ABSTRACT
Human papillomavirus (HPV) vaccination can prevent six types of HPV-related cancers, and approximately, 54.2% of adolescents are up-to-date with the HPV vaccine in the United States. While moderate success has been achieved with provider- and parent-focused interventions, HPV vaccination in the U.S. lags well behind desired goals. In order to maximize HPV vaccination and prevention of HPV-related cancers, it may be prudent to consider state policy approaches, such as school-entry requirements as part of the patchwork of provider, parent, and structural interventions. In this paper, we reviewed the history of efforts to implement school-entry requirements for HPV vaccine, the challenges and benefits associated with implementing these requirements, and the evidence for the effectiveness of school-entry requirements. In addition, we presented new data from Rhode Island’s Immunization Information System (IIS) showing how their school-entry requirement, implemented in 2015, has impacted HPV vaccination rates. These registry data indicate that HPV vaccination rates improved significantly after the 2014–2015 school year and policy implementation, and add to the ongoing evidence supporting the value of school-entry requirements for HPV vaccination. School-entry requirements should be considered alongside other initiatives and policies for promoting HPV vaccine uptake. Taking a comprehensive systems approach to HPV vaccination is needed.
KEYWORDS:
In the U.S., human papillomavirus (HPV) is attributed to approximately 34,800 cancer cases per year for six types of cancers (anal, cervical, oropharyngeal, penile, vaginal, and vulvar).Citation1 It is well established that HPV vaccination prevents pre-cancerous cervical lesions and genital warts.Citation2,Citation3 Further, a recent study out of Sweden shows that vaccination significantly reduces the risk of invasive cervical cancer.Citation4 However, despite the HPV vaccine being licensed and available for over 14 y, HPV vaccination coverage in the U.S. has failed to come close to the Healthy People 2020 goal of 80% series completion.Citation5 The most recent National Immunization Survey-Teen data from 2019 show that only 54.2% of adolescents ages 13–17 y had completed the vaccine series,Citation6 indicating that substantial progress is still needed.
Most efforts at improving HPV vaccine coverage have focused on interventions designed to help healthcare providers make more effective recommendations for the vaccine, with other interventions targeting parental knowledge and attitudes or practice-level factors (e.g., provider prompts). Many of these interventions have proven to be moderately successful; nonetheless, as noted above, HPV vaccination in the U.S. lags well behind desired goals. In order to maximize HPV vaccination and prevention of HPV cancers, it may be prudent to consider state policy approaches, such as school-entry requirements, in addition to provider, parent, and structural interventions. In this paper, we review the history of efforts to implement school-entry requirements for HPV vaccine, the challenges and benefits associated with implementing these requirements, and the evidence for the effectiveness of school-entry requirements. In addition, we present new data from Rhode Island’s Immunization Information System (IIS) showing how their school-entry requirement, implemented in 2015, has impacted HPV vaccination rates.
HPV vaccine school-entry requirements in the U.S
School-entry requirements have had a history of success for other adolescent vaccines, including Tdap and meningococcal ACWY vaccines.Citation7–9 Unfortunately, the HPV vaccine was excluded from school-entry requirements from the beginning, in part because it was considered a “different” type of vaccine largely due to the sexual mode of transmission for this virus, which increased stigma associated with this vaccine.Citation10,Citation11 As a result, the political capital needed to implement a school-entry requirement for HPV vaccination was considerable.
