ABSTRACT
Vaccination is an essential way to prevent the transmission of hepatitis B virus (HBV). Various studies have been published on the cost-effectiveness of HBV vaccination, but since the results vary according to the target population and related health outcomes, this study examined the cost-effectiveness of the universal HBV vaccination in Iran. In this economic evaluation study, a decision tree with the Markov model was used to compare the universal HBV vaccination with a strategy of non-vaccination. Health states used in the model included healthy, chronic hepatitis B, compensated cirrhosis, decompensated cirrhosis, hepatocellular carcinoma, and death. Analyses were performed from a payer’s perspective. Incremental cost-effectiveness ratio (ICER) per life-year gained, and quality-adjusted life-years (QALYs) gained were calculated at a 5% annual discount rate. The sensitivity analysis was conducted using Monte Carlo simulation. Analyses were performed using Microsoft Excel and TreeAge Pro 2011 software. In 2017, the estimated cost per dose for any HBV vaccine was $3.20 USD. The universal HBV vaccination was economically advantageous compared to non-vaccination, and the estimated cost of this program per life-year and QALY gained were $6,319 and negative (-) $1,183.85 USD, respectively. Given the uncertainty of all parameters, the model remained robust and reliable. In Iran, the universal HBV vaccination strategy for both health outcomes of QALY and life-years gained was cost-effective and advantageous. The vaccination strategy saved money, increased life years and improved quality of life. Therefore, it is recommended that this program continues to be provided.
Introduction
Despite advances in health, Hepatitis B virus (HBV) infection remains a major health problem worldwideCitation1,Citation2 and more than 2 billion people have been exposed to the infection.Citation3 HBV is one of the leading causes of chronic hepatitis, cirrhosis, and hepatocellular carcinoma.Citation1 Chronic HBV infection increases the risk of death from liver cirrhosis and hepatocellular carcinoma by 15–25%.Citation4
In 1991, the World Health Organization (WHO) recommended widespread HBV vaccination of infants under the expanded program on immunization (EPI) in all countries to reduce the impact of HBV.Citation5,Citation6 Highly effective HBV vaccines have been introduced since the 1980s.Citation7,Citation8 In 1989, the Iranian Ministry of Health launched a pilot immunization program in four provinces. In 1993, vaccination was expanded to all provinces, and now vaccination coverage in infants is close to 100%.Citation6,Citation9 Despite the national vaccination of infants against HBV, this infection is still a health problem in Iran. One study investigating the prevalence of HBV in the Iranian population,Citation10 showed that the prevalence of HBsAg in was 2.6% and the prevalence of HBcAb was 16.4%. However, a second study showed that the average prevalence of HBsAg in Iran was currently close to 0.9% with a significant reduction in the vaccinated population of 0.6%.Citation11
A vaccine’s effectiveness describes the degree to which the vaccine reduces disease in the general population.Citation12 In high-income countries, many technical advisory groups and relevant national institutions typically consider the results of health economic assessments for vaccine evaluation.Citation13
Although effectiveness measures are often generalizable to different conditions and settings, costs are not easily transferable between different settings and especially between countries. Therefore, the results of economic evaluations conducted outside a country should be used with caution for local conditions and settings.Citation14 Most economic evaluations of HBV immunization have been conducted in industrialized countries.Citation15 However, few have been conducted in Iran. The economic effect of chronic hepatitis B infection has not yet been well identified.Citation16 Given the current economic conditions, lower-cost strategies with high effectiveness are most likely to be of interest to health policymakers.Citation17
The economic evaluation of HBV vaccination in GambiaCitation18 showed that this program was highly cost-effective compared to non-vaccination. The cost-effectiveness ratio of the vaccination program from a payer’s perspective was 47 USD per disability-adjusted life year (DALY). A review of economic evaluations of HBV immunizationCitation19 showed that in countries with very low endemicity, such studies produced contradictory results. Further, in areas with low to high endemicity, the universal vaccination strategy was deemed consistently justifiable.
Various studies on the cost-effectiveness of HBV vaccination have been published, but as cost-effectiveness varies according to the target population selected as well as the outcome measures,Citation4 it remains useful to evaluate the cost-effectiveness of HBV locally. In countries with intermediate endemicity, such as Iran, most cases of HBV transmission are expected to occur in younger age groups.Citation21 In Iran, the national HBV vaccination program for infants has been implemented since 1993,Citation6 and given that no study has yet been conducted on this program, the present study evaluated the cost-effectiveness of this program.
