ABSTRACT
The tetravalent dengue vaccine (CYD-TDV) is approved for use as a 3-dose series for the prevention of dengue in seropositive individuals ≥9 years. A randomized, placebo-controlled, phase II study of a booster dose of CYD-TDV in individuals who completed the 3-dose schedule >5 years previously (NCT02824198), demonstrated that a booster restored neutralizing antibody titers to post-dose 3 levels. We present additional immunogenicity assessments up to 24 months post-booster, and B- and T-cell responses in a participant subset. Participants aged 9–45 years that had received all three doses of CYD-TDV were randomized 3:1 to receive a booster dose of CYD-TDV (n = 89) or placebo (n = 29). Neutralizing antibody levels at Months 1, 6, 12, and 24 post-booster were assessed by plaque reduction neutralization test. In a subset, B-cell responses were assessed by a fluorescent immunospot assay, and T-cells analyzed by flow cytometry at Days 0, 7, 12, Months 1 and 12. We observed an increase of antibody titers Month 1 post-booster, then a gradual decline to Month 24. In the CYD-TDV booster group, an increase in plasmablasts was seen at Day 7 declining by Day 14, an increase in memory B-cells was observed at Day 28 with no persistence at Month 12. CYD-TDV booster recalled a CD8+ T-cell response, dominated by IFN-γ secretion, which decreased 12 months post-booster. This study showed a short-term increase in antibody titers and then gradual decrease following CYD-TDV booster injection >5 years after primary immunization, and the presence of memory B-cells activated following the booster, but with low persistence.
Introduction
The live, attenuated, tetravalent dengue vaccine (CYD-TDV), based on the yellow fever (YF) 17D backbone, has been approved for the prevention of dengue in individuals 9 years or older in over 20 countries, with a schedule of 3 doses, 6 months apart. Only those who have had previous dengue infection (dengue seropositive) should be vaccinated with CYD-TDV due to an excess risk for hospitalized dengue in those who were dengue seronegative at vaccination.Citation1 This indication was based on results from a case-cohort study of three CYD-TDV efficacy studies showing that participants who were not exposed to dengue were at a higher risk of developing severe virologically confirmed dengue (VCD) and hospitalization from VCD following vaccination compared with those who had exposure to dengue before vaccination.Citation2 Following the 3-dose schedule in those aged ≥9 years and dengue seropositive at baseline, the vaccine efficacy against symptomatic dengue over 25 months was found to be 76% (95% CI 64–84).Citation2
There is a potential for the waning of antibody titers following the CYD-TDV vaccine.Citation3 Higher dengue neutralizing antibody levels have been shown to be associated with higher vaccine efficacy,Citation4 and one long-term study of dengue neutralizing antibodies following CYD-TDV vaccination found that they decreased over the 4 years following the third dose.Citation5 However, in another long-term follow-up study, levels of neutralizing antibodies decreased over 1 year following the third dose, but then stabilized at levels higher than baseline, which was maintained over the next 4 years.Citation6 In individuals in Singapore who had completed the 3-dose schedule of CYD-TDV >5 years previously, the use of a booster dose of CYD-TDV restored neutralizing antibody titers at 28 days post-booster to levels seen post-dose 3.Citation7 In a separate study conducted in Latin America, a booster CYD-TDV dose 4–5 years after the primary series increased neutralizing antibody titers to levels at least as high as post-dose 3.Citation8
Humoral immunity is sustained by the differentiation of B-cells into antibodies secreting cells (i.e. plasma cells). The course of an immune response can be divided into the “effector phase” which is associated with either naïve or memory B-cell recruitment and activation, and the “memory phase” associated with a transient germinal center reaction, and maintenance of memory B-cells and long-lived plasma cells for extended periods of time. Newly differentiated memory B-cells and long-lived plasma cells, which sustain protective antibody production, emerge for this dynamic structure. Recall of memory B-cells into an “effector phase” leads to a more specific, stronger, and faster response than naïve B-cell recruitment and activation.Citation9 Protective antibody levels can fall below a protective threshold, potentially due to a decrease in the frequency of long-lived plasma cells. In this scenario, re-activation of memory B cells and their differentiation into antibody-secreting cells upon booster vaccination could provide an accelerated humoral response and protection. Previous studies of cell-mediated immunity following CYD-TDV vaccination in adults and adolescents demonstrated a significant CD8+ T-cell responses against YF 17D, with the predominant secretion of IFN-γ, while natural dengue infection-induced CD4+ T-cell responses with secretion of TNF-α and IFN-γ secretion.Citation10,Citation11
This follow-up study of participants from a phase II study of a booster dose of CYD-TDV >5 years after the primary 3-dose vaccination assessed the persistence of the immune response for 2 years after the booster dose, with additional analyses of B- and T-cell responses conducted in a subset of participants.