Shortly after HPV vaccine was licensed in 2006, several state legislatures introduced legislation to require HPV vaccine for middle school entry (in alignment with Tdap and meningococcal ACWY vaccine requirements), but most of these efforts were unsuccessful. In 2007, the Texas governor, Rick Perry, instituted an executive order for an HPV vaccine school-entry requirement for females. This action was steeped in controversy, as it circumvented the legislature and concerns were raised about lobbying activities initiated by the pharmaceutical company that produced the vaccine (Merck).Citation12 As a result, the executive order was quickly overturned by Texas House Bill 1098.Citation13 Since that time, in response to ongoing criticism, Merck has suspended lobbying efforts with state legislators.Citation11
Shortly after the Texas controversy, Virginia (in 2008) and Washington, DC (in 2009) enacted imperfect 6th grade school-entry requirements for females. Virginia’s policy has remained unchanged over the years, while Washington, DC expanded their policy in 2014 to include males. In both jurisdictions, the procedures for opting out of HPV vaccine are simple and relaxed.Citation14 In Washington, DC, for HPV vaccination only, parents are provided a link to an opt-out form on the government’s website. In Virginia, parents may easily opt out without providing documentation or cause.Citation15
These early policy failures and resulting backlash created barriers for effective and meaningful policy development, leaving HPV vaccine school-entry requirement efforts in hibernation for nearly a decade. In fact, the 2014 report from the President’s Cancer PanelCitation16 concluded that there were too many barriers to implementation for school-entry requirements to be considered a viable option. However, 1 y later, in 2015, Rhode Island implemented gender-neutral HPV vaccine requirements for entry into 7th grade, through public health regulatory authority, not legislation.Citation17 Puerto Rico followed suit in 2017,Citation18 also through a regulatory process. Hawaii is planning to institute a school-entry requirement in 2021;Citation19,Citation20 Hawaii initially planned a 2020 implementation but delayed this action due to the COVID-19 pandemic.
Challenges to, and advantages of, implementation of school-entry requirements
Significant challenges to enact school-entry requirements for HPV vaccine persist, and in many states, effective policies to enact school-entry requirements through legislative or public health regulatory authority are likely not viable for the foreseeable future. The significant push-back from anti-vaccine activists and associated groups has created a political challenge. These groups continue to rally against new (or more rigorous) implementation of legislative school-entry requirements, as was seen in response to California Senate Bill 277, which discontinued religious and personal belief exemptions for vaccines required for school entry.Citation21 Similarly, the activity of HPV vaccine opponents increased substantially leading up to the implementation of Rhode Island’s HPV vaccine requirement.Citation17 In addition, for states with relatively low HPV vaccine coverage, moving to a school-entry requirement may put enormous stress on the schools, health systems, and the HPV vaccine supply. There are lessons to be learned from weak unsuccessful policies (e.g., Virginia) as well as successful policies (e.g., Rhode Island) regarding potential pitfalls and strategies to overcome obstacles.Citation22,Citation23
As evidenced by Rhode Island, the benefits of effective gender-neutral school-entry requirements for HPV vaccination are clear. An effective policy can ensure high vaccine coverage, which protects children’s health, protects society more widely through herd immunity, and helps to ensure health equity and reduction in health disparities associated with HPV-related diseases.Citation24–26 Rhode Island’s universal free vaccine policy also removes financial barriers that might impede the implementation of school-entry requirements. In addition, research suggests that providers would be more comfortable making a strong, presumptive recommendation for HPV vaccination if it is required for school entry.Citation27 This evidence supports a synergistic effect whereby school-entry requirements lead to higher-quality provider recommendations, thereby increasing HPV vaccine acceptance more than either approach alone.
Research evidence on the effects of school-entry requirements
Several studies have documented the ineffectiveness of the Virginia and Washington, DC HPV vaccine school-entry policies.Citation23,Citation28,Citation29 As noted above, the school-entry requirements in these two jurisdictions were implemented in such a way as to almost ensure their failures. The research showing no effect on HPV vaccination rates essentially demonstrates that implementing flawed public health policies leads to a lack of positive public health impact. In 2014, the existing school requirement in the District of Columbia was expanded to include both boys and girls. Also, in 2014, the District of Columbia implemented a communication campaign about the importance of HPV vaccination and about the expanded school requirement. HPV vaccination for three doses increased by 28.6% in girls and 10.9% in boys that year.Citation30
