. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2017;35:3823–29. doi:10.1200/JCO.2017.72.5069.
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi:10.1056/NEJMoa1412082.
- Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, Zahurak M, Yang SC, Jones DR, Broderick S, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–86. doi:10.1056/NEJMoa1716078.
- Ratti M, Lampis A, Hahne JC, Passalacqua R, Valeri N. Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches. Cell Mol Life Sci. 2018;75(22):4151–62. doi:10.1007/s00018-018-2906-9.
- Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu, X Q., Sher, X., Jung, H., Lee, M., et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–58. doi:10.1038/s41591-018-0101-z.
- Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen HZ, Reeser JW, Yu L, Roychowdhury S. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073.
- O’Donnell JS, Long GV, Scolyer RA, Teng MW, Smyth MJ. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev. 2017;52:71–81. doi:10.1016/j.ctrv.2016.11.007.
- Liu Y, Li H, Zhao D, Cheng Y. Differential expression of MMR and PD-L1 in stage II postoperative colorectal cancer. Chin J Colorectal Dis. 2018;7:562–66.
- Mitteldorf C, Berisha A, Pfaltz MC, Broekaert SMC, Schon MP, Kerl K, Kempf, W. Tumor microenvironment and checkpoint molecules in primary cutaneous diffuse large B-cell lymphoma-new therapeutic targets. Am J Surg Pathol. 2017;41:998–1004. doi:10.1097/PAS.0000000000000851.
- Liu F, Chang L, Hu J. Activating transcription factor 6 regulated cell growth, migration and inhibiteds cell apoptosis and autophagy via MAPK pathway in cervical cancer. J Reprod Immunol. 2020;139:103120. doi:10.1016/j.jri.2020.103120.
- Zhuang Y, Liu C, Liu J, Li G. resistance mechanism of PD-1/PD-L1 blockade in the cancer-immunity cycle. Onco Targets Ther. 2020;13:83–94. doi:10.2147/OTT.S239398.
- Garcia AJ, Ruscetti M, Arenzana TL, Tran LM, Bianci-Frias D, Sybert E, Priceman SJ, Wu L, Nelson PS, Smale ST, et al. Pten null prostate epithelium promotes localized myeloid-derived suppressor cell expansion and immune suppression during tumor initiation and progression. Mol Cell Biol. 2014;34(11):2017–28. doi:10.1128/MCB.00090-14.
- Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6(2):202–16. doi:10.1158/2159-8290.CD-15-0283.
- Rimawi MF, De Angelis C, Contreras A, Pareja F, Geyer FC, Burke KA, Herrera S, Wang T, Mayer IA, Forero A, et al. Low PTEN levels and PIK3CA mutations predict resistance to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2 over-expressing breast cancer. Breast Cancer Res Treat. 2018;167(3):731–40. doi:10.1007/s10549-017-4533-9.
- Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018;15(5):273–91. doi:10.1038/nrclinonc.2018.28.
- Costa RLB, Han HS, Gradishar WJ. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review. Breast Cancer Res Treat. 2018;169(3):397–406. doi:10.1007/s10549-018-4697-y.
- Sai J, Owens P, Novitskiy SV, Hawkins OE, Vilgelm AE, Yang J, Sobolik T, Lavender N, Johnson AC, McClain C, et al. PI3K inhibition reduces mammary tumor growth and facilitates antitumor immunity and Anti-PD1 responses. Clin Cancer Res. 2017;23(13):3371–84. doi:10.1158/1078-0432.CCR-16-2142.
- Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20(1):25–39. doi:10.1038/s41577-019-0218-4.
- Jimenez-Sanchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, Gill MB, Park KJ, Zivanovic O, Konner J, et al. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell. 2017;170(5):927–38 e20. doi:10.1016/j.cell.2017.07.025.
- Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–73.
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, et al. Mutations associated with acquired resistance to PD-1 blockade in Melanoma. N Engl J Med. 2016;375(9):819–29. doi:10.1056/NEJMoa1604958.