Methods
Decision analytic model
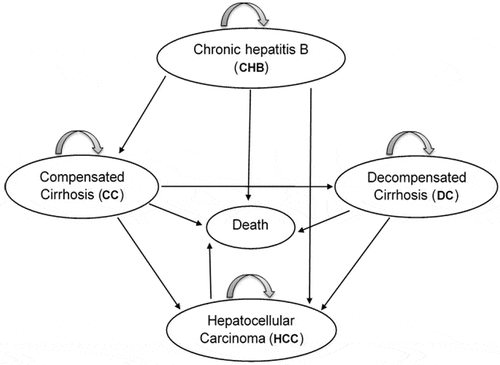
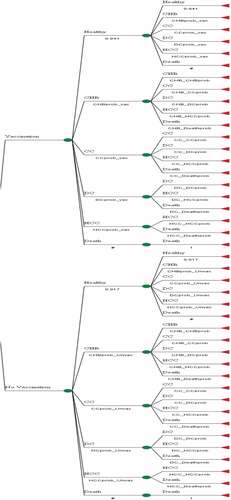
In this study, a decision tree with a Markov model was used to estimate the cost-effectiveness of the universal HBV vaccination strategy. In the Markov model, the natural history of HBV infection had 6 states: healthy, chronic hepatitis B, Compensated Cirrhosis, Decompensated Cirrhosis, Hepatocellular Carcinoma and Death (). The model began with a cohort of 33,204 individuals who were vaccinated through the national HBV vaccination program as infants and a cohort of non-vaccinated individuals in Shiraz.
In the present study, the Markov model had a 1-year cycle that was run for 76 cycles (according to the average life expectancy of Iranian people based on the latest WHO statistics in 2016) to cover the lifetime experiences of the majority of individuals (). When actual parameters were not available, a literature review was conducted of the available Iranian and international literature, and studies that bore greater similarity to the present study (e.g., time and place) and were more reliable were preferred. In the cases that relevant studies were limited, the most appropriate study was selected as the point estimate of each parameter, and other studies were used to construct the confidence intervals of the parameters in the sensitivity analysis.
Compared strategies
In a cost-effectiveness analysis, the costs and health outcomes of various intervention scenarios are calculated and compared with a baseline condition.Citation22 In the Markov model, two strategies were compared, including universal HBV vaccination and non-vaccination. In the universal vaccination strategy, infants receive at least 3 recombinant HBV vaccines (Pasteur Institute of Iran). With respect to the national HBV vaccination program for infants in Iran being implemented in 1993, two cohorts were evaluated; (1) vaccinated individuals born in or after 1994 and (2) non-vaccinated individuals born in 1992 and before. Individuals who were born in 1993 were excluded from the study, due to the potential for problems at the start of the program such as lack of coverage for all infants, or unfamiliarity of staff with the program. Since the coverage of the neonatal vaccination program in Iran is close to 100%, almost all infants born in or after 1993 (the year of the program initiation) were vaccinated. Therefore, unfortunately, it was not possible to select two cohorts within the same time frame. To partially control for age, the age range of participants was limited to between 17 and 50 years in order to minimize the age difference between the two groups.
Vaccine effectiveness
To determine the effectiveness of the HBV vaccination strategy, we conducted a historical cohort study in Shiraz.Citation23 A total of 2,720 individuals were interviewed in two cohorts (vaccinated and non-vaccinated) and their blood samples were taken. The mean effectiveness of the HBV vaccination program for those who received the vaccine three times at 0, 2, and 6 months of age was 29% (95% confidence interval: 6–46%). One of the possible reasons for the low effectiveness compared to other studies is the age of the participants. Aging is recognized as an influential factor in the immune response to vaccination.Citation24 Following a period of 25 years since the introduction of a national HBV vaccination in Iran, some cases of infection are acceptable within a vaccinated population, and subsequently affect the effectiveness of the study.
Estimates of transition probabilities
People with acute hepatitis B infection may recover or develop a chronic condition. About 5% of acute infections in adults progress to the chronic form, and the remaining cases improve.Citation25, Citation26 Chronic HBV can also progress to compensated cirrhosis, hepatocellular carcinoma (HCC), or both. Further, compensated cirrhosis can lead to decompensated cirrhosis and HCC. Any of these states can also lead to death. As there is a variation in the reported transition probabilities of different studies, these probabilities and their ranges are given in . It should be noted that in this model only deaths related to HBV infection are considered, and therefore a healthy person cannot be directly assigned the state of death.
