ABSTRACT
We conducted a systematic review to characterize the incidence rate of herpes zoster (HZ) in the general population, specifically in individuals ≥50 years of age. A total of 69 publications were included in the review. We found a cumulative incidence of HZ ranging from 2.9–19.5 cases per 1,000 population and an incidence rate of HZ ranging from 5.23–10.9 cases per 1,000 person-years. The cumulative incidence (3.22–11.2 versus 2.44–8.0 cases per 1,000 population) and incidence rates (6.05–12.8 versus 4.30–8.5 cases per 1,000 person-years) were higher in females than males. Studies revealed a trend of increasing incidence of HZ with increasing age and over time. Variations in incidence estimates can be attributed to the various study designs, case ascertainments, age distributions of the population and year of the study. HZ is associated with a substantial disease burden and is expected to increase due to population aging.
Introduction
Primary infection with the varicella zoster virus (VZV) often leads to acute varicella or chickenpox, typically in childhood. After recovery from chickenpox, the virus remains dormant in the dorsal root ganglia.Citation1 Age-related decline in immunity or an immunosuppressed condition may lead to the reactivation of VZV causing herpes zoster (HZ), also called shingles. HZ is distinguished by a painful or pruritic, commonly unilateral, blistering rash. Although pain may persist for much longer, the average duration of the HZ rash ranges from 7 to 10 days, with the skin healing completely within approximately 2 to 4 weeks.Citation2
The pain associated with HZ has been described as aching, burning, stabbing, or shock-like. Individuals with HZ may also experience altered sensitivity to touch, pain provoked by trivial stimuli, and unbearable itching.Citation3 The median duration of pain is approximately 32.5 days (the mean duration is 45 days).Citation3 Postherpetic neuralgia (PHN) is frequently defined as pain persisting for at least 3 months after rash onset, and occurs in 5% to 30% of patients.Citation4 Pain associated with PHN can disrupt all aspects of daily life and patients with PHN may experience depression, reduced quality of life, and social withdrawal.Citation2 Other complications associated with HZ include stroke or other cardiovascular events, neurological sequelae, palsy and gastrointestinal ailments.Citation5 Severe cases of the above complications often require hospitalization.Citation5
The lifetime risk of HZ disease without vaccination ranges between 20% and 30%.Citation6,Citation7 Gender, ethnicity, family history, and comorbidities such as systemic lupus erythematosus, asthma, diabetes mellitus and chronic obstructive pulmonary disease are risk factors for HZ.Citation8 An increase in age leads to higher incidence and severity of HZ disease, especially after the age of 50 years, due to age-related decline in immunity.Citation9 Considering the significance of age as a risk factor, the increasing life expectancy in the general population may considerably increase HZ annual cases and disease burden.Citation10 It is becoming crucial for healthcare professionals and health policy-makers to be informed of the latest evidence on the disease burden of HZ. Findings from a previous review conducted in 2014 provides a comprehensive overview of HZ as a significant global health burden.Citation4 To our knowledge, there are no reviews summarizing the evidence from more recently conducted epidemiology and burden of disease studies.
The objective of this review is to provide an up-to-date evidence base on the incidence of HZ. Specifically, this review aims to summarize the incidence rates of HZ in the general population with a focus on individuals ≥50 years of age (YOA). In addition, when available in the literature, the incidence of HZ by risk factors such as gender, age, ethnicity and immunocompetence is described. Trends of HZ incidence stratified by different geographical regions and over time are also presented.
Methods
We performed a systematic review of the literature according to guidelines specified in the Cochrane Handbook for Systematic Reviews of InterventionsCitation11 and Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses (PRISMA)Citation12,Citation13 to obtain relevant information using a reproducible, robust and transparent methodology.
Search sources and strategy
We searched the following online databases: PubMed, Embase, and the Virtual Health Library (VHL) including the Latin American & Caribbean Health Sciences Literature (Lilacs) database. The search strategy was developed using both indexed terms and terms described in the title or abstract. Search terms for the different databases were combined using Boolean operators. Details of the search strategy are provided in Supplementary Table 1. All searches were restricted by publication date from 1 January 2002 onwards and were conducted on 7 December 2018.
Article selection and quality control
Publications identified from the searches were screened in three phases using the inclusion and exclusion criteria provided in Supplementary Table 2.
In the first phase, publications were screened based on the titles and abstracts. All titles and abstracts were screened in duplicate by two independent researchers (HV, EB). The results were compared, and deviations were discussed. In the second phase, the first 10% of eligible full-text publications were checked for relevancy in duplicate by two independent researchers (HV, EB). The results were compared and discussed early in the process to minimize the differences between both researchers with regards to the full-text publications screened in duplicate.
The process of selection of publications was registered in an EndNote library by one of the researchers. The full-text selection was documented per article with reason of exclusion in an Excel file to ensure that a clear overview of all selection steps across all phases was maintained, and reproducibility of the results was assured.
Data extraction, quality assessment and descriptive analyses
After the eligible publications were identified for this review, one researcher (HV) extracted the relevant data from these publications into an Excel database. A reviewer (EB) quality checked the extracted data. Data extraction parameters were established a priori and included publication details, country, study characteristics (design, time period, setting), population characteristics (inclusion and exclusion criteria, sample size, age groups, gender, ethnicity, underlying immunocompromising conditions), methodology (case detection, case definition, type of patients and incidence denominator), incidence of HZ (per person and per person-years separately) by gender, age, ethnicity, year and case definition and information to assess the quality of the study.
There are several well-known and validated checklists available to assess the quality of publications with “classical” study designs (e.g., cohort, case-control, randomized controlled trial, etc.). However, there are no formal validated checklists which focus on incidence studies that are typically designed as surveillance studies. We defined three questions in order to assess the quality of the included HZ incidence studies (Supplementary Table 3).
In this paper, we provide a descriptive overview of the incidence of HZ in the general population ≥50 YOA. Incidence (number of HZ cases per 1,000 population hereafter referred to as cumulative incidence or as number of HZ cases per 1,000 person-years hereafter referred to as incidence rate) is presented for the overall population and for the general population stratified by gender, age, ethnicity, study year and case definition. Where incidence was expressed as a cumulative, we transformed the data from a percentage (i.e., per population) to an incidence rate assuming an exponential distribution (i.e., s(t) = 1-exp(-ƴt) where ƴ is the incidence rate and t is time, assumed to be equal to 1.Citation14 This transformation facilitated the presentation of trends in HZ incidence.
