ABSTRACT
Objectives: We aimed to elucidate public values regarding the use of genomics to improve vaccine development and use (vaccinomics).
Methods: Adults ≥18 years-old were recruited through social media and community organizations, and randomly assigned to one of four nested discussion groups in Boulder, CO and Baltimore, MD. Participants rated their confidence in vaccine safety and effectiveness prior to and after discussing vaccinomics. Before departing, they prioritized funding for vaccinomics versus federal priorities (vaccine safety and efficacy, new vaccines, and free vaccines) and chronic diseases (cancer, heart disease, and diabetes). Grounded Theory-influenced methods were used to identify themes.
Results: Participants broadly supported vaccinomics. Emergent themes: concerns about reduced privacy/confidentiality, increased genetically based stigma/discrimination, and reduced agency to make vaccine-related decisions through genetically based prioritization. Participants supported vaccinomics’ potential for increased personalization. Some participants favored prioritizing others over themselves during a vaccine shortage, while others did not. Some participants worried health insurance companies would discriminate against them based on information discovered through vaccinomics. Participants feared inequitable implementation of vaccinomics would contribute to discrimination and marginalization of vulnerable populations. Discussing vaccinomics did not impact perceptions of vaccine safety and effectiveness. Federal funding for vaccinomics was broadly supported.
Conclusion: Participants supported vaccinomics’ potential for increased personalization, noting policy safeguards to facilitate equitable implementation and protect privacy were needed. Despite some concerns, participants hoped vaccinomics would improve vaccine safety and effectiveness. Policies regarding vaccinomics’ implementation must address public concerns about the privacy and confidentiality of genetic information and potential inequities in access to vaccinomics’ benefits.
Introduction
The emerging field of vaccinomics applies immunology and genomics to the study of vaccine response and development.Citation1 Adversomics, a subfield, applies vaccinomics to the prevention of adverse events following immunization (AEFI).Citation1 Several genomic differences, including biological sex, ancestry, and specific genetic loci, are associated with immune response and vaccine adverse reactions.Citation2–12 Prior to vaccinomics’ implementation in 10–15 years, the ethical, legal, and social implications (ELSI) should be anticipated. Public values and preferences should inform vaccine research and development, licensure, recommendations for use, injury compensation, and communications. Incorporating public values into vaccinomics enhances the likelihood of public participation, informed decision-making, and vaccine acceptance.
Additionally, vaccinomics may positively or negatively impact vaccine hesitancy, which is the delay or refusal of vaccines, despite the availability of vaccination services.Citation13 In 2019, the World Health Organization designated vaccine hesitancy one of the top 10 threats to global health.Citation14 Vaccine safety concerns are common,Citation15–22 despite the rarity of serious reactions like Guillain-Barre Syndrome (GBS).Citation23,Citation24 Individuals with vaccine safety concerns often believe they or their children may be at increased risk of diseases with genetic risk-factors, including allergic reactions, asthma, autoimmune diseases, multiple sclerosis,Citation25 and autism.Citation26 Despite a lack of epidemiological evidence, some believe children’s immune systems could be overloaded by receiving too many vaccines simultaneously.Citation26 Children vaccinated with delay or unvaccinated due to their parents’ concerns often cluster geographically and socially, contributing to vaccine preventable disease outbreaks.Citation27–29 Vaccinomics may alleviate vaccine hesitancy, through personalization of vaccine schedules and improved safety.Citation1 Alternatively, vaccinomics may increase vaccine hesitancy and refusal if individuals who learn they have twice the risk of an adverse outcome compared to others – two in one million versus one in one million – refuse vaccination, even when the absolute risk remains small.Citation24 Vaccinomics may alarm vaccine-hesitant individuals with privacy concerns regarding genomics, or the relative newness of the field.
In 2017, we held a meeting with academic and federal vaccinologists, including representatives from the National Institutes of Health, Food and Drug Administration, Centers for Disease Control and Prevention, and Health Resources and Services Administration (National Vaccine Injury Compensation Program). Our objective was to discuss potential vaccinomics-related policy issues that may emerge and where public input would be useful. Public input on the following questions was expected to be useful:
Who should get access to (possible) personalized vaccines (and at what cost) for public health benefit?
Should prioritization of vaccinomics research be placed on rare, serious, adverse reactions or common, mild reactions? Should subpopulation differences in vaccine response and contagiousness (“more contagious” and “more susceptible” populations) be considered?
Would personalization of vaccines increase confidence in vaccine safety, effectiveness, scheduling, and dosing? Why?
Should vaccinomics be funded over existing federal funding priorities?
These questions were a starting point for discussions with the public. This study aimed to elucidate public values to inform vaccinomics-related policies.
Methods
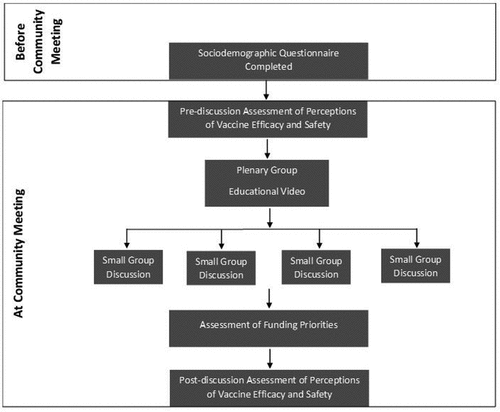
Community meetings comprised of initial plenary sessions followed by facilitated small discussion groups.