Table 1. Parameter values (per year) used in the Markov model and sensitivity analysis
In our study, the initial and transition probabilities for different states of HBV infection were modeled based on a review of the literature published in Iran and elsewhere in the world. It should be noted that in order to improve accuracy, some initial probabilities were extracted from our effectiveness studyCitation24 and from the general Iranian population. For parameters that were less specific to Iran (such as those associated with the natural history of disease) and the uncertainty parameters, data were extracted from the international literature, and it was assumed that the clinical aspects of HBV infection in Iran were comparable to those in other parts of the world. The probabilities for the progression of HBV infection were collected based on published medical literature searches, and are shown in . Death probability was defined as the probability of dying due to the HBV infection and related conditions.
Vaccination costs
The costs of the national HBV vaccination program were calculated based on WHO guidance ,Citation31 and by reviewing the existing documentation as well as by conducting interviews with relevant and knowledgeable persons involved in the program. In this study both recurrent and capital costs were identified. Finally, land, building, water, electricity, gas and telephone costs for health centers, in addition to the personnel costs, vaccine costs, equipment and transportation costs associated with the vaccination program were calculated. After collating these costs, the fraction of the vaccination program that is exclusively pertaining to the HBV vaccination was determined through consultation with the staff and specialists related to the program, and as such the final costs attributed to the hepatitis B vaccination were calculated. In Iran, the hepatitis B vaccine is given to infants in the form of the Pentavalan vaccine (DTP-HepB-Hib), which has been used since November, 2014.Citation32 Therefore, in order to determine the cost of this vaccine, the proportion of the total immunization program that is represented by hepatitis B vaccination was first determined, and then the costs attributed to HBV vaccination were calculated. Other consumable costs such as registration forms and cards were not considered because they were insignificant, as well as the potential for costs resulting from the side effects of the vaccine, due to their scarcity. Finally, the direct and indirect costs incurred by individual vaccine recipients (such as the cost of traveling to the health center) were not considered.
Treatment costs
Many economic evaluations have been performed using data from other studies, such as the cost of clinical evaluation (e.g. clinical trials), or using cost data that are routinely available.Citation33,Citation34 In the present study, the costs associated with different HBV infection states were calculated based on reviewing the published literature. However, due to differences in the cost of the disease state between countries, these costs were identified by reviewing Iranian studies only ().
Effectiveness unit
Life-years gained and quality-adjusted life years (QALY) gained were considered as the units of effectiveness. One way to calculate QALY is to use utility. Health states “utility” measures are usually expressed on a numerical scale from 0 to 1, where 0 represents the “death” state and 1 represents “perfect health”.Citation35 The utility of individuals with chronic hepatitis infection has repeatedly been used in decision-making and cost-effectiveness models.Citation30 In the present study, health state utilities were extracted from published studies ().
Cost-effectiveness
In this cost-effectiveness analysis, since all analyses were performed from a payer’s perspective, direct medical costs were taken into account. All costs were calculated for 2017 at an annual discount rate of 5%, and then cost estimates were converted from Iranian Rials to US Dollars (exchange rate = 34,066 Rials per 1 USD, according to the Central Bank of the Islamic Republic of Iran in 2017).
In the present study, modeling and simulation were used for economic evaluation, and the incremental cost-effectiveness ratio (ICER) was estimated using a probabilistic analysis model with Monte Carlo simulation for related parameters in each Markov cycle.
Sensitivity analysis
Sensitivity analysis was performed to estimate the uncertainty of the parameters. In a two-way sensitivity analysis and probabilistic sensitivity analysis, the effect of combined variations of the parameter on the outcome was investigated.Citation28 Parameters used in the sensitivity analysis included transmission probabilities, costs of disease states, the cost of vaccination, QALYs, and the discount rate.
Statistical methods
After estimating the parameters of cost and effectiveness, these data were entered into Microsoft Excel (Microsoft Office version, Microsoft Corporation, Redmond, WA, USA) and TreeAge Pro (TreeAge Software, Williamstown, MA, USA) software for analysis. Analyses related to life-years gained were performed using Microsoft Excel, and QALY analyses were performed using TreeAge Pro.
The incremental cost-effectiveness ratio and sensitivity analysis were calculated using a Markov model.
Ethical issues
The survey was approved by the Research Ethics Committee of the authors’ institute (Ethical code: IR.SUMS.REC.1397.437) and all elements of the study were in accordance with national regulations.
Results
The cost of national HBV vaccination
In 2017, the cost of inoculation per HBV vaccine in children was 3.20 USD (approximately 52 cents for the each dose of vaccine, and the remainder for service delivery), and the cost of the HBV vaccination program was estimated to be 14.26 USD per fully immunized child ().
