ABSTRACT
Mastocytosis is a rare group of disorders characterized by abnormal accumulation of mast cells in the skin, bone marrow, and internal organs. In particular, patients with systemic mastocytosis are at an increased risk of frequent and severe episodes of anaphylaxis. Hymenoptera venom allergy is the most common trigger of anaphylaxis in these patients. Immunotherapy is an effective and safe therapy recommended for patients with mastocytosis and venom allergy. Although this therapy can be administered according to different protocols, the preferred protocol for patients with mastocytosis remains unclear. Systemic side effects can occur, in particular, during the up-dosing phase of immunotherapy, making progression to the maintenance phase of therapy challenging. This case report presents the diagnosis and ultrarush immunotherapy process ended with anaphylaxis of a 33-y-old male patient with Apis mellifera allergy.
Introduction
Mastocytosis is a rare, heterogeneous group of disorders characterized by abnormal proliferation and accumulation of mast cells in one or more organs. The cutaneous form of mastocytosis is characterized by isolated skin involvement and is often observed in children who have mild symptoms and recover spontaneously during puberty. Systemic mastocytosis (SM) is a severe form of the disease, involving the skin and/or extracutaneous organs such as the liver, spleen, lymph nodes, and bone marrow. The most common form of SM is the indolent SM (ISM) that remains inactive for a long period of time. Local or systemic symptoms occur due to the release of the vasoactive mediators in the mast cells with the effect similar to that of certain stimuli (i.e., venom, drug, food) observed in patients with mastocytosis. Patients may present with symptoms such as a burning sensation in the skin, itching, rash, flushing, abdominal pain, diarrhea, vomiting, dyspepsia, headache, hypotension, and anaphylaxis.Citation1,Citation2
The prevalence of anaphylaxis is 22–49% in the adult patients with SM, where the hymenoptera venom allergy (HVA) is the most common etiological factor.Citation2 Venom immunotherapy (VIT) is the most effective and safe treatment method in the patients at high risk of anaphylaxis due to venom allergy.Citation3 This case report presents an ultrarush VIT course for honeybee in an adult patient with ISM and HVA.
Case presentation
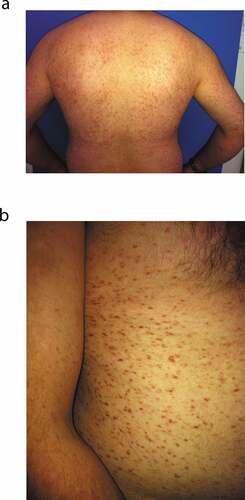
A 33-y-old male patient was referred to our allergy clinic with a history of widespread skin rash, shortness of breath, vomiting, and syncope after sustaining a honeybee sting. The patient reported that he lived in a rural area and had a history of similar reactions for 10–15 min after a honeybee sting that occurred twice during the previous year. Physical examination of the patient revealed pink-red macular lesions on the back, abdomen, and both arms (). These lesions had been present for approximately 10 y, and the patient had been diagnosed with urticaria pigmentosa after a skin biopsy 3 y before presenting at our department. The patient had been advised to take antihistamine tablets when required. Furthermore, the patient was prescribed regular therapy with inhaled formoterol+budesonide and oral antihistamine+leukotriene antagonist for asthma that remained stable during the previous year.
Figure 1. (a) Urticaria pigmentosa (back and both arms). (b) Urticaria pigmentosa (abdomen and right arm)
Hemogram and routine biochemistry tests showed no abnormal findings. Serum tryptase level was 96.8 ng/mL (reference range 0–11.5 ng/mL), and abdominal ultrasonography to detect possible internal organ involvement revealed no abnormal findings. Immunohistochemical staining of the bone marrow biopsy specimens revealed multifocal intense mast cell infiltration (>15 cells/per aggregate) and mast cell tryptase, CD117, and CD25 positivity. The patient was diagnosed with ISM based on anamnesis and laboratory tests.
Laboratory tests for HVA revealed serum total IgE level of 14.1 IU/mL (reference range 0–85 IU/mL), and the IgE levels for wasp and honeybee were found to be negative (reference range < 0.35 kIU/L) (ImmunoCAP, Thermo Fisher Scientific/Phadia, Uppsala, Sweden). Skin prick testing at a 1:1 concentration showed a positive reaction to honeybee venom and a negative reaction to wasp venom (Alyostal, Stallergenes, Antony Cedex, France).