Community meeting recruitment
Three community meetings with nested discussion groups (N = 8) were held in Boulder, CO (n = 2 meetings in March 2018, n = 2 nested discussions each), and Baltimore, MD (n = 1 meeting in April 2018, n = 4 nested discussions). Boulder is a mostly Caucasian (87.9% versus 1.1% African American) urban community with a high prevalence of under-immunized children (3.3% of kindergartners had ≥1 vaccine exemption in 2018–2019).Citation30 Baltimore has a large African American (62.8% versus 31.8% Caucasian) population and is urban (Maryland exemption data are not available).Citation31 These cities were chosen to enroll diverse participants.Citation31
Respondents were purposively recruited using a multipronged strategy so that each city’s sociodemographic profile would be approximately represented according to Census data for age, race/ethnicity, household income, and education. Parents of young children were over-enrolled compared to nonparents, since they make frequent vaccine-related decisions.
Electronic and paper flyers were distributed to invite participants to a “conversation on vaccines and genetics, and what might be possible in an emerging area of science called vaccinomics.” To enroll parents along the vaccine-hesitant continuum and individuals representing the gender, age, and race/ethnicity distributions of each city, hard copy flyers were distributed at public libraries and eateries in both cities. In Boulder, project staff waited outside libraries (per local regulations) and spoke to parents with young children as they came and went. Electronic advertisements were placed with local newspapers and nextdoor.com. Flyers were distributed to retirement communities, churches, and to University of Colorado Boulder science students. In Baltimore, flyers were distributed at public elementary schools, nonprofit community organizations offering GED courses, financial services, and recreation and at a gym adjacent to a day care center. For meeting in both cities, electronic flyers were distributed via social media to parent and anti-vaccine groups. Due to difficulty reaching enrollment targets in Baltimore, a Facebook advertisement was placed to enhance recruitment. This was not necessary in Boulder.
Boulder participants were polled during the meeting regarding their children’s ages (multiple responses allowed: 0–10 years-old: n = 5, 11–18 years-old: n = 4, >18 years-old: n = 7). Baltimore participants reported their children’s ages via the recruitment survey (multiple responses allowed: <5 years-old: n = 9, 15–18 years-old: n = 15, >18: n = 11).
Individuals were offered a 50 USD Visa® gift card to participate in a 2-hour meeting on a weekend day. Parents were offered an additional 30 USD cash incentive for childcare. Refreshments were served at the meetings.
Sociodemographic questionnaire
Interested individuals registered by completing a sociodemographic questionnaire with a staff member by phone or independently online. All questionnaires were completed prior to participating in the community meetings. We compared self-reported sociodemographic information to each city’s Census data based on age, gender, race/ethnicity, education, and household income. Once a category was filled, participants with similar demographic characteristics were no longer recruited but placed on a waitlist. Subsequent recruitment targeted individuals in unfilled sociodemographic categories. Individuals were reminded by phone and/or e-mail about the upcoming meeting two days in advance. If they were no longer available, recruiters attempted to fill their place with someone from the waitlist. Questionnaire data were tabulated by city.
Ethical review
This project was determined not to be human subjects research by the Johns Hopkins Institutional Review Board.
Vaccinomics introduction
Since participants were not expected to be familiar with vaccinomics, we created a four-minute animated video (available from: https://preview.tinyurl.com/vaccinomics) that was shown at the start of each plenary session (Boulder n1 = 30; n2 = 29; Baltimore n3 = 35). After watching the video, a vaccine expert answered questions for approximately 10 minutes.
Small group discussions
Following the plenary sessions, participants (n = 10-15) were randomly assigned to small groups for facilitated discussions. There were four discussion groups with two sets of participants over two days in Boulder and four discussion groups on a—single day in Baltimore. Participants could not hear other groups’ discussions. Trained facilitators led the discussions and elicited feedback from all participants. Additional team members took handwritten notes.
Discussions were conducted using a standardized, semi-structured guide designed to elicit the policy implications of vaccinomics, audio recorded, and professionally transcribed. Facilitators set up hypothetical scenarios to assess participants’ values around genetically based vaccine prioritization during a vaccine shortage and widespread genetic screening to identify the few (approximately one in one million) individuals at risk of serious vaccine reactions.Citation24 Facilitators explained that genetic testing might reveal that some people are more contagious (“super spreaders”) and might be prioritized for vaccination to contain an infectious disease outbreak. Participants’ values regarding how federal funding should be allocated for vaccinomics versus other vaccine and chronic disease-related issues were probed. Open-ended prompts, developed based on the 2017 Stakeholders’ Meeting and team discussions, elicited participants’ values and rationale behind their thinking.
Vaccine confidence
We assessed confidence in vaccine safety and effectiveness prior to educating participants about vaccinomics and at the end of the meeting to test the hypothesis that discussing AEFIs and vaccinomics would not alter vaccine confidence. In the large group setting, participants reported their confidence in vaccines for adults and babies by placing stickers along four spectra ranging from “not effective” to “very effective” and “not safe” to “very safe.” Stickers fell along an unnumbered x-axis with 10 hashmarks. Stickers were assigned a numeric value from 1 to 10 based on the nearest hash mark when rounding up. The values of the pre- and post-discussion stickers were graphed and unpaired t-tests for the difference between the pre- and post-discussion means were estimated for each exercise. Participants noted why they placed their stickers where they did on post-it notes, which were thematically categorized by whether participants wrote them pre/post small group discussions, relevance to vaccine safety/effectiveness, and relevance to babies/adults. Emergent themes were identified through iteratively recategorizing written comments. Data collection methods did not permit comments to be matched to specific scores.