Table 2. Total annual costs of national hepatitis B vaccination program in Iran (2017 USD)
Cost-effectiveness analysis
The incremental cost-effectiveness ratios per QALY gained for an infant over a 76-year lifespan are shown in . The mean QALY achieved per person in the universal HBV vaccination program was 0.91 years more than for non-vaccination (14.28 vs. 13.37 years). The ICER of universal vaccination compared to non-vaccination was negative (-) 1,183 USD per QALY gained, indicating that this strategy was advantageous in comparison to the non-vaccination strategy and increased QALY.
Table 3. Cost-effectiveness analysis between universal hepatitis B vaccination and no-vaccination program
Statistical analysis of life-years showed that the ICER of universal vaccination compared to non-vaccination was 6,319 USD per life-year gained. As such, universal vaccination increased the life-years gained up to 20,385 in the cohort of 33,204 vaccinated infants. In other words, universal vaccination increased the life-years gained by 0.614 in each fully vaccinated infant ().
The costs of universal vaccination in Iran per QALY and per life-year gained were 6,319 USD and negative (-) 1,183.85, USD respectively.
Sensitivity analysis (Uncertainty analysis)
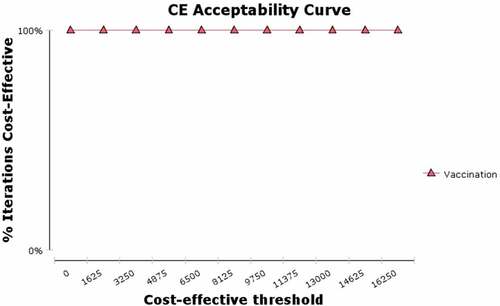
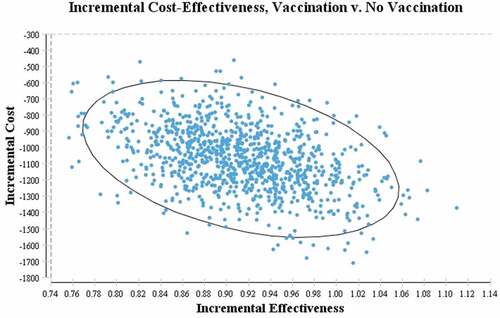
To investigate the uncertainties in the parameters, a probabilistic sensitivity analysis was performed, the result of which was that changing the parameter values had no effect on the cost-effectiveness of vaccination. shows the results of the 1,000 ICER iterations with respect to the parameter uncertainty, where the relevant points are placed on the cost-effectiveness plane (C-E plane). As shown, all ICERs are concentrated in the lower-right quarter (fourth quarter) of the C-E plane, confirming our conclusions regarding the advantage of universal vaccination, and that vaccination reduces costs and increases QALYs. Therefore, given the uncertainty of all parameters (even assuming the worst-case scenario), the model remains robust and reliable.
Figure 3. Results of Probabilistic Sensitivity Analysis. Simulated results of 1000 replicates of incremental cost-effectiveness ratio for vaccination vs. no-vaccination strategies
The results of a probabilistic sensitivity analysis in which the parameters were varied simultaneously in the Monte Carlo simulation show that the advantage of universal vaccination over non-vaccination is almost certain, even for the lowest-cost values used in the model (). In other words, the results of the cost-effectiveness analysis were verified by the acceptability curve.
Discussion
In recent years, Iran has made many efforts to prevent and control hepatitis B infection following the recommendation of the WHO on the immunization of children under the universal vaccination strategy. The present study demonstrated the impact of this program in Iran, showing that the ICER of universal HBV vaccination compared to non-vaccination was negative (-) 1,183 USD per QALY gained and 6,319 USD per life-year gained. The point estimate of the ICER can be compared using different criteria. Gross domestic product (GDP) per capita is often used as an alternative indicator of effectiveness threshold.Citation19 In the present study, the ICER was negative, therefore, universal HBV vaccination in Iran is cost-effective. It should be noted that when the ICER is negative and placed in the fourth quarter of the C-E plane, the program is cost-effective and it is not usually deemed necessary to compare using threshold values.