Materials and methods
Study design and participants
This was a phase II, randomized, placebo-controlled study of a booster dose of CYD-TDV conducted in Singapore, and the design of the study has been described in full previously (CYD63; UTN: U1111-1161-2813, NCT02824198).Citation7 Briefly, participants were enrolled from the CYD28 study if they had received all three doses of CYD-TDV, were aged 9–45 years at first study injection and had sufficient baseline samples for additional analyses. Participants were randomized 3:1 to receive a booster dose of CYD-TDV or placebo at Day 0 of CYD63. The full CYD63 study period was July 2016 to January 2019, with primary vaccination in CYD28 from April 2009 to October 2010 and booster vaccination in CYD63 from July 2016 to February 2017.
The studies were undertaken in compliance with the International Conference on Harmonization (ICH) guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. The protocol and amendments were approved by applicable Independent Ethics Committees/Institutional Review Boards and the regulatory agency as per local regulations. Informed consent was obtained from the participants or their parents/legal guardians before any study procedures were performed.
Immunogenicity
Blood samples were obtained for immunogenicity assessment at visits on Day 0 prior to booster dose, and Day 28, Month 6, Month 12, and Month 24 post-booster; blood samples collected on Day 28 were used in an earlier published analysis, as part of the primary analysis of this study.Citation7 The primary endpoint for CYD63 has been presented previously.Citation7 The additional analyses reported here include the neutralizing antibody levels against each dengue serotype at Months 6, 12, and 24 after booster or placebo injection, as assessed by plaque reduction neutralization test (PRNT) which has been previously described in detail.Citation12 Dengue seropositivity was defined as antibody titers ≥10 (1/dil). Results were also stratified by participants’ dengue serostatus (seropositive or seronegative) before the first dose of CYD-TDV in CYD28.
B- and T-cell responses from the additional immunogenicity test (AIT) subset
A subset of 56 participants (42 in the CYD-TDV group and 14 in the placebo group; additional immunological tests [AIT] subset) were enrolled from two of the study sites and provided additional blood samples for assessment of B-cell and T-cell responses; these samples were obtained prior to booster dose at Day 0, and at Days 7, 14 and 28, and at Month 12 post-booster. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood collected in Lithium Heparin tubes (BD Vacutainer) by density centrifugation over lymphocyte separation medium (Eurobio). The PBMC band was transferred, washed in 0.9% NaCl and then suspended in fetal calf serum (FCS) (Hyclone). PBMCs were frozen in 90% FCS + 10% DMSO using a CoolCell freezing module (Proteigene) at – 70°C. Frozen PBMCs were transferred to liquid nitrogen storage within seven days for long-term storage and shipment to Sanofi Pasteur.
Memory B-cells secreting dengue specific-IgG antibodies were quantified using a fluorescent immunospot (FluoroSpot; Mabtech AB, Stockholm, Sweden), and specific polyfunctional T-cells (cell-mediated immunity [CMI]) were characterized by flow cytometry, at the Human Immunology Platform within the Research and External Innovation Department, Sanofi Pasteur, Marcy l’Etoile, France.
The B-cell response was assessed by two different protocols, either with in vitro pre-incubation for five days with a polyclonal stimulus, namely R848 and IL-2, to expand and activate the B-cells (memory B-cell FluoroSpot), or without pre-incubation for the enumeration of antigen-specific B-cells that have recently been activated in vivo (Plasmablast FluoroSpot). The memory B-cell frequency against each dengue serotype was measured at Day 0 prior to booster and Day 28 and Month 12 post-booster; the plasmablast frequency against each dengue serotype was measured at Day 0 prior to booster and Day 7 and Day 14 post-booster.
Briefly, 96-well plates were pre-treated with 35% ethanol before coating with 5 µg/mL of an anti-dengue antibody (3F11; in-house monoclonal antibody against the envelope of the four serotypes) or an anti-total IgG, and incubated overnight at +5°C. After washing and blocking, the four dengue serotypes (CYD1, CYD2, CYD3, and CYD4; 10 log GEeq/mL) were added to separate wells on each plate and incubated for 1 hour at ambient temperature. The plates were subsequently washed and serially diluted concentrations of either stimulated or non-stimulated PBMCs (B memory cells or plasmablasts, respectively) were added to the wells and incubated for 5 hours in a humidified incubator at +37°C with 5% CO2. Plates were then washed and kept in phosphate-buffered saline (PBS) overnight at +5°C. Plates were incubated for 2 hours at ambient temperature with a fluorochrome-labeled detection antibody anti-IgG 550 (clone MT78/145; Mabtech) diluted in PBS-FCS. The plates were then washed to remove excess fluorochrome-labeled antibody and dried at ambient temperature while protected from light, before counting spots with the FluoroSpot reader (ISpotRobot, AID, Strasburg, Germany).