Funding
We assessed how participants prioritized funding for vaccinomics compared to other health-related options by asking them to allocate 100 USD of play money as if they were a member of Congress. The first activity compared vaccinomics to other vaccine programs (free vaccines for low-income children, development of new vaccines, and studies of vaccine safety and efficacy); the second compared vaccines and vaccinomics in combination to chronic diseases (cancer, heart disease, and diabetes). Participants divided their 100 USD between four jars for each activity. The money in each jar was summed and divided by the total amount of money allocated in each activity, which accounted for some participants not allocating their allotted funds. Data collection procedures are depicted in .
Data analysis
Grounded TheoryCitation32 influenced iterative, thematic analyses of the discussion group transcripts and written comments on vaccine confidence, using Atlas.ti®Citation33 for Windows and Microsoft Office®. Two people independently coded one transcript using open, descriptive codes.Citation32 Their coding was compared, revised, and an agreed upon list was used on all transcripts. Codes were revised a third time and data were iteratively recoded using open, axial, and selective codes. Transcripts were recoded as new codes emerged, and the properties of the code list were refined. Memos described the properties and dimensions of each code and summarized emergent themes. Categories were discussed with the project team and revised.Citation32 Conclusions based on Grounded TheoryCitation32 were compared to thematic notes taken by a coauthor uninvolved in data analysis, to evaluate the consistency of our findings. Quantitative data were analyzed using Stata, Version 16®.Citation34
Results
Study population
Ninety-four participants were enrolled from Baltimore (n = 35) and Boulder (n = 59; ). Seventy-two percent of participants were female (n = 67). Thirty five percent were 18–29 years old, 21% 30–44 years-old, 1% 45–60 years-old, and 21% >60 years-old. Two-thirds were non-Hispanic White and 18% were non-Hispanic Black. Holding a bachelor’s degree (43%) or higher (15%) was common. Half had household annual income <$50,000 (n = 47). Most Non-Hispanic Black participants were from Baltimore. Baltimore registrants were most likely to learn about the event via Facebook (53% versus Boulder: 18%), and most online recruits were “no shows” (Baltimore: 91%, Boulder: 69%; Supplemental Table 1). Results from seven of the eight discussion groups (10–15 people each) are reported (one group’s recording failed).
Table 1. Sociodemographic distribution of the sample
Emergent themes
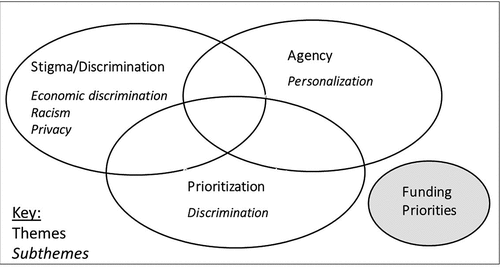
Vaccinomics’ policy implications consisted of four constructs ().
Vaccine prioritization: Prioritization for vaccination, especially during a vaccine shortage, may be based on genetics to maximize effectiveness and safety. Most participants opposed prioritization. Potential for increased discrimination was a subtheme.
Agency: Participants worried vaccinomics would remove their agency to make vaccine-related decisions. Personalization of vaccine schedules was a subtheme, stemming from a facilitator-led scenario.
Stigma/discrimination: Participants worried genetic information collected for the purposes of vaccinomics would not be kept private (a subtheme) and that they might be discriminated against or stigmatized as a result.
Vaccinomics funding: Participants supported funding vaccinomics versus other vaccine and chronic disease-related options.
Vaccine prioritization
This theme consisted of who should be prioritized for vaccination and how participants might react to not being prioritized. Discrimination was a subtheme. One woman said:
I’d be fine prioritizing the other people who were more … at risk of dying from the disease or at risk of spreading the disease. Source: Boulder 4
Though some participants supported prioritizing strangers or their grandchildren over themselves during a vaccine shortage, others said prioritization would limit their agency to make decisions for themselves. Participants identified vaccine access and affordability, maximizing public health benefits, and race-based prioritization as important policy areas. One man thought prioritization bordered on discrimination. He said:
I was going to bring up the trust factor. But who is telling me I can’t get the vaccine when there’s this disease that’s spreading through the population so quickly? … Issues of discrimination come up, issues of priority, and … age group, gender, et cetera. Source: Boulder 3
Another man and many others viewed prioritization as potentially limiting low-income and minority groups’ agency. He said:
… People would freak out … You know, a bunch of White folks get vaccinated, but what happens to the Hispanic and Black populations … ? They didn’t get vaccinated. It could really play into like people suspecting foul play. It’s like, … did they really try to get these super spreaders … ? … That would be an issue when giving power. Source: Boulder 2
When asked how they felt about race/ethnicity-based vaccine prioritization compared to gender-based prioritization, participants expressed fear that race/ethnicity would exacerbate existing inequities in healthcare access and discrimination. Race-based prioritization was considered unacceptable. See Supplemental Table 2 or prioritization quotations.