In the universal vaccination strategy, the ICER per QALY gained for an infant over a 76-year lifespan was -$1,183.85, and placed in the fourth quarter of the C-E plane, indicating the economic advantage of this strategy. This finding was consistent with other economic evaluations. A study in VietnamCitation29 investigated the cost-effectiveness of universal vaccination on 1,639,000 infants born in 2002. The results showed that the program was cost-effective and the ICER per QALY gained was 3.77 USD. In 2002, a study in ChinaCitation36 found universal vaccination over a lifetime in a cohort of 10 million infants to yield more than 620,000 QALYs. Another cost-effectiveness studyCitation19 that used different outcome measures from the present study also showed that childhood vaccination as part of a universal strategy was a cost-effective health intervention. However, a discrepancy was found between existing studies, as not all findings were in agreement. A 2011 study from the perspective of the National Health Service in the UKCitation37 used the Markov model to examined the impact of HBV vaccination using QALYs as the outcome measure. Based on the vaccine price at the time of the study, universal vaccination of infants was not deemed to be cost-effective in the United Kingdom. This could be due to much of the burden of HBV infection in the UK being attributed to immigrants who contracted the infection while outside the UK, before migrating to the UK. As such, the vaccination program had no effect on these people and could not prevent their disease. Another reason may be the difference in price of the vaccine between countries. For example, in 2018, the cost of each vaccine dose in the public sectors of the United States was estimated at 11.60 USD,Citation38 while in the present study the cost was approximately 52 cents.
Vaccination is effective in terms of reduced mortality due to HBV infection, increased life expectancy, and cost savings (by reducing the incidence of complications arising from HBV such as cirrhosis and hepatocellular carcinoma). In the cohort of 33,204 vaccinated infants in the present study, universal HBV vaccination increased the life-years gained to 20,386 years, and the ICER per life-year gained was found to be 6,319 USD. Accordingly, universal HBV vaccination in Iran has been a cost-effective strategy, consistent with studies in other countries. A cost-effectiveness study conducted in ChinaCitation36 showed that universal vaccination against HBV infection significantly reduced the morbidity and mortality of HBV complications. This strategy yielded more than 743,000 life-years gained for the cohort of 10 million new-borns in 2002. A study conducted in Taiwan, a country with a high HBV endemicity,Citation5 found that the mean life-years gained for each person using universal vaccination was 3.89, compared to non-vaccination. A study conducted in Ireland39 showed that universal vaccination was more cost-effective than selective vaccination. In the 80-year period studied, the ICER was between €10,992 and €67,200 per life-year gained for the minimum and maximum cost of the vaccine, respectively.
In the universal vaccination strategy, the mean QALY achieved over a 76-year lifetime for a new-born was 0.91, compared to non-vaccination. One study using data from India, a country with intermediate endemicity,Citation27 showed that universal vaccination compared to non-vaccination had a high cost-effectiveness, and increased the QALY and number of years lived by a birth cohort by 0.221 and 0.173 years, respectively.
Probabilistic sensitivity analysis showed that, even when varying the parameter values, all ICERs were concentrated in the fourth quarter of the cost-effectiveness plane, showing that in all extracted samples, universal vaccination was economically advantageous and cost-effective compared to non-vaccination. Universal vaccination in Iran was advantageous even for the lowest willingness to pay values. A study in ChinaCitation28 evaluated the effect of the HBV catch-up vaccination in 8–15 year-olds (who were not fully vaccinated between 1994 and 2002). The results of probabilistic sensitivity analysis showed that the uncertainty of the parameters had no effect on the results, and in all cases, the catch-up vaccination program was cost-effective and advantageous compared to no catch-up vaccination.
In a review study by Beutels et al.Citation20 all published studies on the economic evaluations of the HBV vaccination between 1994 and 2000 were reviewed, and the results of studies based on the levels of HBV endemicity were discussed. Most of the assessments were in industrialized countries, usually in low to very low endemicity areas. Based on their economic evaluation, universal HBV vaccination was found to be justifiable in areas with all levels of endemicity. Finally, to improve cross-country comparisons, it was recommended that specific guidelines be provided for the economic evaluation of infectious disease prevention programs.
Strengths and weaknesses
As already mentioned, the target population and the associated health outcomes can influence the results of the cost-effectiveness analysis.Citation5 One of the strengths of this study was the calculation of the ICER using two different outcome measures (QALY and life-years gained). This practice adds reliability and robustness to the reported result, as the two outcomes agree that universal HBV vaccination was cost-effective. Another strength of this study is that in order to increase the validity and reliability of the results, some parameters (such as the cost of vaccination program) were calculated using novel data. The use of sensitivity analysis also increases the reliability of the results.
The present study has some weaknesses. As with other economic evaluations, the results depend on the available literature. Estimates of costs are related to current conditions (the natural history of the disease and related treatments, and other influential health policies), which may change in the future, requiring subsequent analyses with updated parameters. Due to the high coverage of the vaccination program in Iran, it was not possible to select two comparable cohorts from the same time period. As such, the control cohort of unvaccinated individuals was selected from those born before the implementation of the national HBV vaccination program. In order to increase comparability, care was taken to ensure that individuals were selected in such a way as to minimize age differences between the two groups, and costs were calculated for both groups based on 2017 data.