Because allergic reactions were associated with honeybee in the medical history, VIT for honeybee was planned for the patient. An ultrarush VIT protocol was selected, considering the experience of our clinic, the patient’s socioeconomic status, long travel time required of the patient for clinic visits, the flexibility of the therapy that allows a rapid switch to a maintenance dose, and the patient’s preferences (subcutaneous injections of 0.1, 1, 10, and 20 μg doses with 30-min intervals and doses of 30 and 40 μg with 1-h intervals in the first day; 50 + 50 μg doses with 1-h interval at day 8; 100 μg doses at day 22).
After monitoring and establishing venous access in the emergency department, the patient was administered subcutaneous doses of Alyostal Apis mellifera venom (Stallergenes, Antony Cedex, France). The process and timeline followed in the present case are shown in . A systemic reaction developed at a dose of 30 μg on the first day. After corticosteroid and antihistamine treatment, the patient’s complaints improved; the patient was subsequently observed for any further changes.
Table 1. Apis mellifera venom immunotherapy (VIT) administration scheme of our case
On the second day, we set the treatment dose intervals as 1 h. We administered the last dose of 20 μg, which was tolerated by the patient, but mild side effects occurred. After antihistamine treatment, we reduced the dose by 20% and applied 15 μg and again 20 μg doses, which were tolerated well and with no side effects. However, the treatment was discontinued because a severe systemic reaction developed at a dose of 30 μg. After the VIT treatment was terminated, the patient was hospitalized for close monitoring; the patient was provided with two adrenaline autoinjectors and an emergency kit (oral antihistamine and corticosteroid) on discharge. The patient refused VIT due to severe side effects and did not undergo pretreatment with omalizumab.
Discussion
VIT is the only treatment method that reduces the risk of severe reactions after a bee sting and increases the quality of life in patients diagnosed with HVA. Mastocytosis is an important risk factor in both the frequency and the severity of anaphylaxis in patients with HVA.Citation2,Citation3 Venom extract-specific IgE (sIgE) levels can be lower in patients with mastocytosis than in the general population.Citation4,Citation5 Moreover, in general, these sIgE levels are below the traditional cutoff value (0.35 kIU/L), in which case there is difficulty in diagnosing HVA. Michel et al.Citation4 showed that in patients with mast cell disorders, usually venom sIgE levels are between 0.10 and 0.35 kIU/L, and the current recommended diagnostic cutoff value is 0.10 kIU/L. A separate study reported that 0.17 kIU/L cutoff value for yellow jacket venom sIgE was more appropriate than 0.35 kIU/L for optimal diagnostic accuracy in patients with mastocytosis.Citation5 As a result, levels between 0.10 and 0.35 kIU/L for venom sIgE in patients with mastocytosis can be considered positive in patients with a convincing history.
Negative standard venom extract sIgE and negative skin tests are quite common in patients with mastocytosis. In these cases, which present significant challenges for HVA diagnosis and VIT, analysis of sIgE to recombinant allergens should be performed.Citation6 Although our patient had no history of a reaction to wasp sting, he did have a history of anaphylaxis after honeybee stings. We did not perform venom allergen sIgE tests because the skin prick test was positive only to the honeybee.