Agency
Intertwined with prioritization, participants believed they had the right to choose whether they or their children were vaccinated. Without prompting, several participants worried that vaccinomics would lead to mandatory vaccination. One woman explained:
My main concern, that is if I’m identified as super spreader, is it forced on. And I don’t want to get the vaccine, then what? That’s my big [concern] … Source: Boulder 1
A woman worried her agency would be limited even if she was not identified as a super spreader, which facilitators defined as someone who might be extra infectious due to their genetics. She explained:
[My concern is] not so much to do [with] genetic testing. If they have a genetic testing to also be able to look at it and determine whether or not [I’m a super spreader], but not have it be mandated by the government saying, “Well, you have this genome you have to have this done.” Source: Boulder 1
Another woman explained that having the information necessary to make an informed decision mattered, not just having agency. She said:
I think what’s important is that risk information be presented in a way people can easily understand. Out of 100 people those who don’t get the vaccine who are like you, versus – it’s just how it’s communicated. … The whole public needs to understand risk better, in general. … It just needs to be correct and simple in the explanation … you have to let people make their own decision if there’s enough vaccine … Source: Boulder 3
One participant explained her decision-making process regarding the human papillomavirus vaccine (Gardasil) and why parents need agency to make vaccine-related decisions. She said:
… If they are a healthcare worker, they should be able to say, “I don’t feel comfortable with taking this because I don’t feel like it’s been tested enough.” I know with my son I don’t want him to get the Gardasil because when he was younger, he got … [inaudible] So I just feel like everybody is different. … How one person reacts is not how another person reacts. … There’s really no way to be able to tell. Source: Baltimore 1
A man noted individual-level agency would complicate vaccine prioritization. He said:
… In a real-world application, you wouldn’t be able to, like, categorize all these people into one system and then … force them to come in to the hospital to get their vaccinations, right … Because personal opinion comes into effect … Especially with anti-vax movements that – like, it would make sense, but it wouldn’t be practical.
Source: Boulder 4
Participants felt they had a right to make vaccination decisions, regardless of the algorithms vaccinomics might suggest. See Supplemental Table 3 for agency quotations.
Personalization of vaccine schedules
A subtheme of agency was that participants were interested in vaccinomics’ potential to personalize vaccine schedules. Facilitators explained that new vaccines would not be created for individuals, rather vaccine schedules would be refined for subgroups of the population based on population-level genomic information. Participants’ comments about personalization were overwhelmingly positive, focused on the individual and community-level benefits of improved vaccine effectiveness. Drawing an analogy to stem cell research, one woman explained:
… It can be individualized … I think what we’re finding now with the stem cell research, the more you can individualize a treatment or a vaccine, the more effective it will be. Source: Boulder 2
A man agreed that personalization could lead to increased effectiveness at the population-level. He said:
… If you’re able to understand their genomics and you’re able to pretty much make this vaccine effective as possible, you’ll be able to enhance the herd immunity effectiveness. Boulder Group 2
In contrast, a woman noted vaccinomics’ individual-level benefit, personalization, was intertwined with its risks. She said:
I’d feel more comfortable if I had genetic testing that says that I’m not going to react adversely … When you’re an infant you don’t know what they’re allergic to or not. You’re just giving them vaccine and be like okay … If you have that genetic testing, my concern is more if that it gets out to insurance companies … [it] can be used adversely against me, and not just for the benefit of my health. Source: Baltimore 1
Participants were told the risk of serious adverse reactions was approximately one in one million.Citation24 A man indicated that the more information gleaned through vaccinomics, the better. He said:
… I think it would kind of ease your mind for a lot of these immunizations there’s risks that are explained … It would ease your mind on those risks to know you aren’t that one in one million … . Boulder Group 2
Regarding the potential for personalization to lead to more complex vaccine schedules, a woman said:
I would just say about the schedule, it’s already really complex … I think if a parent knows that that schedule is customized and catered to their specific child, … it would make them more likely to [vaccinate]. Boulder Group 2
While the woman above thought personalization might appeal to vaccine hesitant parents, another woman thought parents’ desire to amend recommendations would not cease with vaccinomics. She said:
… You’re essentially making it more complex. Each person has an individual schedule. But what if those individuals create their own individual schedule, like many people now make schedules that are different from the recommended schedules. So then there’s even more of a variance from the variance … It seems really complicated … Boulder Group 3
Personalization of vaccines was viewed with cautious optimism but was intertwined with concern that vaccinomics could contribute to stigma/discrimination and vaccine schedule complexity. See Supplemental Table 4 for personalization quotations.
Stigma/discrimination
Participants feared stigma and discrimination would result if genetic information collected to implement vaccinomics was not kept private and confidential (a subtheme). Facilitators explained widespread genetic testing might be used to help prevent serious adverse reactions to vaccines. They said the risk now of someone developing paralysis or dying after vaccination is very rare, afflicting approximately one person out of every one million people vaccinated.Citation24 Despite the potential benefits and without prompting, participants worried vaccinomics would lead to economic and racial discrimination (subthemes), exacerbating inequities in healthcare access. A man explained:
… Who is going to … actually to obtain it? … That could be said for a lot of technologies … Rich folks have it for a while and then over time we can get it to more broke folks. But it’s like more of an immediate issue with vaccines. Source: Boulder 2
One woman feared vaccinomics could exacerbate existing inequalities. She said:
Who gets [vaccinated]? For me, healthcare is between lower end of society are not getting the same level as the very rich. … I think this would become more of an economic thing where it’s the health policy will be driven by pharmaceutical companies and insurance companies. Source: Boulder 1
Another woman worried vaccinomics may only help some racial groups. She said:
… I think it’s probably a predominantly White field, so we have to be careful that the other races are getting what they need and that their risk factors are included in [vaccinomics]. Source: Boulder 4
Participants feared that genetic information collected to implement vaccinomics would be used as the basis for discrimination by the U.S. government and health insurers. They noted historical cases in which individuals’ rights had been violated, such as Henrietta Lacks, whose cervical cells were shared without her consent,Citation35 and personal examples. A man living with AIDS said:
… I’ve been working with AIDS treatment and vaccination … for a long time and one of the issues that has come up is that collecting information about people’s health is great as long as it stays between the doctor and the patient, but that isn’t where it stays, and as soon as there is some record of something about your health eventually government or someone is going to find a way to get in there and find out … Sometimes that’s used for really great reasons, in terms of distributing resources for treatment and prevention … . but there is always this possibility that it could be used against you, and there’s also a certain amount of stigma attached to that. Source: Baltimore 2
Participants worried individuals in power would violate patient confidentiality to access genetic results, which would lead to stigmatization/discrimination. A man worried this would limit his healthcare access. He explained:
… I’ve got a lot of preexisting conditions … I don’t want … them to say, “No, you can’t have it,” or it’s going to be so much it’s I can’t afford it. Source: Boulder 4
Many participants feared health insurance companies would discriminate against them based on their genetics. One woman said genetic testing may not be worthwhile. She suggested:
… It seems like to me DNA is so intensely private and personal … that if there were other areas of study that didn’t require this mass culling of such personal information … Source: Boulder 4
Another participant worried social ostracism could result if he was revealed to be a super spreader. He said:
I think [vaccinomics] would be fantastic and I’d be all in favor of pursuing this. … I can see some people’s concerns would be being identified as a super spreader could ostracize you from a social perspective. Source: Boulder 1
See Supplemental Table 5 for additional stigma/discrimination quotations.