Conclusions
The universal HBV vaccination strategy increased the expected QALY and life years of the cohort, and the cost-effectiveness of this strategy was confirmed for both outcomes. The universal HBV vaccination strategy increased life expectancy, improved quality of life, and reduced costs. The findings of this study, the first economic evaluation of HBV vaccination in Iran, show the impact of the immunization program on HBV infection and will be of interest to those working in healthcare policy. Given the economic advantage of the universal HBV vaccination strategy, it is recommended that this program continues.
Disclosure of Potential Conflicts of Interest
The authors have no conflicts of interest to declare.
Acknowledgments
The present article was extracted from a thesis written by Ali Mohammad Mokhtari (PhD of Epidemiology). This thesis was supervised by Dr Alireza Mirahmadizadeh and financially supported by Shiraz University of Medical Sciences, grant number 1396-01-04-16236. The authors wish to thank the research affairs of Shiraz University of Medical Sciences for financial support
Additional information
Funding
References
- Ben Hadj M, Bouguerra H, Saffar F, Chelly S, Hechaichi A, Talmoudi K, Bahrini A, Chouki T, Hazgui O, Hannachi N, et al. Observational study of vaccine effectiveness 20years after the introduction of universal hepatitis B vaccination in Tunisia. Vaccine. 2018;36:5858–64. doi:10.1016/j.vaccine.2018.08.038.
- Costa CI, Delgado IF, Da Costa JAC, De Carvalho RF, Mouta E Júnior SDS, Vianna COA, De Moraes MTB. Establishment and validation of an ELISA for the quantitation of HBsAg in recombinant hepatitis B vaccines. J Virol Methods. 2011;172:32–37. doi:10.1016/j.jviromet.2010.12.010.
- Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis. 2011;53:68–75. doi:10.1093/cid/cir270.
- Hung HF, Chen TH. Probabilistic cost-effectiveness analysis of the long-term effect of universal hepatitis B vaccination: an experience from Taiwan with high hepatitis B virus infection and hepatitis B E Antigen positive prevalence. Vaccine. 2009;27:6770–76. doi:10.1016/j.vaccine.2009.08.082.
- European Consensus Group on Hepatitis B Immunity. Are booster immunisations needed for lifelong hepatitis B immunity? Lancet. 2000;355:561–65. doi:10.1016/S0140-6736(99)07239-6.
- Rezaee R, Aghcheli B, Poortahmasebi V, Qorbani M, Alavian SM, Jazayeri SM. Prevalence of national responsiveness to HBV vaccine after 22 years of Iranian Expanded Program on Immunization (EPI): a systematic review and meta-analysis study. Hepat Mon. 2015;15:e23618. doi:10.5812/hepatmon.23618.
- Garcia D, Porras A, Rico Mendoza A, Alvis N, Navas MC, De La Hoz F, De Neira M, Osorio E, Valderrama JF. Hepatitis B infection control in Colombian Amazon after 15years of hepatitis B vaccination. Effectiveness of birth dose and current prevalence. Vaccine. 2018;36:2721–26. doi:10.1016/j.vaccine.2017.11.004.
- Liaw Y-F, Chu C-M. Hepatitis B virus infection. Lancet. 2009;373:582–92. doi:10.1016/S0140-6736(09)60207-5.
- Mirahmadizadeh A, Zahmatkesh S, Kashfi Nezhad M, Sayadi M, Tabatabaee H, Mokhtari A. Vaccination coverage in children of Fars Province, 2017: achievement of global vaccine action plan goals. Sadra Med Sci J. 2018;6:251–60. Persian.
- Merat S, Nouraie M, Abolghasemi H, Jamali R, Amini-Kafiabad S, Maghsudlu M, Pourshams A, Malekzadeh R. Seroprevalence and risk factors of hepatitis A virus infection in Iran: a population based study. Arch Iran Med. 2010;13:99.
- Moghadami M, Dadashpour N, Mokhtari AM, Ebrahimi M, Mirahmadizadeh A. The effectiveness of the national hepatitis B vaccination program 25 years after its introduction in Iran: a historical cohort study. Braz J Infect Dis. 2019b;23:419–26. doi:10.1016/j.bjid.2019.10.001.
- Weinberg GA, Szilagyi PG. Vaccine epidemiology: efficacy, effectiveness, and the translational research roadmap. J Infect Dis. 2010;201:1607–10. doi:10.1086/652404.