T-cell CMI response was measured in samples at Day 0 prior to booster, and Day 28 and Month 12 post-booster by intracellular cytokine staining. PBMCs were incubated with YF 17D NS3 peptide pool (a total of 153 15-mer peptides with 11 amino acid overlap), and peptide pools for the envelope of each dengue serotype and dengue NS3, an antibody against the degranulation marker CD107a, and secretion inhibitors to allow the intracellular accumulation of cytokines in activated cells. Cells were then stained with surface markers CD3, CD4, CD8, CD45RA, and CCR7, and, once fixed, were stained with intracellular markers CD154, IFN-γ, TNF-α, IL-2, and MIP-1β and analyzed by flow cytometry, corrected by a negative control medium + DMSO solvent result; samples were acquired on LSR-II (BD Biosciences, San Jose, CA, USA) and data were analyzed using a computational-automated gating pipeline (this pipeline is based on a supervised Machine Learning approach using training datasets gated manually [Altrabio algorithm, www.altrabio.com]). Some unique approaches have been adopted to leverage data pre-processing, feature engineering, transfer learning and data augmentation to overcome the major challenges encountered in building predictive models for cytometry data automated gating. This approach has already been used for several different other academic or industrial studies.Citation13,Citation14 The assessed outcomes were the cytokine secreting CD4+ and CD8+ T-cell counts, T-cell subclass proportion among antigen-specific cells (positive for one or more of the functional markers CD154, IL-2, TNF-α, IFN-γ, CD107a, MIP-1β): (naïve [CD45RA+ and CCR7+], effector [CD45RA- and CCR7-], central [CD45RA- and CCR7+] and terminally differentiated [CD45RA+ and CCR7-] memory T-cells), and the proportion of polyfunctional CD4+ and CD8+ T-cells among antigen-specific cells (T-cells expressing at least one, two, three, or >three markers among: CD154, IL-2, TNF-α, IFN-γ, CD107a, MIP-1β).
Safety
The occurrence of serious adverse events (SAE) and cases of hospitalized dengue were recorded throughout the study follow-up period up to 24 months post-booster injection.
Statistical analyses
All the analyses in this study were descriptive. For categorical data results are presented as number and percentage of participants and 95% confidence intervals (CI), with the 95% CIs for proportions calculated using the exact binomial distribution (Clopper–Pearson method). For continuous data, geometric means and 95% CI were presented; assuming the log10 transformation of the geometric mean titer ratio (GMTR) followed a normal distribution, the mean and 95% CIs were calculated on a log10 scale; antilog transformations were applied to the results of calculations to compute geometric mean titers (GMTs), GMTRs and 95% CIs on their original scale.
Analyses of the differences in the memory B-cell responses were conducted by 2-way ANOVA model, with group and visit or group and age group and their interaction (dual interaction terms) as fixed effects, computed in a mixed model with repeated statement to account for the longitudinal aspect of the study (participant repetition). All data were log10-transformed prior to statistical analysis. P-values were corrected for multiple testing using the False Discovery Rate procedure (q-values are reported). For plasmablast responses a non-parametric Wilcoxon signed-rank test was used; p-values of Signed Rank were reported.
Analyses were performed with SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Immunogenicity analyses were conducted on the per-protocol analysis set (PPAS) which comprised those who received booster dose, had valid post-injection serology results and had no protocol deviations; participants were included according to the group they were randomized. Safety analyses were conducted in the safety analysis set (SafAS) which comprised all participants who received an injection; participants were included according to the vaccine they received.
Results
Study participants
The participants randomized into CYD63 have been described in detail previously.Citation7 All participants had received a primary 3-dose series of CYD-TDV five or more years prior to the booster or placebo injection in this study. Out of 269 eligible subjects, 118 subjects were randomized to the CYD-TDV booster (n = 89) and placebo group (n = 29). Overall, 100 (84.7%) participants completed the 24-month follow-up, 74 in the booster group and 26 in the placebo group (Table S1). A subset of 42 participants from the CYD-TDV booster group and 14 from the placebo group provided additional blood samples for evaluation of B- and T-cell responses.