Funding priorities
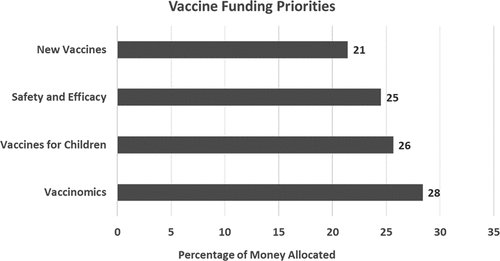
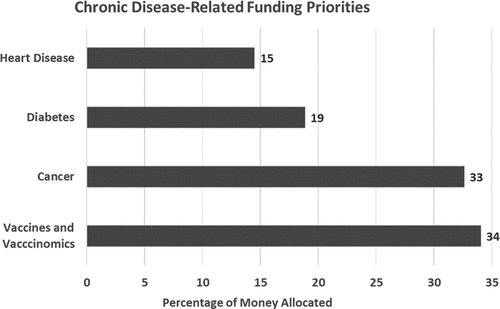
Participants favored funding vaccinomics compared to other vaccine-related priorities (free vaccines for low-income children, development of new vaccines, and studies of vaccine safety and efficacy) and chronic disease-related priorities (cancer, heart disease, and diabetes). In the vaccines-related exercise, 28% of funds were allocated to vaccinomics, 26% to purchasing vaccines for low-income U.S. children, 25% to studies of vaccine safety and efficacy, and 21% to research and development of new vaccines (). In the chronic disease research and development exercise, 34% of funding was allocated to vaccines and vaccinomics, 33% to cancer, 19% to diabetes, and 15% to heart disease (). In making vaccine-related decisions, participants cited government mandates, personal experiences, pandemic prevention, and economic reasons.
Vaccine confidence
Discussing vaccinomics, AEFIs and adverse reactions did not alter vaccine safety and effectiveness perceptions. Using an unpaired t-test to compare the difference in scores before and after discussions, there was no statistical evidence the mean scores differed over time for vaccine effectiveness or safety for children or adults (all p > 0.40; ).
Table 2. Vaccine confidence: two sample t-test for the equality of means
Participants’ written comments support that vaccine confidence was unchanged, though some wrote that the discussions and animation increased their knowledge. Pre discussion, several participants wrote that vaccines are more effective for babies than adults. Post-discussion, most comments reflected no change in beliefs or slightly more hopefulness for the future. Pre-discussion safety themes for adults and babies, respectively, included: personal experience, allergic reactions, manufacturing processes, disease prevention, and scientific rigor () and vaccine benefits outweighing the risks, general safety concerns, AEFI being rare, and scientific rigor (). Post-discussion themes for adults included: interest in vaccinomics’ potential to make vaccines safer and that adverse reactions rarely occur () and for babies: no change and that adverse reactions rarely occur (). Vaccine effectiveness themes pre-discussion for adults included: variance in effectiveness for adults versus children and themes among babies were that vaccines improved immunity and were somewhat effective. Post discussion, adult themes included: effectiveness at disease prevention and for babies: disease prevention and no change.
Table 3. Participants’ written comments explaining pre vs. post-discussion vaccine safety ratings for adults
Table 4. Participants’ written comments explaining pre vs. post-discussion vaccine safety ratings for babies
Table 5. Participants’ written comments explaining pre vs. post-discussion vaccine effectiveness ratings for adults
Table 6. Participants’ written comments explaining pre vs. post-discussion vaccine effectiveness ratings for babies
Discussion
We believe this is the first study to elucidate public values about vaccinomics and the implications of using genomics for infectious disease prevention.Citation36 We found broad support for vaccinomics. Discussions of adverse events following immunization (AEFI) did not impact perceptions of vaccine safety or effectiveness. Personalized vaccine schedules were especially supported by those personally or knew someone who had experienced an AEFI. Some participants feared information collected for vaccinomics would not be kept confidential, potentially leading to stigmatization/discrimination and increased health insurance fees or lost coverage. Others worried vaccination would be mandatory for super spreaders and unvaccinated individuals would be discriminated against, emphasizing the right to agency and opposition for compulsory vaccination.Citation37 Although the Genetic Information Nondiscrimination Act (GINA) prohibits genetic discrimination, it only applies to health insurers and employers.Citation38 The results of home-based genetic testing, which is increasing common, are not protected. Participants appeared unaware of protections afforded by GINA and the Health Insurance Portability and Accountability Act (HIPAA). Increasing awareness of these laws and more comprehensive nondiscrimination policies may enhance vaccinomics participation.Citation39
Vaccinomics may lead to more personalized vaccine schedules. Our findings suggest this may increase vaccine confidence. Compared to non-Hispanic Whites, minority populations are less trusting of vaccines, public health authorities, and pharmaceutical companies.Citation40–42 Low measles-mumps-rubella vaccine coverage among racial/ethnic minorities is evidence of mistrust.Citation43 Participants reported race/ethnicity-based prioritization could exacerbate mistrust of vaccines and public health authorities, and that sex-based prioritization would be preferable.