The implementation in 2015 of Rhode Island’s more stringent school-entry requirements for HPV vaccination provided an opportunity for reevaluation of the impact of this kind of policy. Studies using the difference-in-differences approach, which uses a quasi-experimental design to assess the policy changes as a natural experiment, highlighted that the change in HPV vaccination among boys in Rhode Island was significantly greater than in states without a rigorous school-entry policy.Citation31,Citation32 The same pattern of results was found for both parent-reported data and provider-confirmed data. Girls in Rhode Island already had the highest rates of uptake in the nation, so it was not clear that the school-entry requirement had a unique impact on their vaccination rates. A more recent assessment of all HPV vaccine school-entry policies also supported the improved effect of these requirements on HPV vaccination uptake.Citation33
Rhode Island IIS data analysis
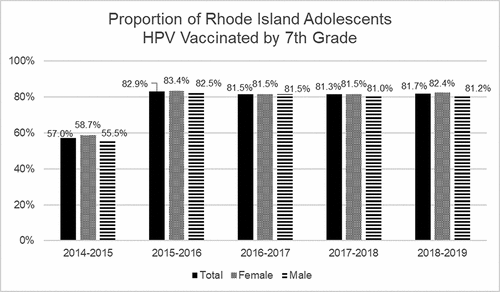
The studies cited above show that Rhode Island’s school-entry requirement policy improved overall vaccine coverage for boys, but not for girls. However, an important additional question is whether HPV vaccine school-entry requirements led to higher coverage at a younger age, such as in 7th grade when the vaccine is required. This effect would be important, as HPV vaccine induces a stronger immune response in younger, compared to older adolescents, and vaccinating younger adolescents helps to ensure that they are protected before exposure to the virus. To answer this question, we examined data from Rhode Island’s IIS database, which is included in KIDSNET. The IIS, maintained by the Rhode Island Department of Health, includes public school enrollment data starting from the 2015–2016 school year. Since the Rhode Island school-entry policy was enacted in 2015 – the same year the registry data were collected – we used vaccination data from 8th graders in the 2015–2016 school year to represent the cohort of 7th graders in 2014–2015. Subsequently, we examined 7th grader HPV vaccination rates for 2015–2016 until the 2018–2019 school year. Each public school student had data on the date of HPV vaccine administration and sex. In 2014–2015, 57% of students had received the HPV vaccine compared to approximately 82% of students in later years after the policy enactment (). Pairwise comparisons by school year were statistically significant for frequency of HPV vaccination comparing the 2014–2015 (pre-policy) school year to all other school years (post-policy), for the full sample, males, and females, respectively (p-values <0.0001). No statistically significant differences were observed between school years after the policy implementation. Thus, these registry data indicate that HPV vaccination rates improved significantly after the 2014–2015 school year and policy implementation.
Conclusion
The evidence is overwhelming that HPV vaccine school-entry requirements, when properly implemented, have a positive impact on HPV vaccine coverage. Building on Rhode Island’s success, we now see other jurisdictions making the transition. In 2018, Puerto Rico required HPV vaccination in schools and demonstrated a 90% success rate, even with turmoil from Hurricane Maria.Citation18 Starting in July 2021, Hawaii will also require the HPV vaccine for school entry.Citation19,Citation20 Continued assessment of these policies is needed to contribute to the existing evidence of school-entry requirements for HPV vaccination.
Cancer is a public health priority with substantial investment in prevention and treatment. Scientific progress has discovered a way to prevent a subset of cancers that cause significant morbidity and mortality. Unfortunately, the connection between HPV and sexual activity continues to plague progress with this vaccine. Consequently, the HPV vaccine is separated from other adolescent vaccines in policies for school entry.
School-entry requirements should be considered alongside other initiatives and policies for promoting HPV vaccine uptake. In fact, Roberts et al. (2018) found that a combination of policies, such as Medicaid expansion, policies for pharmacists to deliver HPV vaccines, school-entry requirements, and sexual education mandates are associated with higher HPV vaccine uptake. In addition to campaigns and interventions to improve provider recommendations for the HPV vaccine, statewide policies can lead to downstream impact on HPV vaccination.Citation34 A recent analysis of Medicaid expansion and HPV vaccine uptake supports improvements in vaccination in states that expanded Medicaid.Citation35 Taking a comprehensive systems approach to HPV vaccination is needed. There is not one avenue, but a patchwork of initiatives and policy, including school-entry requirements, that is needed to be successful in reaching the American Cancer Society’s goal of an HPV vaccination rate of 80% among 13 y olds in 2026–20 y after the initial introduction of the vaccine.Citation36
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The KIDSNET registry data were provided by the Rhode Island Department of Health’s Center for Health Data and Analysis. This analysis was approved by the North Texas Regional Institutional Review Board.
