ABSTRACT
Vaccine hesitancy remains a global health threat. Addressing parental vaccine hesitancy is essential to maintaining high vaccine coverage levels and preventing disease outbreaks; however, it is unknown if administering a vaccine hesitancy screening tool negatively impacts parental vaccine beliefs. We conducted a stratified randomized controlled trial in pediatric primary care practices. English-speaking parents ≥18 years of age seeking routine care for infants <3 months of age were eligible. Participants were randomized to receive 1 of 2 surveys – the Parent Attitudes about Childhood Vaccines (PACV) survey or a placebo survey. Six months after initial enrollment, all participants were asked to complete the PACV, regardless of initial randomization group. Our primary outcome was the proportion of hesitant to non-hesitant parents at 6-months between randomization groups. We examined associations between vaccine hesitancy and participant characteristics. We also evaluated the change in the proportion of vaccine-hesitant parents in the PACV group between baseline and 6-month follow up. We enrolled 1705 parents at baseline. At 6-month follow up, 819 parents completed the PACV (50.2% from PACV group vs. 54.1% from placebo group). The proportion of hesitant parents at 6 months did not differ between PACV and placebo groups (6.6% vs. 6.1%; p = .78) and the odds of hesitancy among PACV group participants was not higher than those in the placebo group (OR = 1.10; 95% CI: 0.63–1.93; p = .743). Race was the only characteristic significantly associated with vaccine hesitancy at 6-month follow up (p = .003). Overall, administration of the PACV did not trigger vaccine hesitancy in this study population.
Introduction
Vaccine coverage levels remain high in the U.S.; however, the number of nonmedical vaccine exemptions continues to increase.Citation1 Outbreaks of vaccine-preventable diseases, notably measles, have surged in the U.S. and across the globe, leading the World Health Organization to identify vaccine hesitancy as one of the top ten global health threats.Citation2 During the 2015 Disneyland measles outbreaks, 80% of measles cases were among unvaccinated individuals or those with unknown vaccine status. Of the infected unvaccinated individuals, 43% reported declining measles-mumps-rubella (MMR) vaccination for nonmedical reasons.Citation3 Similarly, in the U.S. measles outbreaks in 2019, 89% of measles cases were among unvaccinated individuals or those with unknown vaccine status.Citation4 While several factors contribute to decreased vaccine coverage among children, parental vaccine concerns are a leading cause. Thus, addressing vaccine hesitancy is critical to maintaining high vaccine coverage levels and preventing future disease outbreaks.
Vaccine-hesitant parents (VHPs) are reluctant to vaccinate their children and may ultimately delay or refuse some or all vaccines. One recent study found approximately 6% of parents were vaccine-hesitant; among these parents, 68% delayed or refused vaccines for their child.Citation5–8 Another recent study found that nearly 23% of 19 to 35 month olds in the United States followed an alternate vaccine schedule rather than the recommended schedule set forth by the Advisory Committee on Immunization Practices (ACIP).Citation6 VHPs espouse a broad spectrum of vaccine beliefs and are often more amenable to education and reassurance from their child’s pediatric provider than parents who refuse all vaccines.Citation7,Citation8 Given the continued increase in vaccine hesitancy and the related public health implications, there is considerable interest in screening parents for vaccine hesitancy within the clinical setting to help ensure parental vaccine concerns are identified and addressed. Several measures for vaccine hesitancy screening were developed in the last decade; however, it is unknown if administering a screening instrument negatively impacts parental vaccine beliefs by triggering vaccine concerns that were not otherwise present.Citation9It is possible that presenting concepts related to vaccine safety, efficacy and importance to parents who have not been previously exposed to or considered such concepts may lead these parents to explore these ideas and potentially develop vaccine hesitancy. One of the most widely used screening tools for vaccine hesitancy is the Parent Attitudes about Childhood Vaccines (PACV) survey.Citation10 The PACV survey is a valid and reliable tool containing 15-items designed to identify vaccine-hesitant parents.Citation8,Citation10–12 In this study, we examined the impact of administering the PACV survey to parents of newborns receiving pediatric primary care in Houston, TX. Additionally, we characterized the demographics of vaccine-hesitant parents and assessed the prevalence of vaccine hesitancy in this population.