- Wang Y, Wang L. miR-34a attenuates glioma cells progression and chemoresistance via targeting PD-L1. Biotechnol Lett. 2017;39(10):1485–92. doi:10.1007/s10529-017-2397-z.
- Ishibashi M, Tamura H, Sunakawa M, Kondo-Onodera A, Okuyama N, Hamada Y, Moriya K, Choi I, Tamada K, Inokuchi K, et al. Myeloma drug resistance induced by binding of myeloma B7-H1 (PD-L1) to PD-1. Cancer Immunol Res. 2016;4(9):779–88. doi:10.1158/2326-6066.CIR-15-0296.
- Sucker A, Zhao F, Pieper N, Heeke C, Maltaner R, Stadtler N, Real, B., Bielefeld, N., Howe, S., Weide, B., et al. Acquired IFNgamma resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nat Commun. 2017;8:15440. doi:10.1038/ncomms15440.
- Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W, Sandoval S, Torrejon DY, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7(2):188–201. doi:10.1158/2159-8290.CD-16-1223.
- Bai J, Gao Z, Li X, Dong L, Han W, Nie J. Regulation of PD-1/PD-L1 pathway and resistance to PD-1/PD-L1 blockade. Oncotarget. 2017;8(66):110693–707. doi:10.18632/oncotarget.22690.
- Wang H, Fu C, Du J, Wang H, He R, Yin X, Li H, Li X, Wang H, Li K, et al. Enhanced histone H3 acetylation of the PD-L1 promoter via the COP1/c-Jun/HDAC3 axis is required for PD-L1 expression in drug-resistant cancer cells. J Exp Clin Cancer Res. 2020;39(1):29. doi:10.1186/s13046-020-1536-x.
- Cicenas J, Kalyan K, Sorokinas A, Jatulyte A, Valiunas D, Kaupinis A, Valius, M. Highlights of the latest advances in research on CDK inhibitors. Cancers (Basel). 2014;6:2224–42. doi:10.3390/cancers6042224.
- Schaer DA, Beckmann RP, Dempsey JA, Huber L, Forest A, Amaladas N, Li Y, Wang YC, Rasmussen ER, Chin D, et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 2018;22(11):2978–94. doi:10.1016/j.celrep.2018.02.053.
- Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, Khan N, Ubellacker JM, Xie S, Metzger-Filho O, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471–75. doi:10.1038/nature23465.
- Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, Chhabra S, Huang W, Liu H, Aref AR, et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 2018;8(2):216–33. doi:10.1158/2159-8290.CD-17-0915.
- Jerby-Arnon L, Shah P, Cuoco MS, Rodman C, Su MJ, Melms JC, Leeson, R., Kanodia, A., Mei, S., Lin, J-R., et al. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell. 2018;175:984–97 e24. doi:10.1016/j.cell.2018.09.006.
- Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. doi:10.1016/j.canlet.2016.01.043.
- Marty R, Kaabinejadian S, Rossell D, Slifker MJ, van de Haar J, Engin HB, de Prisco N, Ideker T, Hildebrand WH, Font-Burgada J, et al. MHC-I genotype restricts the oncogenic mutational landscape. Cell. 2017;171(6):1272–83 e15. doi:10.1016/j.cell.2017.09.050.
- Huang AC, Orlowski RJ, Xu X, Mick R, George SM, Yan PK, Manne S, Kraya AA, Wubbenhorst B, Dorfman L, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25(3):454–61. doi:10.1038/s41591-019-0357-y.
- McGranahan N, Rosenthal R, Hiley CT, Rowan AJ, Watkins TBK, Wilson GA, Birkbak NJ, Veeriah S, Van Loo P, Herrero J, et al. Allele-specific HLA loss and immune escape in lung cancer evolution. Cell. 2017;171(6):1259–71 e11. doi:10.1016/j.cell.2017.10.001.
- Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–01. doi:10.1056/NEJMc1713444.
- Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, Zhang, T., Adleff, V., Phallen, J., Wali, N., et al. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov. 2017;7:264–76. doi:10.1158/2159-8290.CD-16-0828.