Immunogenicity results
Following the previously reported increase in antibody titers at Day 28, there was a gradual decrease in the neutralizing antibody titers at Month 6, Month 12 and Month 24 post-injection for each of the dengue serotypes in the CYD-TDV booster group ().Citation7 For serotype 3, the GMTs (1/dil) increased from 22.4 pre-booster to 105 at Day 28 post-booster, then gradually decreased to 36.5 at Month 24 post-booster. For serotype 4, the GMTs increased from 28.0 pre-booster to 123 at Day 28 post-booster, then gradually decreased to 50.1 at Month 24 post-booster. Considering the CYD-TDV/placebo ratio, the GMTR between the booster and placebo groups showed a booster effect (lower bound of 95% CI >1) up to 24 months post-booster for serotypes 3 and 4, with ratios of 2.17 (95% CI 1.12, 4.19) and 2.05 (95% CI 1.19, 3.51), respectively, while the GMTRs for serotypes 1 and 2 at 24 months were 1.06 (95% CI 0.52, 2.16) and 1.92 (95% CI 0.77, 4.76), respectively.
Table 1. GMTs against each serotype during the 2-year follow-up in all participants regardless of baseline serostatus (PPAS)
Among the 75 subjects enrolled in the CYD-TVD booster group, 19 (25%) were seropositive at baseline, prior to first injection in CYD28, with 10/28 (36%) seropositive in the placebo group. When assessed by baseline serostatus, GMTs were higher at pre-booster injection in those who were seropositive at baseline ( and Table S2). At Month 24 post-booster, GMTs (1/dil) ranged from 71.7 (serotype 1) to 354 (serotype 2) in those who were seropositive at baseline and from 7.88 (serotype 1) to 39.1 (serotype 4) in those who were seronegative at baseline.
Figure 1. GMTs of neutralizing antibodies against (a) serotype 1, (b) serotype 2, (c) serotype 3 and (d) serotype 4 during the 24-month follow-up in all participants by baseline dengue serostatus before the first dose of CYD-TDV in CYD28 (PPAS)
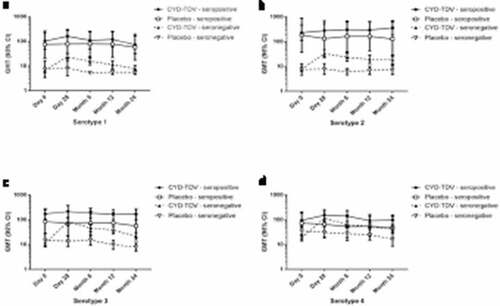
For all participants in the CYD-TDV booster group, the proportion of participants with seropositivity to each dengue serotype increased from Day 0 to Day 28, and then decreased until Month 24, but remained above Day 0 levels (Figure S1A–D). Seropositivity to each serotype in the placebo group remained relatively constant over the 2 years of follow-up.
B- and T-cell responses
Memory B-cell and T-cell analyses were conducted on frozen PBMC. The cell yield of around 106 cells per mL of blood collected was as expected, and the good cell viability (median around 96%) indicated the good quality of the cells and their ability to present good functionality in subsequent testing.
The results from the Additional Immunogenicity Test subset on the plasmablast and memory B-cell and T-cell outcomes could not be determined by baseline dengue serostatus as only 10 subjects out of the 56 recruited were dengue seropositive at baseline.
Plasmablast and memory B-cell results
The plasmablast and memory B-cell FluoroSpot assay could not determine specificity against each dengue serotype. The CYD-specific IgG cross-reactivity between serotypes was too strong and prevented the detection of a dominant serotype.
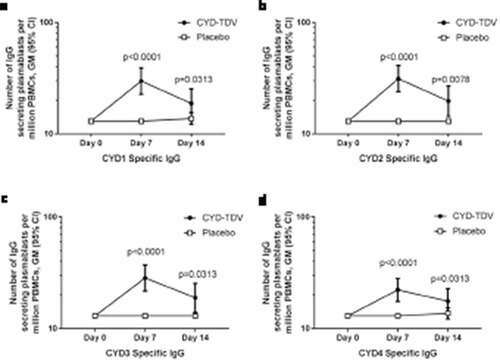
Pre-booster dose, no IgG secreting plasmablasts were detected in either the booster or placebo group. Seven days after the booster dose a significant increase in CYD-TDV-specific plasmablasts was seen in the booster group, and was observed for all four dengue serotypes (; p < .0001). This response declined by 14 days after booster injection but remained above pre-booster levels, with significant differences still seen compared to baseline (CYD1 p = .0313, CYD2 p = .0078, CYD3 p = .0313, CYD4 p = .0313). While there was an increase in dengue-specific plasmablasts in the booster group, this response was not seen in all participants; for example, for CYD1 at Day 7 a response was detected in only 23/41 (56%) of participants in the booster group, and this response was generally low with a GM of 57 × 106 PBMCs/mL.