Methods
We conducted a stratified randomized controlled trial in pediatric primary care practices in Houston, TX between October 2013 and June 2016. The study was registered with clinicaltrials.gov (identifier NCT04450940) as the Impact of PACV Administration on Vaccine Hesitancy trial and approved by the Institutional Review Board at Baylor College of Medicine. This study included a waiver of written documentation of consent. Our aim was to examine the impact of PACV administration on vaccine hesitancy over a 6-month period.
Setting and sample
We enrolled parents and/or guardians of infants less than 3 months of age who received care at a Texas Children’s Pediatrics study site. Texas Children’s Pediatrics (TCP) is the pediatric primary care arm of Texas Children’s Hospital. TCP is a network of over 50 practices that employs more than 250 board-certified pediatricians with approximately one million patient encounters annually; as of 2019, 25% of TCP patients were publicly insured.
Twelve TCP practices participated in this study. Participating practices were selected based on payor mix and practice leaderships’ willingness to participate. We sought to enroll patients with a broad payor mix. Of the 12 sites, 7 serve a primarily publicly insured population with a range of 39 to 80% of patients enrolled in a public insurance program (i.e. Medicaid or CHIP). The remaining 5 sites serve primarily privately insured patients with a range of 83 to 95% of patients enrolled in a private health insurance plan.
Parents/guardians were eligible to participate if they were English-speaking, ≥18 years of age, and the parent/guardian of an infant <3 months of age receiving routine well-child care at a participating TCP study site. TCP staff identified potentially eligible parents using the clinic’s daily schedule and approached them to assess their willingness to participate at the 2-week well child visit. Potentially eligible parents received an enrollment packet containing a self-screening form, cover letter and survey at appointment check-in. The self-screening form was located on the cover of the enrollment packet; the form included instructions as well as a brief decision tree in English and Spanish to determine if the parent/guardian met the study eligibility criteria. If the parent/guardian did not meet eligibility criteria, were Spanish-speaking only or they were not interested in participating, the self-screening form directed them to return the packet to the practice staff. Parents/guardians who met eligibility criteria and were willing to participate in the study were directed to open the enrollment packet. The enrollment packet contained the cover letter and survey. The cover letter provided a description of the study including eligibility criteria and information related to consent.
Randomization and intervention
Participants were randomized to receive one of two surveys – either the PACV survey or a placebo survey. The placebo survey was a 15-item survey on general childhood topics such as breastfeeding, infant formula use, bed sharing, care seat safety and other general childhood health and safety topics. Surveys were randomized by study staff prior to distribution to TCP study sites using a stratified randomization design, with practice sites being the unit of stratification. The allocation ratio was 1:1; surveys (i.e. either the PACV or placebo survey) were placed in envelopes and shuffled for randomization. All study authors and TCP clinic staff were blinded to study arm allocation.
At enrollment, parents completed either the PACV or the placebo survey. Six months after enrollment, all participants were contacted by e-mail (up to 2 times) and phone (up to 3 times) and asked to complete the PACV. Therefore, the PACV was administered to all 6-month follow up participants regardless of baseline randomization group. Study staff administered the PACV at 6 months to participants by phone. Study staff emailed instructions to complete the follow up survey online to participants who failed to respond by phone or as requested by the participant. Parents were enrolled in person at the study site at the time of their child’s 2 week well child visit; however, follow up surveys were administered by phone or e-mail in lieu of written follow up surveys due to logistical obstacles.
Data collection
We asked parents to provide demographic information including age, gender, educational level, marital status, race/ethnicity, household income, relationship to the child and the number of children in the household. Demographic information was included on both the PACV and the placebo survey.
Sample size
Based on a prior study that found 8.2% of expectant parents in Houston, TX were vaccine-hesitant, we estimated 10% of participants in the placebo randomization group would be vaccine-hesitant at 6 months.Citation13 We calculated a sample size of 215 participants per randomization group would be needed to provide ≥80% power to detect a 0.10 unit difference (10% vaccine hesitancy in placebo group vs. 20% in PACV group) in the proportion of vaccine-hesitant parents between randomization groups at 6 months using Fisher’s exact test assuming α = 0.05 (two-sided). Additionally, we calculated a sample size of 400 participants per randomization group would provide >80% probability of estimating the proportion of vaccine-hesitant parents in each group at baseline and 6-months with a 95% exact Binomial confidence interval with a half-width ≤0.03.Citation14 Therefore, this study planned to enroll and randomize 1700 parents to account for 50% non-response of completion for the second survey.