If differences in vaccine safety and efficacy are linked to biological sex, self-reported sex at birth could be used to assign vaccine schedules. Alternatively, genetic testing for rare markers might be used to identify high-risk individuals. For example, the HLA-DQB1*06:02 haplotype was associated with narcolepsy onset following vaccination with the AS03-adjuvanted Pandemrix® against H1N1 in 2009.Citation44 If future studies show similar associations between genomics and adverse reactions, this could inform subgroup-specific vaccine schedules.
Vaccinomics may lead to increased vaccine confidence and acceptance especially among populations with safety concerns. Participants who experienced an AEFI or knew someone who had, indicated they might trust vaccinomics more than current vaccines. By improving vaccine confidence among hesitant populations, vaccinomics may reduce the prevalence and clustering of under-immunized individuals, thereby reducing vaccine-preventable disease outbreaks.Citation27–29 Here, there was no evidence that discussing AEFI reduced participants vaccine confidence. Vaccinomics may make vaccine schedules more complex and its expected use of genomic data raises the potential for privacy violations. Rigorous guidelines, regulations and monitoring of vaccinomics will be essential to ensure the public’s safety and confidence. Robust safety surveillance may bolster public trust and participation.
Much remains to be determined about the feasibility of vaccinomics implementation, and if the policy implications of vaccinomics are viewed differently by racial/ethnic minority communities underrepresented here. This study provides evidence that adults in disparate cities supported vaccinomics, though they worried about its implications. These findings were robust using Grounded Theory-influenced methodsCitation32 and meeting notes taken by a coauthor uninvolved in data analysis.
Limitations
Although our sample was socioeconomically diverse, generalizability is limited by under enrollment of racial/ethnic minorities historically harmed by medical research, and perhaps more skeptical of genetic research than Whites. Research on the policy implications of vaccinomics among racial/ethnic minorities should be conducted. Placing a Facebook advertisement led to more than twice as many Baltimore participants being recruited online and a higher “no show” proportion versus Boulder, leading to under representation of African Americans.
Our methods precluded us from analyzing the qualitative data by discussion group or sociodemographic characteristics. Results from one discussion group are excluded because the recorder was not turned on, although this group’s facilitators and notetaker recorded similar themes. Focus group discussions and activities conducted in group settings, including the vaccine confidence and the funding exercises, are subject to social desirability bias.Citation45 Participants who saw more money in one jar, may have added their money to the same jar. Seeing most respondents rated vaccine confidence highly may have influenced others to do the same. Participants were encouraged to make their selections based on their personal preferences, but they may have felt pressured as others waited their turn to complete the exercise. Participants thought there was overlap among the chronic disease options presented in one funding exercise, questioning whether the Human Papillomavirus (HPV) vaccine fell under “vaccines and vaccinomics” or “cancer.” Facilitators said “vaccines and vaccinomics” included the HPV vaccine and “cancer” included other aspects of prevention and treatment. Nondifferential misclassification may have resulted. Data collection methods precluded paired analyses of pre- and post-discussion vaccine confidence ratings. Unpaired analyses indicated there was no difference between the two time points, which was supported by qualitative data.
Public health implications
The social and economic costs of vaccine refusal may be mitigated by using vaccinomics to increase vaccine acceptance. A 2008 measles outbreak in California led to approximately 10,376 USD/case in public sector spending and 775 USD/case in spending by the affected family.Citation46 Vaccinomics may increase vaccine acceptance, preventing outbreaks. Vaccinomics could implemented in areas at greatest risk of VPD, identified through mathematical models and geospatial statistics,Citation27,Citation28,Citation47–49 minimizing the community-wide effects of intentional under immunization.
Conclusions and next steps
Policies are needed that address constituents’ concerns about vaccinomics, including confidentiality of genetic test results and the potential for increased stigma/discrimination. Despite some apprehensions, participants were hopeful that vaccinomics may improve vaccine safety and effectiveness. Further research into racial/ethnic minorities’ views on the policy implications of vaccinomics is needed. We presented these results to stakeholders to aid them in designing policies that foster vaccinomics participation.
Disclosure of Potential Conflicts of Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: J Brewer, RJ Limaye, A Sutherland, G Geller, and CI Spina have no conflicts and report no financial disclosures. JE Gerber received support for manuscript revisions from RTI International. DA Salmon received consulting and/or research support form Merck, Walgreens, and Janssen.