- Ultsch B, Damm O, Beutels P, Bilcke J, Brüggenjürgen B, Gerber-Grote A, Greiner W, Hanquet G, Hutubessy R, JIT M. Methods for health economic evaluation of vaccines and immunization decision frameworks: a consensus framework from a European Vaccine Economics Community. Pharmacoeconomics. 2016;34:227–44. doi:10.1007/s40273-015-0335-2.
- Haas M. Economic evaluation: a useful research method. Aust J Physiother. 2003;49:85–86. doi:10.1016/S0004-9514(14)60124-0.
- Griffiths UK, Hutton G, Pascoal EDD. The cost-effectiveness of introducing hepatitis B vaccine into infant immunization services in Mozambique. Health Policy Plan. 2005;20:50–59. doi:10.1093/heapol/czi006.
- Eckman MH, Kaiser TE, Sherman KE. The cost-effectiveness of screening for chronic hepatitis B infection in the United States. Clin Infect Dis. 2011:1294–306.
- Zehtab N, Jafari M, Barooni M, Nakhaee N, Goudarzi R, Larry Zadeh MH. Cost-effectiveness analysis of breast cancer screening in Rural Iran. Asian Pac J Cancer Prev. 2016;17:609–14. doi:10.7314/APJCP.2016.17.2.609.
- Kim SY, Salomon JA, Goldie SJ. Economic evaluation of hepatitis B vaccination in low-income countries: using cost-effectiveness affordability curves. Bull World Health Organ. 2007;85:833–42. doi:10.2471/BLT.06.038893.
- Beutels P. Economic evaluations of hepatitis B immunization: a global review of recent studies (1994-2000). Health Econ. 2001;10:751–74.
- Klingler C, Thoumi AI, Mrithinjayam VS. Cost-effectiveness analysis of an additional birth dose of hepatitis B vaccine to prevent perinatal transmission in a medical setting in Mozambique. Vaccine. 2012;31:252–59. doi:10.1016/j.vaccine.2012.08.007.
- Jafarzadeh A, Khoshnoodi J, Ghorbani S, Hazrati SM, Faraj Mazaheri B, Shokri F. Differential immunogenicity of a recombinant hepatitis B vaccine in Iranian neonates: influence of ethnicity and environmental factors. Iran J Immunol. 2004;1:98–104.
- Hahné S, Veldhuijzen I, Wiessing L, Lim T, Salminen M, Van de Laar M. Evidence for the cost-effectiveness of screening for chronic hepatitis B and C among migrant populations: results from a review of the literature. screening. 2013;13:181.
- Moghadami M, Dadashpour N, Mokhtari AM, Ebrahimi M, Mirahmadizadeh A. The effectiveness of the national hepatitis B vaccination program 25 years after its introduction in Iran: a historical cohort study. Braz J Infect Dis. 2019a;23:419–26.
- Zuckerman JN. Nonresponse to hepatitis B vaccines and the kinetics of anti-HBs production. J. Med. Virol. 1996;50(4):283–88.
- Hu Y, Grau LE, Scott G, Seal KH, Marshall PA, Singer M, Heimer R. Economic evaluation of delivering hepatitis B vaccine to injection drug users. Am J Prev Med. 2008;35:25–32. doi:10.1016/j.amepre.2008.03.028.
- Aggarwal R, Ghoshal UC, Naik SR. Assessment of cost-effectiveness of universal hepatitis B immunization in a low-income country with intermediate endemicity using a Markov model. J Hepatol. 2003;38:215–22. doi:10.1016/S0168-8278(02)00382-3.
- Jia Y, Li L, Cui F, Zhang D, Zhang G, Wang F, Gong X, Zheng H, Wu Z, Miao N, et al. Cost-effectiveness analysis of a hepatitis B vaccination catch-up program among children in Shandong Province, China. Hum Vaccin Immunother. 2014;10:2983–91. doi:10.4161/hv.29944.
- Tu HAT, De Vries R, Woerdenbag HJ, Li SC, Le HH, Van Hulst M, Postma MJ. Cost-effectiveness analysis of hepatitis B immunization in vietnam: application of cost-effectiveness affordability curves in health care decision making. Value Health Reg Issues. 2012;1:7–14. doi:10.1016/j.vhri.2012.03.007.
- Woo G, Tomlinson G, Yim C, Lilly L, Therapondos G, Wong DKH, Ungar WJ, Einarson TR, Sherman M, Heathcote JE, et al. Health state utilities and quality of life in patients with hepatitis B. Can J Gastroenterol. 2012;26:445–51. doi:10.1155/2012/736452.