In the general population, VIT prevents subsequent systemic sting reactions in 77–84% and 91–96% of patients treated with honeybee venom and Vespula spp. venom, respectively.Citation2,Citation3 VIT is strongly recommended in patients with mastocytosis and HVA, despite the evidence of a slightly decreased efficacy.Citation2,Citation7
Local and systemic reactions can be observed in relation to VIT. The most important risk factors for such side effects are therapy for honeybee venom and rapid dose increase in the up-dosing phase of the rush/ultrarush protocols. In addition, mastocytosis and/or high serum tryptase levels are considered to be risk factors for side effects associated with VIT. In general, the rate of systemic reactions in VIT is 8–20%.Citation3 Side effects were detected in 38 (18.9%) patients in a study evaluating the safety of VIT in 201 patients with mastocytosis, and most of these side effects were systemic reactions (73.7%).Citation2 Although side effects associated with VIT are more frequently observed in the patients with than without mastocytosis, VIT has often been reported as well tolerated also in this patient group.Citation3,Citation7,Citation8
To date, no preferred VIT protocol for use in patients with HVA and mastocytosis has been established. Each center selects a treatment protocol, depending on their experience and the patient’s condition. Conventional, cluster, rush, and modified rush VIT protocols have been described in the literature.Citation7–9 A total of 5 systemic reactions (3 clusters, 2 conventional) have been reported in a study of 21 patients with ISM undergoing conventional/cluster VIT. In this study, systemic reactions (1 conventional, 1 cluster) occurred in 2 out of 5 patients who received VIT for honeybee, whereas no side effects were observed in the remaining 3 patients (2 conventional, 1 cluster). The authors reported that the last dose had been repeated, or the therapy had been resumed with dose reduction after the occurrence of a systemic reaction in these patients. Of the patients who developed systemic reactions, one refused resume the therapy (wasp venom), whereas the therapy was discontinued in the other patient due to severe side effects (mixed vespid venom and honeybee venom). These researchers have found extremely low basal tryptase levels in the patients who developed systemic reactions.Citation8 These levels were lower than those in our patients.
In another study, which involved a total of 84 Italian and Spanish patients with mastocytosis (91% had ISM), which performed VIT using conventional/rush/modified rush protocols, systemic reactions were reported in 4 patients in the up-dosing phase of the therapy. Three out of these four patients (2 modified rush, 1 conventional) were among 14 (21.4%) patients who underwent VIT for honeybee venom. Following these side effects, dose increments were slowed down, and VIT was continued with oral antihistamine premedication. There was no difference in the type of side effects observed in the conventional and modified rush protocols; however, side effects were more common in patients undergoing the modified rush protocol. Furthermore, in this study, no patient was reported to withdraw from the therapy due to side effects; moreover no side effects occurred in the maintenance phase.Citation7
In a recent study, which is, to our knowledge, the only study employing the ultrarush VIT protocol in patients with mastocytosis, no side effects have been reported in 8 patients (1 honeybee) during the up-dosing and maintenance phases. In this study, all patients had received premedication with oral antihistamine before undergoing VIT. Similar to our patient, one patient receiving VIT for honeybee in this previous study had ISM; however, this patient’s basal serum tryptase level (15.2 ng/mL) was considerably lower than that of our patient.Citation10
In another study evaluating 32 patients with mastocytosis (22 had ISM) and wasp venom allergy, side effects occurred in 8 patients in the up-dosing phase of the VIT that lasted 7 weeks and in 3 patients in the maintenance phase. All patients received premedication with oral antihistamine before VIT, and local reactions and mild systemic reactions have often been observed. However, one patient who underwent simultaneous VIT for wasp and honeybee developed severe anaphylaxis in the maintenance phase. Similar to those observed in our patient, systemic reactions in these patients could be treated only after administration of the second dose of adrenaline; subsequently, omalizumab was added to the therapy, and VIT was continued only for wasp.Citation11 In our patient, severe systemic reactions occurred despite dose reduction, and the therapy was finally discontinued. The patient did not undergo subsequent omalizumab therapy. When systemic reactions occur during VIT, one or more of the such options as switching to another protocol with a lower up-dosing phase, premedication, and omalizumab therapy are recommended.Citation11,Citation12 Concurrently, some patients may not continue VIT due to recurrent anaphylaxis, as was the case with our patient. In addition, therapy can be terminated if the risks of resuming therapy outweigh the benefits.Citation8,Citation13
In conclusion, the ultrarush VIT protocol for honeybee may cause severe systemic reactions in patients with SM and high basal tryptase levels. The VIT protocol for these patients should involve admission to the emergency department of an experienced center; all measures against the development of anaphylaxis should be taken. Every effort must be made to administer an effective dose of VIT that would last a lifetime, considering the high risk of developing severe reactions due to bee stings in patients with mastocytosis. The decision to discontinue the therapy must be made by weighing the potential risks and benefits in a detailed patient-physician interview. Patients who are not undergoing VIT should be prescribed at least two adrenaline autoinjectors and an emergency kit.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank the patient for the contribution to the case report.
References
- Valent P, Akin C, Escribano L, Födinger M, Hartmann K, Brockow K, Castells M, Sperr WR, Kluin-Nelemans HC, Hamdy NA, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37:435–53. doi:10.1111/j.1365-2362.2007.01807.x. PMID: 17537151.