Results
Included studies
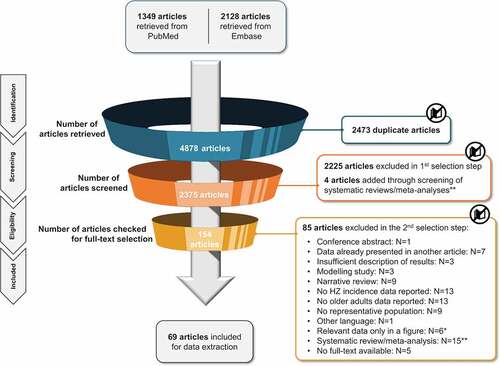
A total of 4,848 publications were identified from the databases. After the removal of duplicates, titles and abstracts of 2,375 publications were screened for eligibility based on the pre-specified inclusion and exclusion criteria (Supplementary Table 2). After excluding 2,225 publications based on title and abstract screening, 150 full-text publications were assessed for full-text eligibility using the same criteria. A total of 69 publications were included in the review .
Figure 1. PRISMA diagram
Study characteristics
The characteristics of the 69 individual studies are provided in . The majority of studies were conducted in the United States of America (USA; n = 14),Citation6,Citation15–27 followed by Canada (n = 6),Citation28–33 the United Kingdom (UK; n = 6),Citation34–39 Germany (n = 5),Citation40–44 Japan (n = 5),Citation45–49 the Netherlands (n = 5),Citation36,Citation50–53 Spain (n=5),Citation54–58 Taiwan (n = 4),Citation59–62 Australia (n = 3),Citation63–65 China (n = 3),Citation66–68 Italy (n = 3),Citation69–71 France (n = 2),Citation72,Citation73 New Zealand(n = 2),Citation74,Citation75 Sweden (n = 2),Citation76,Citation77 Denmark (n = 1),Citation78 Israel (n = 1),Citation79 Norway (n = 1),Citation80 Poland (n = 1),Citation81 and South Korea (n = 1).Citation82 One study presented data for both the Netherlands and the UK separately;Citation36 this study is captured within the individual countries ; .
Figure 2. Distribution of publications (N = 69) by (A) Study design, (B) Case identification method, (C) Incidence data stratification.
The total number of studies add up to 77 studies as 8 studies utilized more than one method to detect HZ (see )
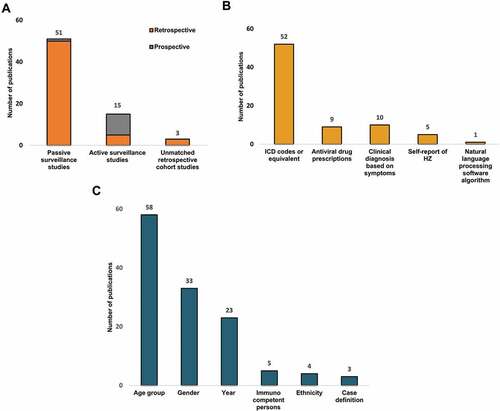
Table 1. Characteristics of included studies (N = 69)
provides the distribution of the 69 included studies by study design. Most studies (n = 51) were passive surveillance studies, with either a retrospective (n = 50) or prospective (n = 1)Citation63 design. Furthermore, 15 active surveillance studies (10 prospective [] and 5 retrospectiveCitation15,Citation26,Citation66–68), and 3 unmatched retrospective cohort studiesCitation21,Citation24,Citation34 were found.
provides the distribution of the 69 included studies by the method used for HZ case identification. Fifty-two studies used the International Classification of Diseases (ICD) codes or equivalent codes to define HZ cases (), while 9 studies also used antiviral drug prescriptions (), 10 studies used a clinical diagnosis based on symptoms (), 5 studies used a self-report of HZ (), and one study used a natural language processing software algorithm ().
provides the distribution of the studies by the type of incidence data reported. More studies (n = 42; ) expressed the HZ incidence as cumulative incidence than as incidence rate (n = 27; ). While 6 studies gave only one overall HZ incidence (), 58 studies stratified the incidence by age (), 33 studies by gender (), 23 studies by study year (), and 4 studies by ethnicity.Citation15,Citation17,Citation21,Citation38 In 5 studies, the HZ incidence was reported for the overall general population as well as the immunocompetent population only.Citation6,Citation21,Citation24,Citation41,Citation71 Lastly, 3 studies reported the HZ incidence for two different case definitions of HZ.Citation21,Citation64,Citation65
Thirty-six of the included studies were nationwide studies or claimed to be representative of the national population, while the remaining studies were conducted in one or several country regions. Study periods varied, with the oldest data reported for the period 1980–1989Citation19 and the most recent data for the year 2017.Citation49 The number of studies including outpatients only (n = 32) was similar to the number of studies including both inpatients and outpatients (n = 37) (). The HZ incidence was reported for a study population ≥50 YOA in 42 studies (). In 12, 2 and 6 studies, the study population was ≥45 YOA (), ≥60 YOACitation49,Citation53 and ≥65 YOA (), respectively. In the remaining studies, the HZ incidence was described for a study population of ≥70 YOA,Citation80 >80 YOA,Citation60 60–89 YOA,Citation34 50–74 YOA,Citation45 50–64 YOA,Citation20 70–79 YOA,Citation46 or 68–79 YOA.Citation39
Overview of the incidence of HZ
Overall incidence of HZ in the general population
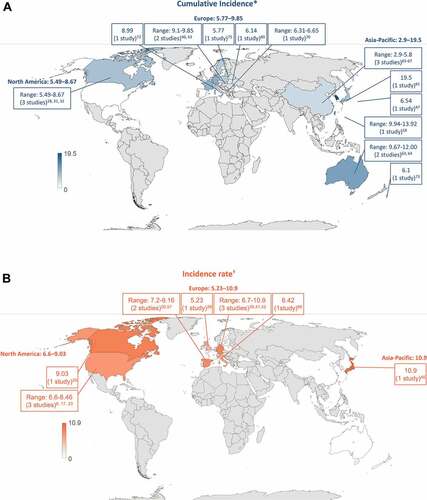
The overall HZ incidence in the general population ≥50 YOA was reported in 30 of 42 studies (). When comparing the geographical regions, the cumulative incidence ranges were 5.49–8.67 per 1,000 population for North America, 5.77–9.85 per 1,000 population for Europe and 2.9–19.5 per 1,000 population in the Asia-Pacific region. Incidence rates were 6.6–9.03 per 1,000 person-years for North America, 5.23–10.9 per 1,000 person-years for Europe and 10.9 per 1,000 person-years in the Asia-Pacific region.