- Walker D. Cost and cost-effectiveness guidelines: which ones to use? Health Policy Plan. 2001;16:113–21. doi:10.1093/heapol/16.1.113.
- Berangi Z, Karami M, Mohammadi Y, Nazarzadeh M, Zahraei SM, Javidrad H, Heidari S. Epidemiological profile of meningitis in Iran before pentavalent vaccine introduction. BMC Pediatr. 2019;19:370. doi:10.1186/s12887-019-1741-y.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford university press; 2015. https://books.google.co.uk/books?id=lvWACgAAQBAJ
- Fox-Rushby J, Cairns J. Economic evaluation. Open University Press: McGraw-Hill Education (UK); 2005.
- Prieto L, Sacristan JA. Problems and solutions in calculating quality-adjusted life years (QALYs). Health Qual Life Outcomes. 2003;1:80. doi:10.1186/1477-7525-1-80.
- Lu SQ, Mcghee SM, Xie X, Cheng J, Fielding R. Economic evaluation of universal newborn hepatitis B vaccination in China. Vaccine. 2013;31:1864–69. doi:10.1016/j.vaccine.2013.01.020.
- Siddiqui MR, Gay N, Edmunds WJ, Ramsay M. Economic evaluation of infant and adolescent hepatitis B vaccination in the UK. Vaccine. 2011;29:466–75. doi:10.1016/j.vaccine.2010.10.075.
- Hall EW, Rosenberg ES, Trigg M, Nelson N, Schillie SJPHR. Cost analysis of single-dose hepatitis B revaccination among infants born to hepatitis B surface antigen-positive mothers and not responding to the initial vaccine series. Public Health Rep. 2018;133(3):338–46.
- Tilson L, Thornton L, O’Flanagan D, Johnson H, barry M. Cost effectiveness of hepatitis B vaccination strategies in Ireland: an economic evaluation. Eur J Public Health. 2008;18:275–82. doi:10.1093/eurpub/ckm123.
- Chinnaratha MA, Kaambwa B, Woodman RJ, Fraser RJ, Wigg AJ. Assessing the clinical and economic impact of increasing treatment uptake in chronic hepatitis B infection using a Markov model. J Gastroenterol Hepatol. 2017;32(7):1370–7.
- Ke W, Zhang C, Liu L, Gao Y, Yao Z, Ye X, et al. Cost-effectiveness analysis of tenofovir disoproxil fumarate for treatment of chronic hepatitis B in China. Hepatol Int. 2016;10(6):924–36.
- Wiens A, Lenzi L, Venson R, Pedroso ML, Correr CJ, Pontarolo R. Economic evaluation of treatments for chronic hepatitis B. Braz J Infect Dis. 2013;17(4):418–26.
- Lee D, Park SM. Cost-effectiveness analysis of hepatitis B vaccination strategies to prevent perinatal transmission in North Korea: selective vaccination vs. universal vaccination. PloS One. 2016;11(11):e0165879.
- Kavosi Z, Zare F, Jafari A, Fattahi MR. Economic burden of hepatitis B virus infection in different stages of disease; a report from southern iran. Middle East J Digest Dis. 2014;6(3):156.
- Keshavarz K, Kebriaeezadeh A, Alavian SM, Akbari Sari A, Abedin Dorkoosh F, Keshvari M, et al. Economic burden of hepatitis B virus-related diseases: evidence from iran. Hepat Mon. 2015;15(4):e25854.
- Adibi P, Rezailashkajani M, Roshandel D, Behrouz N, Ansari S, Somi MH, et al. An economic analysis of premarriage prevention of hepatitis B transmission in Iran. BMC Infect Dis. 2004;4(1):31.
- Hajarizadeh B, Rashidian A, Haghdoost A, Alavian S. Estimating the costs of the mass vaccination campaign against hepatitis B in Iranian adolescents. GOVARESH. 2009;14(1):27–34.
- Levy AR, Kowdley KV, Iloeje U, Tafesse E, Mukherjee J, Gish R, et al. The impact of chronic hepatitis B on quality of life: a multinational study of utilities from infected and uninfected persons. Value Health. 2008;11(3):527–38.
- Wu B, Li T, Chen H, Shen J. Cost-effectiveness of nucleoside analog therapy for hepatitis B in China: a Markov analysis. Value Health. 2010;13(5):592–600.
- Edejer T-T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans D, et al. WHO guide to cost-effectiveness analysis. Geneva: World Health Organization; 2003.
- Hutton DW, So SK, Brandeau ML. Cost-effectiveness of nationwide hepatitis B catch-up vaccination among children and adolescents in China. Hepatology. 2010;51(2):405–14.