Data analysis
We scored the PACV using methods established by Opel et al.Citation11 Final PACV scores were dichotomized as ≥50 (hesitant) and <50 (non-hesitant). This method of scoring is consistent with prior studies demonstrating that more children of parents with PACV scores ≥50 were under immunized compared to children of parents with PACV scores <50.Citation8,Citation12
We summarized demographic characteristics using mean with standard deviation, median with 25th and 75th percentiles and frequency with percentage. The summary statistics were stratified in several ways: 1) randomization group (PACV or placebo) at baseline and 6-month follow up, 2) 6-month follow up status (completed 6-month follow up or lost to follow up) and 3) vaccine-hesitancy status (hesitant vs. non-hesitant) at 6-month follow up.
Our primary outcome was to compare the proportion of hesitant to non-hesitant parents between randomization groups (PACV vs. placebo) at 6-month follow up. We used Fisher’s exact test to measure this outcome as well as multivariable logistic regression to estimate the odds of vaccine hesitancy at 6-months. To explore associations between vaccine hesitancy and participant characteristics, we used independent and multivariable logistic regression. In the multivariable analysis, we adjusted for randomization group as well as patient characteristics found to be significant in the independent logistic regression model. Using Sidak’s method, we adjusted for pairwise comparisons for race/ethnicity. We also performed a sensitivity analysis to account for the variation due to correlation within sites using Generalized Estimating Equations.
We also sought to analyze the change in the proportion of vaccine-hesitant parents in the PACV randomization group between baseline and 6-month follow up using McNemar’s test. We also performed a mixed effects logistic regression model to assess the change in hesitancy in the PACV group, accounting for within person and site correlation. In this analysis, we adjusted for patient characteristics that were different between participants who completed 6-month follow up and those who were lost to follow up.
Results
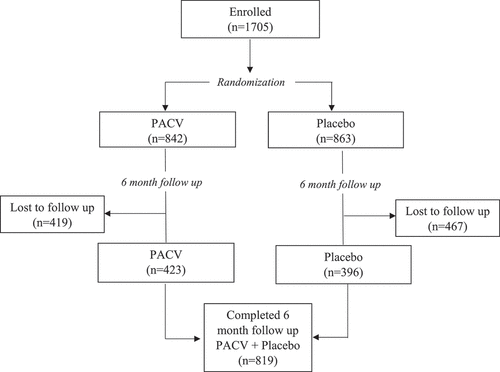
We enrolled 1705 parents/guardians. Of these, 49.4% (n = 842) received the PACV survey at baseline and 50.6% (n = 863) received the placebo survey at baseline. At 6 months, 423 of the 842 PACV group participants completed the follow up survey with the remainder (50.2%; n = 419) lost to follow up. Within the placebo group, 396 completed the follow up survey with the remainder (54.1%; n = 46 7) lost to follow up. Overall, 819 (48%) of the 1,705 baseline participants completed the PACV at 6-month follow up ().
Examining overall participant characteristics at baseline, participants were primarily white (46.8%), married (77.2%), 30 years of age or older (56.6%) and reported an income of 75,000 USD or more (54.0%). Comparing PACV participants to placebo participants at baseline, no significant demographic differences were observed (). Comparing all 6-month follow up participants to participants lost to follow up, those who completed 6-month follow up were more likely to be older (p = .004), white (p < .001), married (p < .001), have more education (p < .001) and a higher income (p < .001) ().