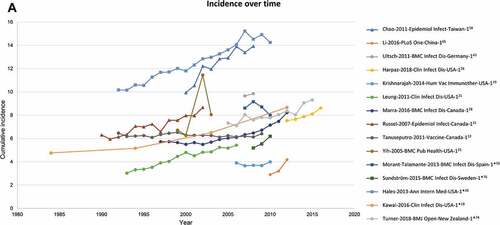
Trends in cumulative incidence, over time
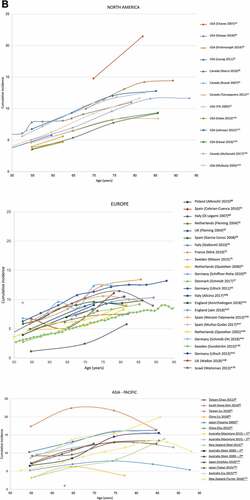
shows the cumulative incidence of HZ which is seen to be increasing over time. It should be noted that these studies do not all cover the same age groups, hence a wide difference in incidence estimates is observed. Most studies report the observed increase of HZ incidence over time. One study by Yih et al. specifically presented the relationship between varicella vaccination introduction and HZ incidence increase over time.Citation26
Incidence of HZ in the general population, by gender
The HZ incidence in the general population ≥50 YOA stratified by gender was reported in 14 studies. All 14 studies reported a higher incidence of HZ in females compared to males. In females, the cumulative incidence in the general population ≥50 YOA ranged from 3.22 cases per 1,000 population in 2010 in ChinaCitation66 to 11.2 cases per 1,000 population in 2004 in Germany.Citation41 In males, the corresponding incidence ranged from 2.44 per 1,000 persons to 8.0 per 1,000 persons in the same studies.Citation41,Citation66 The incidence rate in females ranged from 6.05 cases per 1,000 person-years in the period 2006–2012 in the UKCitation37 to 12.8 cases per 1,000 person-years in the period 2008–2012 in Japan.Citation47 In males, the corresponding incidence ranged from 4.30 cases per 1,000 person-years to 8.5 cases per 1,000 person-years in the same studies.Citation37,Citation47
Twenty-eight studies reported a higher incidence of HZ in females than for in males in age groups besides the ≥50 years group. However, Chaves et al. reported a higher HZ incidence in males in the ≥65 years group, but corresponding confidence intervals were wide and overlapped.Citation15 In two studies a higher incidence in males was found in the highest age groups only.Citation23,Citation74
Incidence of HZ in the general population, by age
Almost all studies (n = 58) reported the HZ incidence for different age groups (Supplementary Table 4). In 35 studies, the incidence increased with increasing age. However, in 14 studies a drop in incidence was reported for the highest age groups, i.e., ≥70 YOA,Citation67,Citation82 ≥75 YOA,Citation56 ≥80 YOA,Citation42,Citation47,Citation48,Citation57,Citation61,Citation71 or ≥85 YOA.Citation37,Citation62,Citation69,Citation75,Citation79 In 8 studies, both scenarios (i.e. increasing incidence with increasing age and a decline in incidence for the highest age group) were reported during different time periods.Citation17,Citation23,Citation31–33,Citation55,Citation59,Citation64 Moreover, in one study the incidence of HZ decreased in individuals of 60–69 YOA compared to those of 50–59 YOA, after which it increased in individuals ≥70 YOA.Citation54 Except for one Japanese study that did not report the incidence for persons ≥75 YOACitation45 and one Chinese study,Citation66 all Asian studies reported a drop in incidence for the highest age groups.Citation47,Citation48,Citation59,Citation61,Citation62,Citation67,Citation82 Furthermore, 2 Italian studies,Citation69,Citation71 2 Spanish studies,Citation56,Citation57 one UK studyCitation37 and one study from New ZealandCitation75 reported a decline in the incidence for the highest age groups. From the other European countries, Australia, Canada and the USA, no studies were found that solely reported a declining incidence in the highest age groups.
Trends in cumulative incidence, by age
The cumulative incidence for all geographical regions by age is provided in (data for all individual studies is provided in Supplementary Figure 1). Most studies depict a steady increasing trend in HZ incidence with age. Four studies covering North America,Citation15 EuropeCitation70 and two studies from the Asia-Pacific regionCitation68,Citation82 report incidence estimates that deviate from the overall trend.Citation15,Citation68,Citation70,Citation82
Incidence of HZ in immunocompetent persons
Nine studies reported the HZ incidence in immunocompetent persons only.Citation6,Citation21,Citation24,Citation37,Citation39,Citation41,Citation58,Citation71,Citation73 In seven studies, the HZ incidence for those ≥50 YOA was reported, with a range of 6.31–9.5 cases per 1,000 personsCitation41,Citation71,Citation73 and a range of 5.23–7.2 cases per 1,000 person-years.Citation6,Citation24,Citation37,Citation58 In five studies, the HZ incidence was separately reported for the overall general population and the immunocompetent population only.Citation6,Citation21,Citation24,Citation41,Citation71 In all five studies, estimates of incidence in the overall general population were numerically higher compared to the immunocompetent population.