- Thommen DS, Schreiner J, Muller P, Herzig P, Roller A, Belousov A, Umana, P., Pisa, P., Klein, C., Bacac, M., et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res. 2015;3:1344–55. doi:10.1158/2326-6066.CIR-15-0097.
- Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi, L., Redig, A J., Rodig, S J., Asahina, H., et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi:10.1038/ncomms10501.
- Le Mercier I, Lines JL, Noelle RJ. Beyond CTLA-4 and PD-1, the generation Z of negative checkpoint regulators. Front Immunol. 2015;6:418. doi:10.3389/fimmu.2015.00418.
- Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19(6):1189–201. doi:10.1016/j.celrep.2017.04.031.
- Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24:26. doi:10.1186/s12929-017-0329-9.
- Anderson ES, Postow MA, Wolchok JD, Young RJ, Ballangrud A, Chan TA, Yamada, Y., Beal, K. Melanoma brain metastases treated with stereotactic radiosurgery and concurrent pembrolizumab display marked regression; efficacy and safety of combined treatment. J Immunother Cancer. 2017;5:76. doi:10.1186/s40425-017-0282-x.
- Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, Garon, E B., Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903. doi:10.1016/S1470-2045(17)30380-7.
- De Ruysscher D. Radiotherapy and PD-L1 inhibition in metastatic NSCLC. Lancet Oncol. 2017;18(7):840–42. doi:10.1016/S1470-2045(17)30354-6.
- Maity A, Mick R, Huang AC, George SM, Farwell MD, Lukens JN, Berman AT, Mitchell TC, Bauml J, Schuchter LM, et al. A phase I trial of pembrolizumab with hypofractionated radiotherapy in patients with metastatic solid tumours. Br J Cancer. 2018;119(10):1200–07. doi:10.1038/s41416-018-0281-9.
- Theelen W, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts J, Dumoulin, D W., Bahce, I., Niemeijer, A.L.N., de Langen, A J., et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019. doi:10.1001/jamaoncol.2019.1478.
- Shevtsov M, Sato H, Multhoff G, Shibata A. Novel approaches to improve the efficacy of immuno-radiotherapy. Front Oncol. 2019;9:156. doi:10.3389/fonc.2019.00156.
- Sato H, Okonogi N, Nakano T. Rationale of combination of anti-PD-1/PD-L1 antibody therapy and radiotherapy for cancer treatment. Int J Clin Oncol. 2020;25(5):801–09. doi:10.1007/s10147-020-01666-1.
- Iijima M, Okonogi N, Nakajima NI, Morokoshi Y, Kanda H, Yamada T, Kobayashi Y, Banno K, Wakatsuki M, Yamada S, et al. Significance of PD-L1 expression in carbon-ion radiotherapy for uterine cervical adeno/adenosquamous carcinoma. J Gynecol Oncol. 2020;31(2):e19. doi:10.3802/jgo.2020.31.e19.
- Permata TBM, Hagiwara Y, Sato H, Yasuhara T, Oike T, Gondhowiardjo S, Held, K D., Nakano, T., Shibata, A. Base excision repair regulates PD-L1 expression in cancer cells. Oncogene. 2019;38:4452–66. doi:10.1038/s41388-019-0733-6.
- Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5(9):915–19. doi:10.1158/2159-8290.CD-15-0563.
- Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu, Y-X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi:10.1172/JCI67313.
- Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–65. doi:10.1038/nature22079.
- Kordbacheh T, Honeychurch J, Blackhall F, Faivre-Finn C, Illidge T. Radiotherapy and anti-PD-1/PD-L1 combinations in lung cancer: building better translational research platforms. Ann Oncol. 2018;29(2):301–10. doi:10.1093/annonc/mdx790.
- Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin. 2017;67:65–85.
- Takahashi Y, Yasui T, Tamari K, Minami K, Otani K, Isohashi F, Seo Y, Kambe R, Koizumi M, Ogawa K, et al. Radiation enhanced the local and distant anti-tumor efficacy in dual immune checkpoint blockade therapy in osteosarcoma. PLoS One. 2017;12(12):e0189697. doi:10.1371/journal.pone.0189697.