Table 1. Proportion (%) of baseline participant characteristics by randomization group*
Table 2. Proportion (%) of baseline participant characteristics by follow up status*
In bivariate analysis, the proportion of hesitant parents at 6 months did not differ between the PACV and placebo randomization groups (6.6% vs. 6.1%; p = .78; ). The odds of vaccine hesitancy among PACV group participants was not significantly higher than placebo group participants (OR = 1.10; 95% CI: 0.63–1.93; p = .743). Race was the only characteristic significantly associated with vaccine hesitancy at 6-month follow up (p = .003). In multivariable analysis adjusting for race, the odds of vaccine hesitancy among PACV group participants remained not statistically significant (adjusted OR = 1.13; 95% CI: 0.64–2.01; p = .675). After adjusting for randomization group, race continued to be significantly associated with vaccine hesitancy (p = .003). The odds of being vaccine-hesitant were 3.06 times greater among blacks compared to whites (95% CI: 1.5–6.3; p = .002) and 3.16 times greater among parents who reported their race/ethnicity as other compared to whites (95% CI: 1.3–7.5; p = .009) (). Additionally, the odds of being vaccine-hesitant were 2.8 times higher among blacks (95% CI: 1.1–7.0; p = .025) and 2.9 times higher among other race/ethnicities compared to Hispanics (95% CI: 1.0–8.2; p = .042). When compared to Asian participants, the odds of vaccine hesitancy were 8.6 times and 8.9 times higher among black participants (95% CI: 1.1–67.1; p = .040) and participants of other race/ethnicities, respectively (95% CI: 1.1–73.4; p = .043). Self-reported other race/ethnicities included Middle Eastern, Pakistani, Native American, Indian, Italian, Egyptian and Brazilian. After adjusting for multiple pairwise comparisons between all race/ethnicities, only one comparison remained statistically significant – the odds of being vaccine-hesitant were 3.1 times higher in blacks versus whites (95% CI: 1.1–8.5; p = .02). Finally, accounting for variation due to correlation within sites using Generalized Estimating Equations models did not change overall results (data not shown).
Table 3. Proportion of vaccine-hesitant parents (VHPs) by randomization group and study time point
Table 4. The odds of vaccine hesitancy (i.e. scoring ≥50 on the PACV) at 6-month follow-up
Within the PACV group, the proportion of hesitant parents did not significantly change between baseline and 6-month follow up (6.9% vs. 6.6%; p = .87). Using mixed effects logistic regression to account for correlation within patients and sites, the odds of vaccine hesitancy among PACV group participants did not change from baseline to 6-month follow up (OR = 0.93; 95% CI: 0.50–1.67; p = .809). Adjusting for patient characteristics that differed between participants who completed 6-month follow up and those who were lost to follow up (maternal age, race/ethnicity, marital status, education, and income) did not significantly impact the odds of hesitancy in PACV group participants (OR = 0.92; 95% CI: 0.50–1.67; p = .776).
Discussion
We conducted a stratified randomized controlled trial to examine the impact of PACV survey administration on vaccine hesitancy in parents of newborns in Houston, TX. We found that the proportion of vaccine-hesitant parents between randomization groups (PACV and placebo) did not differ at 6 months. Moreover, within the PACV group, the proportion of vaccine-hesitant parents at baseline and 6-month follow up was not significantly different. These findings suggest that administration of the PACV did not trigger vaccine hesitancy in this study population. Given the interest in screening for vaccine-hesitant parents to assist providers in their efforts to address vaccine concerns, confirming administration of the PACV did not induce vaccine hesitancy is reassuring. Assessing vaccine status at 6 months would provide additional validation that the PACV did trigger vaccine hesitancy; however, such an undertaking was outside the scope of this project. Currently, it remains unclear if screening is an effective intervention in addressing and reducing vaccine hesitancy as research in this area is limited. A recent study by Opel et al assessed the impact of pre-visit vaccine hesitancy screening on vaccine uptake and reported that no significant impact occurred.Citation15 Further research is needed to assess if screening is a beneficial tool for providers or if other confounding factors contributed to the null effect in Opel’s study.