Incidence of HZ in the general population, by ethnicity
In 4 studies, the HZ incidence was reported by ethnicity.Citation15,Citation17,Citation21,Citation38 In 3 studies, the highest incidence was reported in Caucasians, while in the fourth study, persons with an American Indian/Alaskan native ethnicity were found to have the highest HZ incidence.Citation17 The lowest HZ incidence was generally found among persons reported as black, except in the study of Chaves et al. where those with either Hispanic or other ethnicity had the lowest HZ incidence.Citation15
Incidence of HZ in the general population, by study year
In 23 studies the HZ incidence was reported for different years, and in 17 of these studies the HZ incidence increased during the years. In addition, Amirthalingam et al. also reported such an increase until the introduction of the zoster vaccine in the UK.Citation34 Harpaz et al. also found an increase in HZ incidence over time, but specifically reported a decline in the rate of increase among older adults from 2006 through 2016; reasons for this trajectory over time for older adults could not be confirmed as a consequence of the impact of HZ vaccination introduction.Citation27 In the remaining five studies, no clear increasing trend over the years was reported.Citation20,Citation32,Citation33,Citation57,Citation68
Incidence of hz in the general population, by case definition
In 3 studies, the HZ incidence was compared for different case definitions of HZ. Langan et al. reported the HZ incidence based on ICD codes only and based on these ICD codes in combination with the use of antivirals within 7 days before or after the diagnostic code for HZ.Citation21 Among those individuals ≥65 YOA, the incidence was much lower when using the latter definition (9.9 per 1,000 person-years) compared to ICD codes only (15.0 per 1,000 person-years). The lower incidence using the latter definition remained when the data were stratified for different age, gender and ethnicity groups. Two Australian studies compared the HZ incidence based on general practitioner (GP) visits with incidence estimates based on antiviral prescriptions. In both studies, no clear difference was found between both case definitions.Citation64,Citation65
Quality assessment of the included studies
The methodological quality of each publication was assessed using the quality assessment tool provided in Supplementary Table 3. The majority of studies had a valid case definition for the diagnosis of HZ and the denominator to calculate incidence was properly defined. However, the majority of studies did not capture individuals that were representative of the target population (Supplementary Table 5).
Discussion
This review provides an overview of the worldwide incidence of HZ in the general population with data from 69 studies. It also provides important contemporary insights on the incidence of HZ by gender, age, immunocompetent status, ethnicity, study year and case definition since the last published systematic review on this topic by Kawai et al.Citation4 in 2014. It should be noted that this review found little to no evidence for the regions of Eastern Europe, Middle East, South America or Africa. HZ may be a low health priority in many of these countries; however, the proportion of older adults is projected to double over the next several decades,Citation14 and the numbers of HZ cases may increase worldwide. The evidence base from Kawai et al.Citation4 together with this review provides a comprehensive overview of how an appropriate study methodology leads to consistent methods being used for worldwide incidence studies to help deliver reliable estimates of disease burden. These updated data could also be utilized in the context of healthcare policy surrounding the implementation of effective preventive measures such as vaccination against HZ.
Overall, the cumulative incidence of HZ ranged from 2.9 to 19.5 cases per 1,000 population and from 5.23 to 10.9 cases per 1,000 person-years in the general population ≥50 YOA. Among geographical regions, the highest and lowest incidence rates were both reported in the Asia-Pacific countries, although incidence estimates overlap, and the lowest incidence came from the same region. In the general population ≥50 YOA, the cumulative incidence (3.22–11.2 versus 2.44–8.0 cases per 1,000 population) and incidence rates (6.05–12.8 versus 4.30–8.5 cases per 1,000 person-years) were higher in females than in males. The incidence of HZ was higher in Caucasians compared to persons of Black ethnicity, but this was examined in only a few studies and could be attributed to under-reporting. Only a few studies reported incidence data based on these stratifications. Across regions, a trend of steadily increasing HZ incidence over time is observed.
Variations in incidence estimates summarized in this review could be related to several factors and warrant discussion in the interpretation of the overall review findings. Methodological variations related to geographical spread, sample size, diagnostic methodology, time period and age of the study population were observed among studies. This made comparisons between studies difficult while simultaneously affecting generalizability. Specifically, studies varied in terms of study setting (ranging from a single region to national surveillance), and sample size (ranging from 2,135 to 31,943,930 individuals) with information on sample size lacking in 32 studies. In some studies, antiviral prescriptions were used to define HZ. Individuals with a mild case of HZ may not receive formal treatment but may choose to obtain over-the-counter treatment; it is also expected that treatment uptake differs by age which could have led to differential case ascertainment. These factors could have led to an underestimation of the overall HZ incidence. To the contrary, the overall HZ incidence may have been overestimated if only antivirals were used to confirm HZ diagnosis, attributable to the fact that antivirals are also prescribed for other diseases. Additionally, the study period also differed between publications, with the oldest data reporting for the period 1980–1989 and the most recent data for the year 2017. As HZ incidence tends to increase over the years and differs by age, the study period and the age of the study population should be kept in mind when comparing studies.
Most studies revealed a trend of increasing incidence of HZ with increasing age. A few deviations were seen on this trend in different regions, where the highest age groups (i.e. >80 YOA) reported a drop in incidence. Reasons for this observation could be that older adults have pain and rash due to other conditions and, as such, HZ may be under-diagnosed, or the fact that many subjects in insurance databases represent a healthier cohort of individuals.Citation45 In addition, GP visits for individuals who are institutionalized or in nursing homes are not always captured in traditional databases. In this review, half of the studies included outpatient-only settings, which lends bias toward the representation of subjects with milder disease (i.e. hospitalization not required). It is well documented that the risk of hospitalization increases with age,Citation9 thereby consequently under-representing the incidence in older adults. Other studies can be considered as outliers when looking at the overall trend with age, as they report either lower or higher incidences. The deviations could generally be explained by the type of study design, choice of population, case definition and healthcare-seeking behavior of individuals in a specific setting. Some of these studies used different case definitions, for example; self-reporting using a random digit dialing survey;Citation15 use of clinical confirmation only via GP clinics;Citation70 and self-reporting via surveying individuals door to doorCitation68 and a national database study that captures mild cases with an over-representation of femalesCitation82).