Supplemental Material
Download MS Word (67.9 KB)Acknowledgments
This work was supported by grant number RM1HG009038 from the National Human Genome Research Institute (all authors). The funder had no involvement in the design, implementation, analysis, interpretation, or reporting of this study. We thank our participants for their time and contributions.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Poland GA, Kennedy RB, McKinney BA, Ovsyannikova IG, Lambert ND, Jacobson RM, Oberg AL. Vaccinomics, adversomics, and the immune response network theory: individualized vaccinology in the 21st century. Semin Immunol. 2013;25(2):89–103. doi:10.1016/j.smim.2013.04.007.
- Dhakal S, Klein SL, Coyne CB. Host factors impact vaccine efficacy: implications for seasonal and universal influenza vaccine programs. J Virol. 2019;93(21). doi:10.1128/JVI.00797-19.
- Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):9–15. doi:10.1093/trstmh/tru167.
- Halsey NA, Griffioen M, Dreskin SC, Dekker CL, Wood R, Sharma D, Jones JF, LaRussa PS, Garner J, Berger M, et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: reports to VAERS. Vaccine. 2013;31(51):6107–12. doi:10.1016/j.vaccine.2013.09.066.
- Griffioen M, Halsey N. Gender differences in immediate hypersensitivity reactions to vaccines: a review of the literature. Public Health Nurs. 2014;31(3):206–14. doi:10.1111/phn.12073.
- Zent O, et al. Immediate allergic reactions after vaccinations–a post-marketing surveillance review. Eur J Pediatr. 2002;161(1):21–25. doi:10.1007/s00431-001-0853-0.
- Zerbo O, et al. Parental risk factors for fever in their children 7-10 days after the first dose of measles-containing vaccines. Hum Vaccin Immunother. 2019;1–6.
- Tartof SY, Tseng HF, Liu AL, Qian L, Sy LS, Hechter RC, Michael Marcy S, Jacobsen SJ. Exploring the risk factors for vaccine-associated and non-vaccine associated febrile seizures in a large pediatric cohort. Vaccine. 2014;32(22):2574–81. doi:10.1016/j.vaccine.2014.03.044.
- Vestergaard M, Hviid A, Madsen KM, Wohlfahrt J, Thorsen P, Schendel D, Melbye M, Olsen J. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA. 2004;292(3):351–57. doi:10.1001/jama.292.3.351.
- Berg AT, Shinnar S, Shapiro ED, Salomon ME, Crain EF, Hauser WA. Risk factors for a first febrile seizure: a matched case-control study. Epilepsia. 1995;36(4):334–41. doi:10.1111/j.1528-1157.1995.tb01006.x.
- Klein NP, Lewis E, McDonald J, Fireman B, Naleway A, Glanz J, Jackson LA, Donahue JG, Jacobsen SJ, Weintraub E, et al. Risk factors and familial clustering for fever 7–10 days after the first dose of measles vaccines. Vaccine. 2017;35(12):1615–21. doi:10.1016/j.vaccine.2017.02.013.
- Feenstra B, Pasternak B, Geller F, Carstensen L, Wang T, Huang F, Eitson JL, Hollegaard MV, Svanström H, Vestergaard M. Common variants associated with general and MMR vaccine–related febrile seizures. Nat Genet. 2014;46(12):1274–82. doi:10.1038/ng.3129.
- World Health Organization, Report of the SAGE Working Group on Vaccine Hesitancy. 2014.
- World Health Organization, Ten threats to global health in 2019. 2019.
- RJ R Fewer in U.S. Continue to See Vaccines as Important. 2020.
- Kennedy A, LaVail K, Nowak G, Basket M, Landry S. Confidence about vaccines in the United States: understanding parents’ perceptions. Health Aff (Millwood). 2011;30(6):1151–59. doi:10.1377/hlthaff.2011.0396.
- Salmon DA, et al. Vaccine hesitancy: causes, consequences, and a call to action. Am J Prev Med. 2015;49(6 Suppl 4):S391–8. doi:10.1016/j.amepre.2015.06.009.
- Salmon DA, Dudley MZ, Glanz JM, Omer SB. Vaccine hesitancy: causes, consequences, and a call to action. Vaccine. 2015;33(Suppl 4):D66–71. doi:10.1016/j.vaccine.2015.09.035.
- Enkel SL, Attwell K, Snelling TL, Christian HE. ‘Hesitant compliers’: qualitative analysis of concerned fully-vaccinating parents. Vaccine. 2018;36(44):6459–63. doi:10.1016/j.vaccine.2017.09.088.
- McHale P, Keenan A, Ghebrehewet S. Reasons for measles cases not being vaccinated with MMR: investigation into parents’ and carers’ views following a large measles outbreak. Epidemiol Infect. 2016;144(4):870–75. doi:10.1017/S0950268815001909.
- Thompson EL, Rosen BL, Vamos CA, Kadono M, Daley EM. Human papillomavirus vaccination: what are the reasons for nonvaccination among U.S. adolescents? J Adolesc Health. 2017;61(3):288–93. doi:10.1016/j.jadohealth.2017.05.015.
- Wooten KG, Wortley PM, Singleton JA, Euler GL. Perceptions matter: beliefs about influenza vaccine and vaccination behavior among elderly white, black and Hispanic Americans. Vaccine. 2012;30(48):6927–34. doi:10.1016/j.vaccine.2012.08.036.
- Dudley MZ, et al. The clinician’s vaccine safety resource guide: optimizing prevention of vaccine-preventable diseases across the lifespan. Cham (Switzerland): Springer Nature; 2018
- Salmon DA, Proschan M, Forshee R, Gargiullo P, Bleser W, Burwen DR, Cunningham F, Garman P, Greene SK, Lee GM, et al. Association between Guillain-Barré syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: a meta-analysis. Lancet. 2013;381(9876):1461–68. doi:10.1016/S0140-6736(12)62189-8.