References
- Senkomago V, Henley SJ, Thomas CC, Mix JM, Markowitz LE, Saraiya M. Human papillomavirus-attributable cancers - United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2019;68(33):724–28. doi:10.15585/mmwr.mm6833a3.
- Smith LM, Strumpf EC, Kaufman JS, Lofters A, Schwandt M, Lévesque LE. The early benefits of human papillomavirus vaccination on cervical dysplasia and anogenital warts. Pediatrics. 2015;135(5):e1131–1140. doi:10.1542/peds.2014-2961.
- Garland SM, Kjaer SK, Muñoz N, Block SL, Brown DR, DiNubile MJ, Lindsay BR, Kuter BJ, Perez G, Dominiak-Felden G, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis. 2016;63(4):519–27. doi:10.1093/cid/ciw354.
- Lei J, Ploner A, Elfström KM, Wang J, Roth A, Fang F, Sundström K, Dillner J, Sparén P. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383(14):1340–48. doi:10.1056/NEJMoa1917338.
- US Department of Health and Human Services. Immunization and infectious disease. 2014 [accessed 2019 Oct 22]. https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases.
- Elam-Evans LD, Yankey D, Singleton JA, Sterrett N, Markowitz LE, Williams CL, Fredua B, McNamara L, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(33):1109–16. doi:10.15585/mmwr.mm6933a1.
- Moss JL, Reiter PL, Truong YK, Rimer BK, Brewer NT. School entry requirements and coverage of nontargeted adolescent vaccines. Pediatrics. 2016;138(6):e20161414–e20161414. doi:10.1542/peds.2016-1414.
- Kharbanda EO, Stockwell MS, Colgrove J, Natarajan K, Rickert VI. Changes in Tdap and MCV4 vaccine coverage following enactment of a statewide requirement of Tdap vaccination for entry into sixth grade. Am J Public Health. 2010;100(9):1635–40. doi:10.2105/AJPH.2009.179341.
- Bugenske E, Stokley S, Kennedy A, Dorell C. Middle school vaccination requirements and adolescent vaccination coverage. Pediatrics. 2012;129:1056–63.
- Zimet GD, Rosberger Z, Fisher WA, Perez S, Stupiansky NW. Beliefs, behaviors and HPV vaccine: correcting the myths and the misinformation. Prev Med. 2013;57(5):414–18. doi:10.1016/j.ypmed.2013.05.013.
- Haber G, Malow RM, Zimet GD. The HPV vaccine mandate controversy. J Pediatr Adolesc Gynecol. 2007;20(6):325–31. doi:10.1016/j.jpag.2007.03.101.
- Tanne JH. Texas governor is criticised for decision to vaccinate all girls against HPV. BMJ. 2007;334:332–33.
- H.B. 1098. 80th Texas Legislator; 2007.
- Hoss A, Meyerson BE, Zimet GD. State statutes and regulations related to human papillomavirus vaccination. Hum Vaccin Immunother. 2019;15(7–8):1519–26. doi:10.1080/21645515.2019.1627817.
- Virginia Department of Health. School Requirements. 2020; [accessed2020 Oct 6]. https://www.vdh.virginia.gov/immunization/requirements/.
- President’s Cancer Panel. Accelerating HPV vaccine uptake: urgency for action to prevent cancer. Bethesda (MD): National Cancer Institute; 2014.
- Washburn T, Devi Wold A, Raymond P, Duggan-Ball S, Marceau K, Beardsworth A. Current initiatives to protect Rhode Island adolescents through increasing HPV vaccination. Hum Vaccin Immunother. 2016;12(6):1633–38. doi:10.1080/21645515.2016.1161460.
- PR HPV Success Story. 2020; [accessed 2020 Oct 6] https://wicancer.org/wp-content/uploads/2020/09/Puerto-Rico-HPV-Success-Story-FULL-DOCUMENT-FINAL.pdf.
- State of Hawaii Department of Health. 2020–2021 school health requirements. 2020; [accessed 2020 October 6]. https://health.hawaii.gov/docd/vaccines-immunizations/school-health-requirements/sy-20-21/.