The results of the quality assessment of the included studies revealed that a majority of the 69 studies had a valid case definition for the diagnosis of HZ and the denominator to calculate incidence was generally properly defined. However, most studies did not capture individuals that were representative of the national population. These methodological aspects are seldom reported in the individual studies yet useful in contextualizing and interpreting data from epidemiological studies. In this review, it was often unclear from the individual studies whether these regional studies were representative of the overall national population due to several reasons. First, 36 of the included studies were nationwide studies or claimed to be representative of the national population, while the remaining studies were conducted in one or several regions. Second, in all studies, baseline characteristics of the older adults were generally lacking, as the focus of most of the studies was on the total population instead of older adults. Third, most of the included studies in this review were passive surveillance studies that utilized a retrospective design which could have issues surrounding quality of data or incomplete datasets. Information coming from these databases is often dependent on the quality of the information reported by the physicians, with the possibility of mis-coding or under-diagnosis. Fourth, few of these studies reported using a validated algorithm to detect HZ while many of the studies noted misclassification of HZ as a potential limitation. Many of the active surveillance studies failed to provide the proportion of eligible patients that were finally enrolled, making it difficult to state whether the results were generalizable to the overall population. In some of these studies a HZ case was defined by self-reporting, the accuracy of which was never verified, as self-report of HZ is subject to recall bias. Additionally, misclassification of HZ disease due to other rashes (e.g., herpes simplex) or other rashes classified as HZ based on ICD codes may also have occurred. Thus, misclassification of HZ could inadvertently lead to an underestimation or overestimation of HZ cases in the overall population.
Review limitations
Several limitations of this review are worth noting in the interpretation of the overall findings. The search strategy was not designed to find publications on the epidemiology or disease burden of HZ in general. Therefore, some publications that did not specify incidence in the title or abstract or to which an incidence medical subject heading term was not assigned, may not have been captured in this review. However, by screening all systematic reviews and meta-analyses for potentially relevant publications, we feel that this limitation has largely been overcome. A time limit was applied to the searches to identify publications beginning from 1 January 2002. This was considered appropriate by the authors for the update of the evidence base on incidence as a previous review by Kawai et al. summarizes the incidence data from studies that covers time periods from as early as 1945 until 2012.Citation4 Another limitation of this review is the use of an invalidated checklist to perform a quality check of the included studies. However, the quality check performed in this review is simplistic but sheds light on important gaps in the study methods and results reported in the individual studies. Performing such quality checks could drive improvement and harmonization of reporting standards for these types of publications in the future.
Conclusion
Over the last few decades, the incidence of HZ has increased with increasing age due to the aging of the population worldwide, and this trend is visible independent of geographic location.Citation10 Independently of geographic location, the world’s population is aging: the number of older persons is rising, and older age groups constitute a growing share of the population in nearly every country, with implications foreseen for the healthcare sector among others. The aim of many healthcare systems around the world is to focus on promoting healthy aging to prevent diseases and chronic conditions. In this context, the occurrence of HZ, and its associated complications, is expected to place an additional burden, especially in older patients who already have health problems to cope with in their everyday life. Effective vaccines to prevent HZ are available and are known to have a substantial positive impact on improving the quality of life and on decreasing the burden of complications associated with HZ in older individuals.
Figure 4. Cumulative HZ incidence (number of new HZ cases per 1,000 population) by (A) Time, (B) Age (region).Source: Figure 2A: Studies with at least 2 years of data are depicted*Incidence rate (number of HZ cases per 1,000 person-years) converted to cumulative incidence (number of HZ cases per 1,000 population)HZ, herpes zosterNote: While 42 publications reported cumulative incidence, only 30 of these presented an overall incidence for those ≥50 years and are depicted here
Plain language summary
What is the context?
Herpes Zoster (HZ) occurs due to the reactivation of the varicella zoster virus and is common among adults ≥50 years of age (YOA) as cell-mediated immunity declines due to aging.
Age is a significant risk-factor related to HZ and an increase in life expectancy of the general population may considerably increase the number of HZ cases.
In the context of the world’s aging population, the occurrence of HZ is expected to place an additional burden on patients ≥50 YOA who might already have other health problems to cope with in their everyday life.
What is new?
This systematic review of 69 full-text publications demonstrates that the worldwide incidence of HZ is high among individuals ≥50 YOA with little variation in the different regions of the world.
When comparing the geographical regions, the highest incidences were mostly reported in the Asia-Pacific countries.
Our results suggest that the incidence of HZ is higher in females than in males.
The majority of studies also reported a higher incidence of HZ with increasing age.
Incidence rates did not seem to differ when different case definitions were used.
By ethnicity, incidence rates were the highest among Caucasians and the lowest among Black, Hispanic or other ethnicities.
Studies which reported HZ incidence by year mostly showed an increase in incidence over time.
What is the impact?
This review provides a comprehensive overview of HZ disease burden, that could be considered during the planning and implementation of preventive measures such as vaccination against HZ.
Health care systems around the world are increasingly focusing on promoting healthy aging to prevent diseases and chronic conditions. In this context, the prevention of HZ among the ≥50 YOA population could bring about a reduction in the risks to their health and improve their quality of life.
Contributorship
All authors were involved in the design of the study. EB, DC, DVO, and HV collected or generated the data. All authors analyzed and/or interpreted the data and participated to the development of this manuscript and in its critical review with important intellectual contributions. All authors had full access to the data and gave approval of the final manuscript before submission. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The work described was carried out in accordance with ICMJE recommendations for conduct, reporting, editing and publications publishing of scholarly work in medical journals. The corresponding author had the final responsibility to submit for publication.
Conflicts of interest
DVO, DC and JDD are employed by the GSK group of companies. DC and DVO hold shares in the GSK group of companies. BY reports personal fees from the GSK group of companies during the conduct of the study and personal fees from the GSK group of companies outside the submitted work. HV and EB report grants from the GSK group of companies during the conduct of the study and grants from the GSK group of companies and Sanofi Pasteur outside the submitted work.
Disclosures
Trademark
Shingrix is a trademark owned by or licensed to the GSK group of companies.
Zostavax is a trademark owned by or licensed to Merck Sharp & Dohme Corp.
Supplemental Material
Download PDF (312.9 KB)Acknowledgments
The authors would like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Maxime Bessières coordinated manuscript development and editorial support. Amrita Ostawal (Arete Communication UG) provided writing support for this literature review.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Centers for Disease Control and Prevention. Shingles (herpes zoster) overview. 2018 Accessed 2020 Apr 28. https://www.cdc.gov/shingles/about/index.html
- Harpaz R, Ortega-Sanchez IR, Seward JF. Advisory committee on immunization practices centers for disease c, prevention. prevention of herpes zoster: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2008;57:1–30. quiz CE32-34.