While the PACV did not trigger vaccine hesitancy in this population, we found 6.1 to 7.1% of parents were vaccine-hesitant. This finding is similar to other studies that administered the PACV to parents of newborns in the U.S. which reported 8.9 to 15.2% of parents of newborns were vaccine-hesitant.Citation8,Citation12,Citation16 While the proportion of hesitant parents in Houston, TX was lower than these other estimates, we found vaccine hesitancy to be higher in blacks and other minority parents. In prior assessments, the overwhelming majority of participants were white (76.0–84.0%) whereas our study included a substantially lower proportion of whites (53.7% at 6-month follow up). Additionally, our study population included a higher proportion of Hispanics (19.0%) and blacks (13.0%) at 6-month follow up than these prior studies (3.9–6% Hispanics and 3.9–9.0% blacks, respectively).Citation8,Citation12,Citation15 Given the low proportion of blacks and Hispanics represented in these prior studies, differences in vaccine hesitancy among these populations may have gone undetected. In our study, race was the only characteristic consistently associated with vaccine hesitancy even after adjusting for multiple factors. Other non-PACV evaluations with more diverse study populations reported findings similar to ours. For instance, a recent study by Boyle et al found black and Hispanic parents were more likely to endorse elements of vaccine hesitancy.Citation17 Although vaccine beliefs and behaviors among black and Hispanic parents have yet to be fully described, vaccine hesitancy among black and Hispanic adults is more established, especially in regard to the influenza vaccine.Citation18–21 Moreover, recent public polls suggest potentially concerning hesitancy and distrust of the forthcoming COVID-19 vaccines, particularly among black adults. One recent survey reported a substantially higher proportion of black adults stated they would not receive a COVID-19 vaccine compared to Hispanic and white adults.Citation22
Our study had several limitations. First, significant differences between participants who completed 6-month follow up and those who were lost to follow up suggest possible response bias. We took steps to account for these differences by adjusting for multiple factors in the regression models. In the logistic regression model, we adjusted for race as it was the only significant patient characteristic while in the mixed effects analysis, we adjusted for the characteristics which differed between participants who completed 6-month follow up and who were lost to follow up, specifically maternal age, race/ethnicity, marital status, education and income. Additionally, we enrolled a convenience sample of parents/guardians and likely missed potential participants which may have resulted in further response bias. Due to our enrollment procedures and number of study sites, it was insurmountably difficult to enroll on site and track all potentially eligible participants; however, there is no reason to believe that missed potential participants were more or less hesitant than those enrolled. Second, this study was conducted in Houston, TX and the results may not be generalizable to other geographical areas in the U.S. Third, there may also have been baseline demographic differences among our study sites. Although we adjusted for variation between sites, there may be other unknown differences between study sites for which we are unable to account. Fourth, we did not distinguish between race and ethnicity which may have resulted in misclassification of these participant characteristics; however, we believe our findings suggest that minority parents may represent a population with vaccine hesitancy. Future studies should take measures to distinguish between race and ethnicity to correctly identify which populations, if any, demonstrate vaccine hesitancy. Fifth, we did not attempt to measure vaccine status which would have further validated our findings. Lastly, the follow up survey was administered in a different mode than the initial survey which may have resulted in response bias.
Conclusion
Administration of the PACV to parents of newborns does not appear to contribute to development of vaccine hesitancy. We also found the proportion of vaccine-hesitant parents of newborns in Houston, TX ranged from 6.1 to 7.1% which is slightly lower than prior estimates that also used the PACV in parents of newborns. Additionally, blacks were significantly more likely to be vaccine-hesitant than whites. Additional research is needed to further understand how hesitancy rates differ geographically and explore potentially shifting beliefs in minority populations. Understanding vaccine hesitancy among black parents is especially critical given the widespread public health implications of forthcoming COVID-19 vaccines.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors gratefully acknowledge Katherine Tittle, RN, President of TCP and Stanley Spinner, MD, Vice President and Chief Medical Officer of TCP, who allowed us to conduct this research in the TCP practices. The authors also express their sincere gratitude towards the staff of the Texas Children’s Pediatrics study sites without whose support this study would not have been possible. The authors thank Dr. Leila C. Sahni for her assistance throughout study implementation, enrollment, manuscript drafting and review.
References
- Seither R, Loretan C, Driver K, Mellerson JL, Knighton CL, Black CL. Vaccination coverage with selected vaccines and exemption rates among children in kindergarten – united States, 2018-19 school year. MMWR Morb Mortal Wkly Rep. 2019;68:905–12. doi:10.15585/mmwr.mm6841e1.
- World Health Organization. Ten threats to global health in 2019 – january 18, 2019. [accessed 2020 Feb 27]. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019
- Clemons NS, Gastanaduy PA, Fiebelkorn AP, Redd SB, Wallace GS. Measles – United States, January 4 – April 2, 2015. MMWR Morb Mortal Wkly Rep. 2015; 64(14):373–76. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6414a1.htm
- Patel M, Lee AD, Clemmons NS, Redd SB, Poser S, Blog D, Zucker JR, Leung J, Link-Gelles R, Pham H, et al. National update on measles cases and outbreaks – United States, January 1-October 1, 2019. MMWR Morb Mortal Wkly Rep. 2019;68:893–96. doi:10.15585/mmwr.mm6840e2.
- Kempe A, Saville AW, Albertin C, Zimet G, Breck A, Helmkamp L, Vangala S, Dickinson LM, Rand C, Humiston S, et al. Parental hesitancy about routine childhood and influenza vaccinations: a national survey. Pediatrics. 2020;146(1):1–12. doi:10.1542/peds.2019-3852.