- Hawaii Department of Health. Amendment and compilation of chapter 11-157 Hawaii administrative rules; 2019.
- DeDominicis K, Buttenheim AM, Howa AC, Delamater PL, Salmon D, Omer SB, Klein NP. Shouting at each other into the void: a linguistic network analysis of vaccine hesitance and support in online discourse regarding California Law SB277. Soc Sci Med. 2020;266:113216. doi:10.1016/j.socscimed.2020.113216.
- Barraza L, Weidenaar K, Campos-Outcalt D, Yang YT. Human papillomavirus and mandatory immunization laws: what can we learn from early mandates? Public Health Rep. 2016;131(5):728–31. doi:10.1177/0033354916663184.
- Perkins RB, Lin M, Wallington SF, Hanchate AD. Impact of school-entry and education mandates by states on HPV vaccination coverage: analysis of the 2009-2013 National Immunization Survey-Teen. Hum Vaccin Immunother. 2016;12(6):1615–22. doi:10.1080/21645515.2016.1150394.
- Bayefsky MJ, Gostin LO. Requiring Human Papilloma Virus Vaccination for School Entry. JAMA Pediatr. 2019;173(2):123–24. doi:10.1001/jamapediatrics.2018.4283.
- North AL, Niccolai LM. Human papillomavirus vaccination requirements in US Schools: recommendations for moving forward. Am J Public Health. 2016;106(10):1765–70. doi:10.2105/AJPH.2016.303286.
- Daley E, Thompson E, Zimet G. Human papillomavirus vaccination and school entry requirements: politically challenging, but not impossible. JAMA Pediatr. 2019;173(1):6–7. doi:10.1001/jamapediatrics.2018.3327.
- Niccolai LM, North AL, Footman A, Hansen CE. Lack of school requirements and clinician recommendations for human papillomavirus vaccination. J Public Health Res. 2018;7:1324.
- Pierre-Victor D, Page TF, Trepka MJ, Stephens DP, Li T, Madhivanan P. Impact of Virginia’s school-entry vaccine mandate on human papillomavirus vaccination among 13-17-year-old females. J Women Health. 2017;26(3):266–75. doi:10.1089/jwh.2016.5869.
- Cuff RD, Buchanan T, Pelkofski E, Korte J, Modesitt SP, Pierce JY. Rates of human papillomavirus vaccine uptake amongst girls five years after introduction of statewide mandate in Virginia. Am J Obstet Gynecol. 2016;214:752.e751-756.
- Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Singleton JA, Curtis CR, MacNeil J, Markowitz LE, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years–United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784–92. doi:10.15585/mmwr.mm6429a3.
- Thompson EL, Livingston MD 3rd, Daley EM, Zimet GD. Human papillomavirus vaccine initiation for adolescents following Rhode Island’s school-entry requirement, 2010-2016. Am J Public Health. 2018;108(10):1421–23. doi:10.2105/AJPH.2018.304552.
- Thompson EL, Livingston III MD, Daley EM, Saslow D, Zimet GD. Rhode Island human papillomavirus vaccine school entry requirement using provider-verified report. Am J Prev Med. 2020;59(2):274–77. doi:10.1016/j.amepre.2020.02.022.
- Ko JS, Goldbeck CS, Baughan EB, Klausner JD. Association between human papillomavirus vaccination school-entry requirements and vaccination initiation. JAMA Pediatr. 2020;174(9):861. doi:10.1001/jamapediatrics.2020.1852.
- Roberts MC, Murphy T, Moss JL, Wheldon CW, Psek W. A qualitative comparative analysis of combined state health policies related to human papillomavirus vaccine uptake in the United States. Am J Public Health. 2018;108(4):493–99. doi:10.2105/AJPH.2017.304263.
- Hoff BM, Livingston III MD, Thompson EL. The association between state Medicaid expansion and human papillomavirus vaccination. Vaccine. 2020;38(38):5963–65. doi:10.1016/j.vaccine.2020.07.024.
- American Cancer Society. Our HPV vaccination initiatives. 2020; [accessed 2020 Sept 8]. https://www.cancer.org/health-care-professionals/hpv-vaccination-information-for-health-professionals/our-hpv-vaccination-initatives.html.