- Drolet M, Brisson M, Levin MJ, Schmader KE, Oxman MN, Johnson RW, Camden S, Mansi JA. A prospective study of the herpes zoster severity of illness. Clin J Pain. 2010;26:656–66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833. doi:10.1136/bmjopen-2014-004833.
- Volpi A. Severe complications of herpes zoster. Herpes. 2007;14:35–39.
- Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20:748–53. doi:10.1111/j.1525-1497.2005.0150.x.
- Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M, Roos LL, De Serres G. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect. 2001;127:305–14. doi:10.1017/S0950268801005921.
- Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017;92:1806–21. doi:10.1016/j.mayocp.2017.10.009.
- Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20.
- Varghese L, Standaert B, Olivieri A, Curran D. The temporal impact of aging on the burden of herpes zoster. BMC Geriatr. 2017;17:30. doi:10.1186/s12877-017-0420-9.
- Higgins JPTG, S. cochrane handbook for systematic reviews of interventions version 5.0.0 (updated february 2008). The Cochrane Collaboration; 2008 Accessed 2020 Sept 11. www.cochrane-handbook.org
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi:10.1371/journal.pmed.1000100.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi:10.1371/journal.pmed.1000097.
- Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. Pharmacoeconomics. 2007;25:3–6. doi:10.2165/00019053-200725010-00002.
- Chaves SS, Santibanez TA, Gargiullo P, Guris D. Chickenpox exposure and herpes zoster disease incidence in older adults in the U.S. Public Health Rep. 2007;122:155–59. doi:10.1177/003335490712200204.
- Chen SY, Suaya JA, Li Q, Galindo CM, Misurski D, Burstin S, Levin MJ. Incidence of herpes zoster in patients with altered immune function. Infection. 2014;42:325–34. doi:10.1007/s15010-013-0550-8.
- Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med. 2013;159:739–45.
- Johnson BH, Palmer L, Gatwood J, Lenhart G, Kawai K, Acosta CJ. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis. 2015;15:502. doi:10.1186/s12879-015-1262-8.
- Kawai K, Yawn BP, Wollan P, Harpaz R. Increasing incidence of herpes zoster over a 60-year period from a population-based study. Clin Infect Dis. 2016;63:221–26. doi:10.1093/cid/ciw296.
- Krishnarajah G, Carroll C, Priest J, Arondekar B, Burstin S, Levin M. Burden of vaccine-preventable disease in adult Medicaid and commercially insured populations: analysis of claims-based databases, 2006-2010. Hum Vaccin Immunother. 2014;10:2460–67. doi:10.4161/hv.29303.
- Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10:e1001420. doi:10.1371/journal.pmed.1001420.
- Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52:332–40. doi:10.1093/cid/ciq077.
- Mullooly JP, Riedlinger K, Chun C, Weinmann S, Houston H. Incidence of herpes zoster, 1997-2002. Epidemiol Infect. 2005;133:245–53. doi:10.1017/S095026880400281X.
- Suaya JA, Chen SY, Li Q, Burstin SJ, Levin MJ. Incidence of herpes zoster and persistent post-zoster pain in adults with or without diabetes in the United States. Open Forum Infect Dis. 2014;1:ofu049. doi:10.1093/ofid/ofu049.
- Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–49. doi:10.4065/82.11.1341.
- Yih WK, Brooks DR, Lett SM, Jumaan AO, Zhang Z, Clements KM, Seward JF. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998-2003. BMC Public Health. 2005;5:68. doi:10.1186/1471-2458-5-68.
- Harpaz R, Leung JW. The epidemiology of herpes zoster in the united states during the era of varicella and herpes zoster vaccines: changing patterns among older adults. Clin Infect Dis. 2019;69(2):341–344. doi:10.1093/cid/ciy953.
- Edgar BL, Galanis E, Kay C, Skowronski D, Naus M, Patrick D. The burden of varicella and zoster in British Columbia 1994-2003: baseline assessment prior to universal vaccination. Can Commun Dis Rep. 2007;33:1–15.
- Marra F, Chong M, Najafzadeh M. Increasing incidence associated with herpes zoster infection in British Columbia, Canada. BMC Infect Dis. 2016;16:589. doi:10.1186/s12879-016-1898-z.
- McDonald BM, Dover DC, Simmonds KA, Bell CA, Svenson LW, Russell ML. The effectiveness of shingles vaccine among Albertans aged 50years or older: a retrospective cohort study. Vaccine. 2017;35:6984–89. doi:10.1016/j.vaccine.2017.10.067.
- Russell ML, Dover DC, Simmonds KA, Svenson LW. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine. 2014;32:6319–24. doi:10.1016/j.vaccine.2013.09.018.
- Russell ML, Schopflocher DP, Svenson L, Virani SN. Secular trends in the epidemiology of shingles in Alberta. Epidemiol Infect. 2007;135:908–13. doi:10.1017/S0950268807007893.
- Tanuseputro P, Zagorski B, Chan KJ, Kwong JC. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011;29:8580–84. doi:10.1016/j.vaccine.2011.09.024.
- Amirthalingam G, Andrews N, Keel P, Mullett D, Correa A, de Lusignan S, Ramsay M. Evaluation of the effect of the herpes zoster vaccination programme 3 years after its introduction in England: a population-based study. Lancet Public Health. 2018;3:e82–e90. doi:10.1016/S2468-2667(17)30234-7.
- Brisson M, Edmunds WJ. Epidemiology of varicella-zoster virus in England and wales. J Med Virol. 2003;70(Suppl 1):S9–14. doi:10.1002/jmv.10313.
- Fleming DM, Bartelds A, Chapman RS, Cross KW. The consistency of shingles and its significance for health monitoring. Eur J Epidemiol. 2004;19:1113–18. doi:10.1007/s10654-004-2219-1.
- Gauthier A, Breuer J, Carrington D, Martin M, Remy V. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect. 2009;137:38–47. doi:10.1017/S0950268808000678.
- Jain A, van Hoek AJ, Walker JL, Forbes HJ, Langan SM, Root A, Smeeth L, Thomas SL. Inequalities in zoster disease burden: a population-based cohort study to identify social determinants using linked data from the U.K. Clinical practice research datalink. Br J Dermatol. 2018;178:1324–30. doi:10.1111/bjd.16399.