- Hargreaves AL, Nowak G, Frew P, Hinman AR, Orenstein WA, Mendel J, Aikin A, Nadeau JA, McNutt L-A, Chamberlain AT, et al. Adherence to timely vaccinations in the United States. Pediatrics. 2020;145(3):1–11. doi:10.1542/peds.2019-0783.
- Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics. 2008;122:718–25. doi:10.1542/peds.2007-0538.
- Opel DJ, Taylor JA, Zhou C, Catz S, Myaing M, Mangione-Smith R. The relationship between parent attitudes about childhood vaccines survey scores and future child immunization status: A validation study. JAMA Pediatr. 2013;167(11):1065–71. doi:10.1001/jamapediatrics.2013.2483.
- Oduwole EO, Pienaar ED, Mahomed H, Wiysonge CS. Current tools available for investigating vaccine hesitancy: a scoping review protocol. BMJ Open. 2019;9:1–7. doi:10.1136/bmjopen-2019-033245.
- Opel DJ, Taylor JA, Mangione-Smith R, Solomon C, Zhao C, Catz S, Martin D. Validity and reliability of a survey to identify vaccine-hesitant parents. Vaccine. 2011;29:6598–605. doi:10.1016/j.vaccine.2011.06.115.
- Opel DJ, Mangione-Smith R, Taylor JA, Korfiatis C, Wiese C, Catz S, Martin DP. Development of a survey to identify vaccine-hesitant parents: the parent attitudes about childhood vaccines survey. Hum Vaccines. 2011;7(4):419–25.
- Williams SE, Morgan A, Opel D, Edwards K, Weinberg S, Rothman R. Screening tool predicts future underimmunization among a pediatric practice in Tennessee. Clin Pediatr. 2016. Epub. doi:10.1177/0009922815615823.
- Cunningham RM, Minard CG, Guffey D, Swaim LS. Prevalence of vaccine hesitancy among expectant parents in Houston, TX. Acad Pediatr. 2017;18(2):154–60. doi:10.1016/j.acap.2017.08.003.
- Byrt T, Bishop J, Carlin JB. Bias, prevalence, and kappa. J Clini Epidemiol. 1993;46(5):423–29. doi:10.1016/0895-4356(93)90018-V.
- Opel DJ, Henrikson N, Lepere K, Hawkes R, Zhou C, Dunn J, Taylor JA. Previsit screening for parental vaccine hesitancy: a cluster randomized trial. Pediatrics. 2019;144(5):e20190802. doi:10.1542/peds.2019-0802.
- Henrickson NB, Opel DJ, Grothaus L, Nelson J, Scrol A, Dunn J, Faubion T, Roberts M, Marcuse EK, Grossman DC, et al. Physician communication training and parental vaccine hesitancy: a randomized trial. Pediatrics. 2015;136(1):70–79. doi:10.1542/peds.2014-3199.
- Boyle J, Berman L, Nowak IR, Iachan R, Middleton D, Deng Y. An assessment of parents’ childhood immunization beliefs, intentions, and behaviors using a smartphone panel. Vaccine. 2020;38:2416–23. doi:10.1016/j.vaccine.2020.01.032.
- Wooten KG, Wortley PM, Singleton JA, Euler GL. Perceptions matter: beliefs about influenza vaccine and vaccination behavior among elderly white, black and Hispanic Americans. Vaccine. 2012;30:6927–34. doi:10.1016/j.vaccine.2012.08.036.
- Quinn S, Jamison A, Musa D, Hilyard K, Freimuth V. Exploring the continuum of vaccine hesitancy between African American and White adults: results of a qualitative study. PLoS. 2016;8:1–30.
- Quinn S, Jamison A, An J, Freimuth VS, Hancock GR, Musa D. Breaking down the monolith: understanding flu vaccine uptake among African Americans. SSM-Popul Health. 2018;4:25–36. doi:10.1016/j.ssmph.2017.11.003.
- Lindley MC, Wortley PM, Winston CA, Bardenheier BH. The role of attitudes in understanding disparities in adult influenza vaccination. Am J Prev Med. 2006;31(4):281–85. doi:10.1016/j.amepre.2006.06.025.
- Gramlich J, Funk C Pew research center. [accessed 2020 July 29]. https://www.pewresearch.org/fact-tank/2020/06/04/black-americans-face-higher-covid-19-risks-are-more-hesitant-to-trust-medical-scientists-get-vaccinated/