Figure 2. Geometric means of the number of IgG secreting plasmablasts per million PBMCs specific to (a) CYD1, (b) CYD2, (c) CYD3, and (d) CYD4 by FluoroSpot (AIT subset)
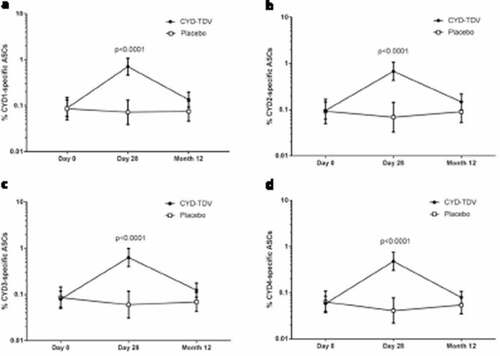
Similarly, pre-booster dose, there was a very low IgG memory B-cell response in both groups, and a significant increase in CYD-specific memory B-cells was demonstrated 28 days after the booster dose in the CYD-TDV group, which was observed for all four serotypes (; p < .0001). The proportion of CYD-specific memory B cells was significantly higher in the vaccine group compared to the placebo group for all serotypes 28 days after booster dose (p < .0001). There was no persistence of vaccine-induced memory B-cells 12 months after the booster dose. The number of memory B-cells at Day 28 post-booster was significantly higher in participants aged 18–45 years than in those aged 9–17 years (Figure S2A–D; p < .0001 for CYD3 and CYD4, and p = .0001 for CYD1 and CYD2). There were five baseline seropositive participants in the 18–45 years age group and one in the 9–17 year age group; if we exclude the seropositive participants from this analysis, there remains a statistically significant difference between age groups at Day 28 (p = .0002 for CYD1 and p < .0001 for CYD2, CYD3, and CYD4).
Figure 3. Percentage of IgG secreting memory B-cells specific to (a) CYD1, (b) CYD2, (c) CYD3, and (d) CYD4 out of total IgG secreting memory B-cells by FluoroSpot (AIT subset)
T-cell results
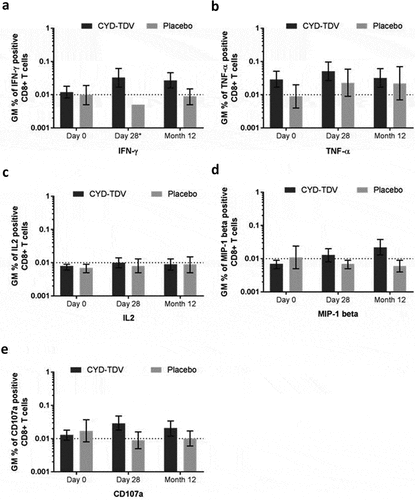
Booster vaccination recalled a YF 17D NS3-specific CD8+ T-cell response, dominated by the secretion of IFN-γ, this response decreased 12 months after booster (). There was no notable change in secretion of TNF-α (), IL-2 (), or MIP-1 β () after CYD-TDV booster. The CD107a expression on CD8+ T-cells correlated with the IFN-γ response, suggesting an activation of cytotoxic CD8+ T-cells induced by YF 17D NS3 stimulation (). There was no CD4+ T-cell response detected (Figure S3A–F). We did not detect any response against the envelope peptide pools (data not shown).
Figure 4. Geometric means of percentage of CD8+ T-cells positive for (a) IFN-γ, (b) TNF-α, (c) IL2, (d) MIP-1 β, and (e) CD107a by intracellular cytokine staining after YF 17D NS3 stimulation (AIT subset)
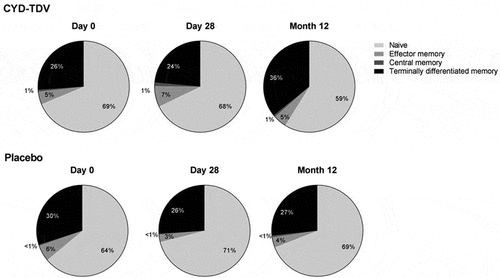
The activated YF 17D NS3-specific CD8+ T-cells had a polyfunctional profile, with the booster dose increasing the proportion of double, triple, and >triple positive CD8+ T-cells (). The analysis of the activated YF 17D NS3-specific CD8+ T-cell subpopulations showed that the booster dose induced a predominantly CD8+ T effector memory cell (CD45RA- and CCR7–) response after 28 days and a terminally differentiated memory cell (CD45RA+ and CCR7–) response after 12 months ().