- Walker JL, Andrews NJ, Amirthalingam G, Forbes H, Langan SM, Thomas SL. Effectiveness of herpes zoster vaccination in an older United Kingdom population. Vaccine. 2018;36:2371–77. doi:10.1016/j.vaccine.2018.02.021.
- Hillebrand K, Bricout H, Schulze-Rath R, Schink T, Garbe E. Incidence of herpes zoster and its complications in Germany, 2005-2009. J Infect. 2015;70:178–86. doi:10.1016/j.jinf.2014.08.018.
- Schiffner-Rohe J, Jow S, Lilie HM, Koster I, Schubert I. [Herpes zoster in Germany. A retrospective analyse of SHL data]. MMW Fortschr Med. 2010;151:193–97.
- Schmidt-Ott R, Schutter U, Simon J, Nautrup BP, von Krempelhuber A, Gopala K, Anastassopoulou A, Guignard A, Curran D, Matthews S, et al. Incidence and costs of herpes zoster and postherpetic neuralgia in German adults aged >/=50 years: A prospective study. J Infect. 2018;76:475–82. doi:10.1016/j.jinf.2018.02.001.
- Ultsch B, Koster I, Reinhold T, Siedler A, Krause G, Icks A, Schubert I, Wichmann O. Epidemiology and cost of herpes zoster and postherpetic neuralgia in Germany. Eur J Health Econ. 2013;14:1015–26. doi:10.1007/s10198-012-0452-1.
- Ultsch B, Siedler A, Rieck T, Reinhold T, Krause G, Wichmann O. Herpes zoster in Germany: quantifying the burden of disease. BMC Infect Dis. 2011;11:173. doi:10.1186/1471-2334-11-173.
- Imafuku S, Matsuki T, Mizukami A, Goto Y, de Souza S, Jegou C, Bianco V, Rosillon D, Ito C, Curran D, et al. Burden of herpes zoster in the Japanese population with immunocompromised/chronic disease conditions: results from a cohort study claims database from 2005-2014. Dermatol Ther (Heidelb). 2019;9(1):117–133. doi:10.1007/s13555-018-0268-8.
- Shiraki K, Toyama N, Daikoku T, Yajima M. Herpes zoster and recurrent herpes zoster. Open Forum Infect Dis. 2017;4:ofx007. doi:10.1093/ofid/ofx007.
- Takao Y, Miyazaki Y, Okeda M, Onishi F, Yano S, Gomi Y, Ishikawa T, Okuno Y, Mori Y, Asada H, et al. Incidences of herpes zoster and postherpetic neuralgia in Japanese adults aged 50 years and older from a community-based prospective cohort study: the SHEZ study. J Epidemiol. 2015;25:617–25.
- Toyama N, Shiraki K. Epidemiology of herpes zoster and its relationship to varicella in Japan: A 10-year survey of 48,388 herpes zoster cases in Miyazaki prefecture. J Med Virol. 2009;81:2053–58.
- Toyama N, Shiraki K. Universal varicella vaccination increased the incidence of herpes zoster in the child-rearing generation as its short-term effect. J Dermatol Sci. 2018;92:89–96. doi:10.1016/j.jdermsci.2018.07.003.
- Opstelten W, Mauritz JW, de Wit NJ, van Wijck AJ, Stalman WA, van Essen GA. Herpes zoster and postherpetic neuralgia: incidence and risk indicators using a general practice research database. Fam Pract. 2002;19:471–75. doi:10.1093/fampra/19.5.471.
- Opstelten W, van Essen GA, Moons KG, van Wijck AJ, Schellevis FG, Kalkman CJ, Verheij TJ. Do herpes zoster patients receive antivirals? A dutch national survey in general practice. Fam Pract. 2005;22:523–28. doi:10.1093/fampra/cmi055.
- Opstelten W, Van Essen GA, Schellevis F, Verheij TJ, Moons KG. Gender as an independent risk factor for herpes zoster: a population-based prospective study. Ann Epidemiol. 2006;16:692–95. doi:10.1016/j.annepidem.2005.12.002.
- Pierik JG, Gumbs PD, Fortanier SA, Van Steenwijk PC, Postma MJ. Epidemiological characteristics and societal burden of varicella zoster virus in the Netherlands. BMC Infect Dis. 2012;12:110. doi:10.1186/1471-2334-12-110.
- Cebrian-Cuenca AM, Diez-Domingo J, Rodriguez MS, Puig-Barbera J, Navarro-Perez J. Epidemiology of herpes zoster infection among patients treated in primary care centres in the Valencian community (Spain). BMC Fam Pract. 2010;11:33.
- Esteban-Vasallo MD, Gil-Prieto R, Dominguez-Berjon MF, Astray-Mochales J, Gil de Miguel A. Temporal trends in incidence rates of herpes zoster among patients treated in primary care centers in Madrid (Spain), 2005-2012. J Infect. 2014;68:378–86. doi:10.1016/j.jinf.2013.09.035.
- Garcia Cenoz M, Castilla J, Montes Y, Moran J, Salaberri A, Elia F, Floristan Y, Rodrigo I, Irisarri F, Arriazu M, et al. [Varicella and herpes zoster incidence prior to the introduction of systematic child vaccination in Navarre, 2005-2006]. An Sist Sanit Navar. 2008;31:71–80.
- Morant-Talamante N, Diez-Domingo J, Martinez-Ubeda S, Puig-Barbera J, Aleman-Sanchez S, Perez-Breva L. Herpes zoster surveillance using electronic databases in the Valencian Community (Spain). BMC Infect Dis. 2013;13:463. doi:10.1186/1471-2334-13-463.
- Munoz-Quiles C, Lopez-Lacort M, Ampudia-Blasco FJ, Diez-Domingo J. Risk and impact of herpes zoster on patients with diabetes: a population-based study, 2009-2014. Hum Vaccin Immunother. 2017;13:2606–11. doi:10.1080/21645515.2017.1368600.
- Chao DY, Chien YZ, Yeh YP, Hsu PS, Lian IB. The incidence of varicella and herpes zoster in Taiwan during a period of increasing varicella vaccine coverage, 2000-2008. Epidemiol Infect. 2012;140:1131–40. doi:10.1017/S0950268811001786.