- Offit PA, Hackett CJ. Addressing parents’ concerns: do vaccines cause allergic or autoimmune diseases? Pediatrics. 2003;111(3):653–59. doi:10.1542/peds.111.3.653.
- Hotez PJ, Nuzhath T, Colwell B. Combating vaccine hesitancy and other 21st century social determinants in the global fight against measles. Curr Opin Virol. 2020;41:1–7. doi:10.1016/j.coviro.2020.01.001.
- Atwell JE, Van Otterloo J, Zipprich J, Winter K, Harriman K, Salmon DA, Halsey NA, Omer SB. Nonmedical vaccine exemptions and pertussis in California, 2010. Pediatrics. 2013;132(4):624–30. doi:10.1542/peds.2013-0878.
- Omer SB, Enger KS, Moulton LH, Halsey NA, Stokley S, Salmon DA. Geographic clustering of nonmedical exemptions to school immunization requirements and associations with geographic clustering of pertussis. Am J Epidemiol. 2008;168(12):1389–96. doi:10.1093/aje/kwn263.
- Patel M, Lee AD, Clemmons NS, Redd SB, Poser S, Blog D, Zucker JR, Leung J, Link-Gelles R, Pham H, et al. National update on measles cases and outbreaks — United States, January 1, 2019. MMWR Morb Mortal Wkly Rep. 2019;68(40):893–96. doi:10.15585/mmwr.mm6840e2.
- Colorado Department of Public Health and Environment. School and child care immunization data 2018-2019 information for partners. 2019. https://www.cohealthdata.dphe.state.co.us/Data/Details/899902.
- U.S. Census Bureau: United States. Quick Facts. 2018 [ accessed 12 Apr 2019]. https://www.census.gov/quickfacts/fact/table/US/PST040218.
- Strauss A, Corbin JM. Basics of qualitative research: grounded theory procedures and techniques. Sage Publications, Inc; 1990.
- ATLAS.ti 8. 2018. https://atlasti.com/.
- StataCorp LLC. Stata 16. 2019. https://www.stata.com/.
- Skloot R. The immortal life of Henrietta Lacks. Broadway Books; 2017.
- Ozdemir V, Pang T, Knoppers BM, Avard D, Faraj SA, Zawati MH, Kolker E. Vaccines of the 21st century and vaccinomics: data-enabled science meets global health to spark collective action for vaccine innovation. Omics. 2011;15(9):523–7.ack. doi:10.1089/omi.2011.03ed.
- Einsiedel EF. Publics and vaccinomics: beyond public understanding of science. Omics. 2011;15(9):607–14. doi:10.1089/omi.2010.0139.
- Senate and House of Representatives of the United States of America in Congress, The Genetic Information Nondiscrimination Act of 2008. 2008.
- Prince AER. Political economy, stakeholder voices, and saliency: lessons from international policies regulating insurer use of genetic information. J Law Biosci. 2018;5(3):461–94. doi:10.1093/jlb/lsz001.
- Freimuth VS, Jamison AM, An J, Hancock GR, Quinn SC. Determinants of trust in the flu vaccine for African Americans and Whites. Soc Sci Med. 2017;193:70–79. doi:10.1016/j.socscimed.2017.10.001.
- Quinn SC, Jamison A, Freimuth VS, An J, Hancock GR, Musa D. Exploring racial influences on flu vaccine attitudes and behavior: results of a national survey of White and African American adults. Vaccine. 2017;35(8):1167–74. doi:10.1016/j.vaccine.2016.12.046.
- Liu BF, Quinn SC, Egnoto M, Freimuth V, Boonchaisri N. Public Understanding of Medical Countermeasures. Health Secur. 2017;15(2):194–206. doi:10.1089/hs.2016.0074.
- Hill HA, et al. Vaccination coverage among children aged 19-35 months - United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(40):1123–28. doi:10.15585/mmwr.mm6740a4.
- Edwards K, Lambert PH, Black S. Narcolepsy and pandemic influenza vaccination: what we need to know to be ready for the next pandemic. Pediatr Infect Dis J. 2019;38(8):873–76. doi:10.1097/INF.0000000000002398.
- Stewart DW, Shamdasani PN, Rook DW. Analyzing focus group data, in focus groups: theory and practice. Thousand Oaks: SAGE Publications, Ltd.; 2007.
- Sugerman DE, Barskey AE, Delea MG, Ortega-Sanchez IR, Bi D, Ralston KJ, Rota PA, Waters-Montijo K, LeBaron CW. Measles outbreak in a highly vaccinated population, San Diego, 2008: role of the intentionally undervaccinated. Pediatrics. 2010;125(4):747–55. doi:10.1542/peds.2009-1653.
- Cadena J, Falcone D, Marathe A, Vullikanti A. Discovery of under immunized spatial clusters using network scan statistics. BMC Med Inform Decis Mak. 2019;19(1):28. doi:10.1186/s12911-018-0706-7.
- Klein NP, Fireman B, Yih WK, Lewis E, Kulldorff M, Ray P, Baxter R, Hambidge S, Nordin J, Naleway A, et al. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics. 2010;126(1):e1–8. doi:10.1542/peds.2010-0665.
- Aloe C, Kulldorff M, Bloom BR. Geospatial analysis of nonmedical vaccine exemptions and pertussis outbreaks in the United States. Proc Natl Acad Sci U S A. 2017;114(27):7101–05. doi:10.1073/pnas.1700240114.