Figure 5. Proportion of simple, double, triple and >triple positive CD8+ T-cells stimulated by YF 17D NS3 peptide pool at Day 0, Day 28 and Month 12, shown as the proportion of the geometric means (AIT subset)
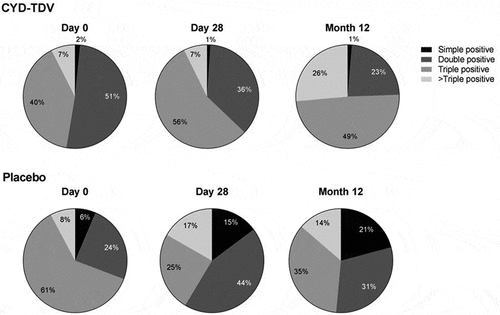
Figure 6. Memory subpopulations of activated CD8+ T-cells* stimulated by YF 17D NS3 at Day 0, Day 28 and Month 12, shown as the proportion of the geometric means (AIT subset)
Safety
There were five SAEs over the study period in the CYD-TDV booster group, none of them were related to the study vaccine (). One SAE occurred within 28 days of the booster dose; a deep cut following a road traffic accident. In those who experienced SAEs between 28 days and 24 months after booster, the SAEs were: hospitalization due to influenza, hospitalization due to abdominal pain, hospitalization due to chronic pelvic pain, and hospitalization due to pre-eclampsia. There were no SAEs in the placebo group. There were no deaths or hospitalized dengue cases in either group over the duration of the study.
Table 2. Safety overview for the 24-month follow-up (SafAS)
Discussion
Following a booster dose of CYD-TDV more than 5 years after the 3-dose primary immunization, antibody titers for each dengue serotype increased at Day 28,Citation7 and then gradually declined over the 24 months of follow-up. Pre-booster antibody titers were higher in those who were seropositive at baseline (before the first dose in the primary study CYD28), than in those who were seronegative. In both seropositive and seronegative participants, the same pattern of an initial increase in titers after booster dose followed by a decline over 2 years was observed. Decrease in antibody titers following the initial increase was more apparent in those who were seronegative while the antibody titers remained relatively higher in those who were seropositive. This suggests a potential value of the booster for management of outbreak situations in both areas of low and high dengue endemicity, but especially in the latter. For all participants, seropositivity rates to each dengue serotype remained higher than the pre-booster levels, ranging from 38% for serotype 1 to 89% for serotype 4 at 24 months post-booster.
A decline in immunogenicity had also been previously documented after the primary CYD-TDV vaccination series in different populations.Citation3,Citation5,Citation6 The similarly designed CYD64 study assessed a booster dose of CYD-TDV 4–5 years after primary vaccination in five countries in Latin America where most of subjects were baseline dengue seropositive; approximately 72% in the CYD-TVD group and approximately 67% in the placebo group. The CYD64 study demonstrated an increase, followed by a decline, in neutralizing antibody titers after a booster dose of CYD-TDV,Citation15 similar to that seen in this study. Compared to this study where only 25% in the CYD-TDV group and 36% in the placebo group were seropositive at baseline, CYD64 found greater antibody titers at both pre-booster and post-booster injection. The increases of immune response post booster were clearer in CYD64, but the decline also seems to have been more marked (although this was likely to simply be a reflection of the relatively greater increase).Citation15 Despite the decline observed in the CYD64 study, neutralizing antibody titers remained at high levels post-booster in participants who were seropositive at baseline (442, 375, 511, and 171 1/dil for serotypes 1, 2, 3, and 4, respectively, at Month 24 post-booster dose). Although differences in baseline dengue seropositivity likely explain the different results observed in the CYD63 and CYD64 studies, we cannot rule out the impact of the presence of Zika virus in Brazil at the time of the CYD64 study, which could potentially have caused boosting of the dengue GMTs through cross-reactivity, which was not the case in Singapore where CYD63 was conducted.Citation15
Memory B-cells and long-lived plasma cells play a major role in the long-term humoral immunity elicited by most vaccines and it is, therefore, important to be able to track memory B-cells as an independent parameter of antigen-specific immune memory. In this study, an expansion of plasmablasts was only seen in about 50% of the CYD-TDV booster group, peaking at Day 7. This expansion would support the presence of a memory B-cell reservoir linked to the primary vaccination, but the frequency of this population was low with an increase of approximately 3-fold compared to the baseline. These observations, with a lack of response in almost 50% of the samples, could be partially explained by technical issues, e.g. the PBMCs used in these tests were frozen, and all steps around cell manipulation like shipment, travel, defrosting could have affected circulating plasmablasts which are a very fragile cell population.Citation16 Additionally, assessing the function of these plasmablasts at Day 7 may have been late, during the decline of the plasmablast burst,Citation17 when they are even more fragile.