- Bockel S, Antoni D, Deutsch E, Mornex F. [Immunotherapy and radiotherapy]. Cancer Radiother. 2017;21:244–55. doi:10.1016/j.canrad.2016.12.005.
- Walle T, Martinez Monge R, Cerwenka A, Ajona D, Melero I, Lecanda F. Radiation effects on antitumor immune responses: current perspectives and challenges. Ther Adv Med Oncol. 2018;10:1758834017742575. doi:10.1177/1758834017742575.
- Gong J, Le TQ, Massarelli E, Hendifar AE, Tuli R. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J Immunother Cancer. 2018;6:46.
- Cao Y, Li W, Wang Z, Pang H. Potential and unsolved problems of anti-PD-1/PD-L1 therapy combined with radiotherapy. Tumori. 2020;300891620940382. [published online ahead of print, 2020 Jul 31].
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–68. doi:10.1158/0008-5472.CAN-14-1258.
- Chen L, Douglass J, Kleinberg L, Ye X, Marciscano AE, Forde PM, Brahmer J, Lipson E, Sharfman W, Hammers H, et al. Concurrent immune checkpoint inhibitors and stereotactic radiosurgery for brain metastases in non-small cell lung cancer, melanoma, and renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2018;100(4):916–25. doi:10.1016/j.ijrobp.2017.11.041.
- Jabbour SK, Berman AT, Decker RH, Lin Y, Feigenberg SJ, Gettinger SN, Aggarwal, C., Langer, C J., Simone, C B., Bradley, J D., et al. Phase 1 trial of pembrolizumab administered concurrently with chemoradiotherapy for locally advanced non-small cell lung cancer: a nonrandomized controlled Trial. JAMA Oncol. 2020;6:848–55.
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29. doi:10.1056/NEJMoa1709937.
- Ahmed KA, Stallworth DG, Kim Y, Johnstone PA, Harrison LB, Caudell JJ, Yu HHM, Etame AB, Weber JS, Gibney GT, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol. 2016;27(3):434–41. doi:10.1093/annonc/mdv622.
- Kotecha R, Kim JM, Miller JA, Juloori A, Chao ST, Murphy ES, Peereboom DM, Mohammadi AM, Barnett GH, Vogelbaum MA, et al. The impact of sequencing PD-1/PD-L1 inhibitors and stereotactic radiosurgery for patients with brain metastasis. Neuro Oncol. 2019;21(8):1060–68. doi:10.1093/neuonc/noz046.
- Lim SH, Hong M, Ahn S, Choi YL, Kim KM, Oh D, Ahn, Y.C., Jung, S.-H., Ahn, M.-J., Park, K., et al. Changes in tumour expression of programmed death-ligand 1 after neoadjuvant concurrent chemoradiotherapy in patients with squamous oesophageal cancer. Eur J Cancer. 2016;52:1–9. doi:10.1016/j.ejca.2015.09.019.
- Chen TW, Huang KC, Chiang SF, Chen WT, Ke TW, Chao KSC. Prognostic relevance of programmed cell death-ligand 1 expression and CD8+ TILs in rectal cancer patients before and after neoadjuvant chemoradiotherapy. J Cancer Res Clin Oncol. 2019;145:1043–53. doi:10.1007/s00432-019-02874-7.
- Xie G, Gu D, Zhang L, Chen S, Wu D. A rapid and systemic complete response to stereotactic body radiation therapy and pembrolizumab in a patient with metastatic renal cell carcinoma. Cancer Biol Ther. 2017;18(8):547–51. doi:10.1080/15384047.2017.1345389.
- Gong X, Li X, Jiang T, Xie H, Zhu Z, Zhou F, Zhou, C. Combined Radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J Thorac Oncol. 2017;12:1085–97. doi:10.1016/j.jtho.2017.04.014.