- Jih JS, Chen YJ, Lin MW, Chen YC, Chen TJ, Huang YL, Chen CC, Lee DD, Chang YT, Wang WJ, et al. Epidemiological features and costs of herpes zoster in Taiwan: a national study 2000 to 2006. Acta Derm Venereol. 2009;89:612–16. doi:10.2340/00015555-0729.
- Lin YH, Huang LM, Chang IS, Tsai FY, Lu CY, Shao PL, Chang LY. Disease burden and epidemiology of herpes zoster in pre-vaccine Taiwan. Vaccine. 2010;28:1217–20. doi:10.1016/j.vaccine.2009.11.029.
- Lu WH, Lin CW, Wang CY, Chen LK, Hsiao FY. Epidemiology and long-term disease burden of herpes zoster and postherpetic neuralgia in Taiwan: a population-based, propensity score-matched cohort study. BMC Public Health. 2018;18:369. doi:10.1186/s12889-018-5247-6.
- Liu B, Heywood AE, Reekie J, Banks E, Kaldor JM, McIntyre P, Newall AT, Macintyre CR. Risk factors for herpes zoster in a large cohort of unvaccinated older adults: a prospective cohort study. Epidemiol Infect. 2015;143:2871–81. doi:10.1017/S0950268814003653.
- MacIntyre R, Stein A, Harrison C, Britt H, Mahimbo A, Cunningham A. Increasing trends of herpes zoster in Australia. PLoS One. 2015;10:e0125025. doi:10.1371/journal.pone.0125025.
- Stein AN, Britt H, Harrison C, Conway EL, Cunningham A, Macintyre CR. Herpes zoster burden of illness and health care resource utilisation in the Australian population aged 50 years and older. Vaccine. 2009;27:520–29. doi:10.1016/j.vaccine.2008.11.012.
- Li Y, An Z, Yin D, Liu Y, Huang Z, Xu J, Ma Y, Tu Q, Li Q, Wang H. Disease burden due to herpes zoster among population aged >/=50 years old in China: a community based retrospective survey. PLoS One. 2016;11:e0152660. doi:10.1371/journal.pone.0152660.
- Lu L, Suo L, Li J, Pang X. A retrospective survey on herpes zoster disease burden and characteristics in Beijing, China. Hum Vaccin Immunother. 2018;1–4. doi:10.1080/21645515.2018.1489193.
- Zhu Q, Zheng H, Qu H, Deng H, Zhang J, Ma W, Lin Y, Xie X, Qiu Q, Huang Z. Epidemiology of herpes zoster among adults aged 50 and above in Guangdong, China. Hum Vaccin Immunother. 2015;11:2113–18. doi:10.1080/21645515.2015.1016672.
- Alicino C, Trucchi C, Paganino C, Barberis I, Boccalini S, Martinelli D, Pellizzari B, Bechini A, Orsi A, Bonanni P, et al. Incidence of herpes zoster and post-herpetic neuralgia in Italy: results from a 3-years population-based study. Hum Vaccin Immunother. 2017;13:399–404. doi:10.1080/21645515.2017.1264834.
- Di Legami V, Gianino MM, Ciofi Degli Atti M, Massari M, Migliardi A, Tomba GS, Zotti C. Epidemiology and costs of herpes zoster: background data to estimate the impact of vaccination. Vaccine. 2007;25:7598–604. doi:10.1016/j.vaccine.2007.07.049.
- Gialloreti LE, Merito M, Pezzotti P, Naldi L, Gatti A, Beillat M, Serradell L, Di Marzo R, Volpi A. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis. 2010;10:230. doi:10.1186/1471-2334-10-230.
- Gonzalez Chiappe S, Sarazin M, Turbelin C, Lasserre A, Pelat C, Bonmarin I, Chosidow O, Blanchon T, Hanslik T. Herpes zoster: burden of disease in France. Vaccine. 2010;28:7933–38. doi:10.1016/j.vaccine.2010.09.074.
- Mick G, Gallais JL, Simon F, Pinchinat S, Bloch K, Beillat M, Serradell L, Derrough T. [Burden of herpes zoster and postherpetic neuralgia: incidence, proportion, and associated costs in the French population aged 50 or over]. Rev Epidemiol Sante Publique. 2010;58:393–401. doi:10.1016/j.respe.2010.06.166.
- Reid JS, Ah Wong B. Herpes zoster (shingles) at a large New Zealand general practice: incidence over 5 years. N Z Med J. 2014;127:56–60.
- Turner NM, MacRae J, Nowlan ML, McBain L, Stubbe MH, Dowell A. Quantifying the incidence and burden of herpes zoster in New Zealand general practice: a retrospective cohort study using a natural language processing software inference algorithm. BMJ Open. 2018;8:e021241. doi:10.1136/bmjopen-2017-021241.
- Nilsson J, Cassel T, Lindquist L. Burden of herpes zoster and post-herpetic neuralgia in Sweden. BMC Infect Dis. 2015;15:215. doi:10.1186/s12879-015-0951-7.
- Sundstrom K, Weibull CE, Soderberg-Lofdal K, Bergstrom T, Sparen P, Arnheim-Dahlstrom L. Incidence of herpes zoster and associated events including stroke–a population-based cohort study. BMC Infect Dis. 2015;15:488. doi:10.1186/s12879-015-1170-y.
- Schmidt SAJ, Vestergaard M, Baggesen LM, Pedersen L, Schonheyder HC, Sorensen HT. Prevaccination epidemiology of herpes zoster in Denmark: quantification of occurrence and risk factors. Vaccine. 2017;35:5589–96.
- Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of Herpes Zoster and its complications. J Infect. 2013;67:463–69. doi:10.1016/j.jinf.2013.06.016.
- Rimseliene G, Vainio K, Gibory M, Salamanca BV, Flem E. Varicella-zoster virus susceptibility and primary healthcare consultations in Norway. BMC Infect Dis. 2016;16:254. doi:10.1186/s12879-016-1581-4.
- Albrecht P, Patrzalek M, Gorynski P. The burden of Herpes Zoster and its complications in Poland in according to the age. Przegl Epidemiol. 2015;69:841–693.
- Kim YJ, Lee CN, Lim CY, Jeon WS, Park YM. Population-based study of the epidemiology of herpes zoster in Korea. J Korean Med Sci. 2014;29:1706–10. doi:10.3346/jkms.2014.29.12.1706.