- Bonadonna P, Scaffidi L. Hymenoptera anaphylaxis as a clonal mast cell disorder. Immunol Allergy Clin North Am. 2018;38:455–68. doi:10.1016/j.iac.2018.04.010. PMID: 30007463.
- Sturm GJ, Varga EM, Roberts G, Mosbech H, Bilò MB, Akdis CA, Antolín-Amérigo D, Cichocka-Jarosz E, Gawlik R, Jakob T, et al. EAACI guidelines on allergen immunotherapy: hymenoptera venom allergy. Allergy. 2018;73:744–64. doi:10.1111/all.13262. PMID: 28748641.
- Michel J, Brockow K, Darsow U, Ring J, Schmidt-Weber CB, Grunwald T, Blank S, Ollert M. Added sensitivity of component-resolved diagnosis in hymenoptera venom-allergic patients with elevated serum tryptase and/or mastocytosis. Allergy. 2016;71:651–60. doi:10.1111/all.12850. PMID: 26836051.
- Vos BJPR, van Anrooij B, van Doormaal JJ, Dubois AEJ, Oude Elberink JNG. Fatal anaphylaxis to yellow jacket stings in mastocytosis: options for identification and treatment of at-risk patients. J Allergy Clin Immunol Pract. 2017;5:1264–71. doi:10.1016/j.jaip.2017.03.019. PMID: 28499778.
- Cifuentes L, Vosseler S, Blank S, Seismann H, Pennino D, Darsow U, Bredehorst R, Ring J, Mempel M, Spillner E, et al. Identification of hymenoptera venom-allergic patients with negative specific IgE to venom extract by using recombinant allergens. J Allergy Clin Immunol. 2014;133:909–10. doi:10.1016/j.jaci.2013.09.047. PMID: 24290287.
- Bonadonna P, Gonzalez-de-Olano D, Zanotti R, Riccio A, De Ferrari L, Lombardo C, Rogkakou A, Escribano L, Alvarez-Twose I, Matito A, et al. Venom immunotherapy in patients with clonal mast cell disorders: efficacy, safety, and practical considerations. J Allergy Clin Immunol Pract. 2013;1:474–78. doi:10.1016/j.jaip.2013.06.014. PMID: 24565619.
- González de Olano D, Alvarez-Twose I, Esteban-López MI, Sánchez-Muñoz L, de Durana MD, Vega A, García-Montero A, González-Mancebo E, Belver T, Herrero-Gil MD, et al. Safety and effectiveness of immunotherapy in patients with indolent systemic mastocytosis presenting with Hymenoptera venom anaphylaxis. J Allergy Clin Immunol. 2008;121:519–26. doi:10.1016/j.jaci.2007.11.010. PMID: 18177694.
- Verburg M, Oldhoff JM, Klemans RJ, Lahey-de Boer A, de Bruin-weller MS, Röckmann H, Sanders C, Bruijnzeel-Koomen CA, Pasmans SG, Knulst AC. Rush immunotherapy for wasp venom allergy seems safe and effective in patients with mastocytosis. Eur Ann Allergy Clin Immunol. 2015;47:192–96. PMID: 26549336.
- Gruzelle V, Ramassamy M, Bulai Lidiveanu C, Didier A, Mailhol C, Guilleminault L. Safety of ultra-rush protocols for hymenoptera venom immunotherapy in systemic mastocytosis. Allergy. 2018;73:2260–63. doi:10.1111/all.13557. PMID: 29984458.
- Jarkvist J, Salehi C, Akin C, Gülen T. Venom immunotherapy in patients with clonal mast cell disorders: igG4 correlates with protection. Allergy. 2020;75:169–77. doi:10.1111/all.13980. PMID: 31306487.
- Golden DBK. Venom Immunotherapy: questions and controversies. Immunol Allergy Clin North Am. 2020;40:59–68. doi:10.1016/j.iac.2019.09.002. PMID: 31761121.
- Kors JW, van Doormaal JJ, de Monchy JG. Anaphylactoid shock following Hymenoptera sting as a presenting symptom of systemic mastocytosis. J Intern Med. 1993;233:255–58. doi:10.1111/j.1365-2796.1993.tb00984.x. PMID: 8450293.