- Yuan Z, Fromm A, Ahmed KA, Grass GD, Yang GQ, Oliver DE, Dilling, T J., Antonia, S J., Perez, B A. Radiotherapy rescue of a nivolumab-refractory immune response in a patient with PD-L1-negative metastatic squamous cell carcinoma of the lung. J Thorac Oncol. 2017;12:e135–e6. doi:10.1016/j.jtho.2017.04.029.
- Sharabi A, Kim SS, Kato S, Sanders PD, Patel SP, Sanghvi P, Weihe E, Kurzrock R. Exceptional response to nivolumab and stereotactic body radiation therapy (SBRT) in neuroendocrine cervical carcinoma with high tumor mutational burden: management considerations from the center for personalized cancer therapy at UC San Diego moores cancer center. Oncologist. 2017;22:631–37.
- Michot JM, Mazeron R, Dercle L, Ammari S, Canova C, Marabelle A, Rose, S., Rubin, E., Deutsch, E., Soria, J-C., et al. Abscopal effect in a Hodgkin lymphoma patient treated by an anti-programmed death 1 antibody. Eur J Cancer. 2016;66:91–94. doi:10.1016/j.ejca.2016.06.017.
- Yu Y, Zeng D, Ou Q, Liu S, Li A, Chen Y, Lin, D., Gao, Q., Zhou, H., Liao, W., et al. Association of survival and immune-related biomarkers with immunotherapy in patients with non-small cell lung cancer: a meta-analysis and individual patient-level analysis. JAMA Netw Open. 2019;2:e196879. doi:10.1001/jamanetworkopen.2019.6879.
- Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US food and drug administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):278. doi:10.1186/s40425-019-0768-9.
- Sampath S, Rahmanuddin S, Sahoo P, Frankel P, Boswell S, Wong J, Rotter, A., Rockne, R., Wong, J., Park, J M., et al. Change in apparent diffusion coefficient is associated with local failure after stereotactic body radiation therapy for non-small cell lung cancer: a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2019;105(3):659–63. doi:10.1016/j.ijrobp.2019.06.2536.
- Ahn HK, Lee H, Kim SG, Hyun SH. Pre-treatment (18)F-FDG PET-based radiomics predict survival in resected non-small cell lung cancer. Clin Radiol. 2019;74:467–73. doi:10.1016/j.crad.2019.02.008.
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20. doi:10.1056/NEJMoa1500596.
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13. doi:10.1126/science.aan6733.
- Luo H, Ge H, Cui Y, Zhang J, Fan R, Zheng A, Zheng, X., Sun, Y. Systemic inflammation biomarkers predict survival in patients of early stage non-small cell lung cancer treated with stereotactic ablative radiotherapy - a single center experience. J Cancer. 2018;9:182–88. doi:10.7150/jca.21703.
- Yamaguchi O, Kaira K, Hashimoto K, Mouri A, Miura Y, Shiono A, Nishihara, F., Murayama, Y., Noda, S-E., Kato, S., et al. Radiotherapy is an independent prognostic marker of favorable prognosis in non-small cell lung cancer patients after treatment with the immune checkpoint inhibitor, nivolumab. Thorac Cancer. 2019;10:992–1000. doi:10.1111/1759-7714.13044.
- Mauclet C, Duplaquet F, Pirard L, Rondelet B, Dupont M, Pop-Stanciu C, Vander Borght T, Remmelink M, D’Haene N, Lambin S, et al. Complete tumor response of a locally advanced lung large-cell neuroendocrine carcinoma after palliative thoracic radiotherapy and immunotherapy with nivolumab. Lung Cancer. 2019;128:53–56. doi:10.1016/j.lungcan.2018.12.006.
- Komori T, Otsuka A, Irie H, Horiguchi A, Honda T, Kabashima K. Drastic effect on giant lung metastatic melanoma by sequential administration of nivolumab with ipilimumab/radiation combination therapy. J Dermatol. 2018;45(1):e7–e8. doi:10.1111/1346-8138.14078.