More than 5 years after 3-dose primary CYD-TDV vaccination, a low memory B-cell frequency was still detected, which allowed the CYD-TDV booster to elicit a memory B-cell response in the vaccine group at 28 days post-booster. The memory B-cell response decreased after 1 year in both groups, showing a low persistence of the vaccine-induced response. A similar pattern of memory B-cell response was seen in response to CYD-TDV booster in the CYD64 study.Citation15 In our study, a greater memory B-cell response was observed for those aged 18–45 years than adolescents aged 9–17 years, this is not due to the unequal distribution of seropositive participants in the Additional Immunogenicity Test subset. In fact, in the Additional Immunogenicity Test subset we only had six baseline seropositive participants; five in the 18–45 year age group and only one in 9–17 year age group. However, if we exclude the seropositive participants from the analysis, we still see a difference statistically significant at Day 28 between age groups. The difference between age groups is largely described by the B-cell pool changes over a lifespan, including the ones against dengue.Citation18,Citation19 In early life, the B-cell pool contains a large number of naïve B-cells of diverse specificity and a small number of memory B-cell clones; with age, the production of naïve B-cells decreases, contributing to the decrease in capacity to respond to new antigens, and memory B-cell accumulate with limited specificities.Citation18 Nevertheless, this difference at Day 28 after booster was not the precursor of a more marked persistence of response, as there was no difference between age groups at Month 12.
In this trial, as observed in previous trials, the YF 17D NS3 response was seen exclusively in the CD8+ T-cell population.Citation11,Citation15,Citation20 As no cross-reactions exist at the backbone level for CD8+ or CD4+ responses between YF 17D NS3 and DEN NS3,Citation10 the CD8+ IFN-γ response measured at Day 28 post-booster in the vaccine group is a response induced by CYD-TDV booster. Although the quantified parameters were weak, the profile of induced response was similar to what we have shown before in response to the YF 17D NS3 peptide pool stimulation: an IFN-γ/TNF-α profile in favor of IFN-γ only in the CD8+ population.Citation11 This IFN-γ response correlated with the CD107a expression, suggesting an activation of cytotoxic CD8+ T-cells. A focus on the polyfunctional cells in this study highlighted that the booster vaccination increased the proportion of double, triple, and >triple positive CD8+ cells, and this polyfunctionality has been shown to be a correlate of T-cell efficacy.Citation21,Citation22 The memory response measured in CD8+ cells in response to the YF 17D NS3 peptide pool was mainly driven by effector memory T-cells 28 days after booster vaccination, and terminally differentiated memory cells 1 year after the booster.
Together the humoral and cellular immunogenicity findings from this study illustrate a short-lived response following a booster dose of CYD-TDV in the population of a country where dengue circulates, but where levels of endemicity are relatively low. The rapid recall of memory B-cells suggests that a booster dose of CYD-TDV may be valuable in response to localized outbreaks of dengue to optimize protection of the population. However, the changes in the levels of neutralizing antibodies in those who were seropositive for dengue before the first vaccine dose was small and short-lived, and therefore the potential benefit of a booster dose might be limited, but difficult to predict due to a relatively small number of participants who were seropositive at baseline in this study. A further consideration is the role of natural boosting of the immune system of those living in dengue endemic areas, and the potential benefits of this may be bigger in a highly endemic population.
There were a number of limitations to this study. Few participants of CYD28 were eligible for inclusion into CYD63, and, as a result, CYD63 was smaller than planned. The assessment of B- and T-cell response was also only conducted in a small subset of participants and may not be representative of a larger population. This study was also only conducted in a single country in South East Asia where dengue endemicity is relatively lower compared to other countries in the region, so may not be applicable to other regions.
In conclusion, this study showed that a booster dose of CYD-TDV more than 5 years after the primary vaccination increased neutralizing antibody levels to post-dose 3 levels, which then declined over the following two years. Evidence from the analyses in the Additional Immunity Test subset indicated there is a presence of memory B-cells in peripheral blood, that are activated following booster dose but show limited persistence. This study highlights the importance of combining the serological, and B- and T-cell-mediated analysis to evaluate and better understand the immunogenicity and the effects of a dengue vaccine booster.
Disclosure of potential conflicts of interest
JP, HW, MB, CF, AP, FJ-B, SB and SG-F are employees of Sanofi Pasteur. AB was an employee of Sanofi Pasteur at the time this study was conducted. SA has no conflict of interest to declare. M-LHO has no conflict of interest to declare. LPS was the study principal investigator. She received no direct payment from the study sponsor for her contributions.
Author contributions
JP was involved in the study concept and design, SA, M-LHO, LPS, AP, FJ-B, SB, and SG-F were involved in data acquisition, all authors were involved in data analysis or interpretation, critical revision of the manuscript, and final approval of the manuscript for submission.
Data sharing statement
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi Pasteur’s data-sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com
Supplemental Material
Download MS Word (591 KB)Acknowledgments
The authors wish to thank the participants and their parents, the investigators, coordinators, and study teams. Editorial assistance with the preparation of the manuscript was provided by Nicola Truss PhD, inScience Communications, Springer Healthcare Ltd, London, UK. Funding for this assistance was provided by Sanofi Pasteur. The authors would like to thank Pascal Blanc, Daniel Larocque for scientific advice, Joseline Ruiz for statistical analysis, and Jean-Sébastien Persico for editorial assistance and manuscript coordination on behalf of Sanofi Pasteur.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- World Health Organization. Dengue vaccine: WHO position paper – september 2018. Wkly Epidemiol Rec 2018;36:457–76.
- Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, Savarino S, Zambrano B, Moureau A, Khromava A, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018;379(4):327–40. doi:10.1056/NEJMoa1800820.
- Vigne C, Dupuy M, Richetin A, Guy B, Jackson N, Bonaparte M, Hu B, Saville M, Chansinghakul D, Noriega F, et al. Integrated immunogenicity analysis of a tetravalent dengue vaccine up to 4 y after vaccination. Hum Vaccin Immunother. 2017;13(9):2004–16. doi:10.1080/21645515.2017.1333211.
- Moodie Z, Juraska M, Huang Y, Zhuang Y, Fong Y, Carpp LN, Self SG, Chambonneau L, Small R, Jackson N, et al. Neutralizing antibody correlates analysis of tetravalent dengue vaccine efficacy trials in Asia and Latin America. J Infect Dis. 2018;217(5):742–53. doi:10.1093/infdis/jix609.
- Tran NH, Chansinghakul D, Chong CY, Low CY, Shek LP, Luong CQ, Frago C, Wartel TA, Sun S, Skipetrova A, et al. Long-term immunogenicity and safety of tetravalent dengue vaccine (CYD-TDV) in healthy populations in Singapore and Vietnam: 4-year follow-up of randomized, controlled, phase II trials. Hum Vaccin Immunother. 2019;15(10):2315–27. doi:10.1080/21645515.2019.1578595.
- Capeding MR, Laot TM, Boaz M, Wartel TA, Crevat D. Immunogenicity and safety of a tetravalent dengue vaccine during a five-year follow-up period. Trials Vaccinol. 2015;4:19–23. doi:10.1016/j.trivac.2015.03.002.
- Park J, Archuleta S, Oh M-LH, Shek LP-C, Jin J, Bonaparte M, Frago C, Bouckenooghe A. Immunogenicity and safety of a dengue vaccine given as a booster in Singapore: a randomized Phase II, placebo-controlled trial evaluating its effects 5–6 years after completion of the primary series. Hum Vaccin Immunother. 2020;16:523–29. doi:10.1080/21645515.2019.1661204.
- Coronel D, Garcia-Rivera EJ, Rivera M, Arredondo-Garcia JL, Dietze R, Perroud AP, Cortés M, Bonaparte M, Zhao J, Tila M, et al. Dengue vaccine booster in healthy adolescents and adults in Latin America: evaluation 4–5 years after a primary 3-dose schedule. Pediatr Infect Dis J. 2019;38(5):e90–e5. doi:10.1097/INF.0000000000002286.
- Palm A-KE, Henry C. Remembrance of things past: long-term B cell memory after infection and vaccination. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.01787.
- Guy B, Nougarede N, Begue S, Sanchez V, Souag N, Carre M, Chambonneau L, Morrisson DN, Shaw D, Qiao M, et al. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine. 2008;26(45):5712–21. doi:10.1016/j.vaccine.2008.08.019.
- Harenberg A, Begue S, Mamessier A, Gimenez-Fourage S, Ching Seah C, Wei Liang A, Li Ng J, Yun Toh X, Archuleta S, Wilder-Smith A, et al. Persistence of Th1/Tc1 responses one year after tetravalent dengue vaccination in adults and adolescents in Singapore. Hum Vaccin Immunother. 2013;9(11):2317–25. doi:10.4161/hv.25562.
- Timiryasova TM, Bonaparte MI, Luo P, Zedar R, Hu BT, Hildreth SW. Optimization and validation of a plaque reduction neutralization test for the detection of neutralizing antibodies to four serotypes of dengue virus used in support of dengue vaccine development. Am J Trop Med Hyg. 2013;88:962–70. doi:10.4269/ajtmh.12-0461.
- Lee H, Sun Y, Patti-Diaz L, Hedrick M, Ehrhardt AG. High-throughput analysis of clinical flow cytometry data by automated gating. Bioinform Biol Insights. 2019;13:1177932219838851. doi:10.1177/1177932219838851.
- Le Lann L, Jouve P-E,Alarcón-Riquelme M, Jamin C, Pers J-O, Alvarez-Errico D, Azevedo N, Barbarroja N, Buttgereit A, et al. Standardization procedure for flow cytometry data harmonization in prospective multicenter studies. Sci Rep. 2020;10(1):11567. doi:10.1038/s41598-020-68468-3.
- Coronel D, García-Rivera E, Rivera D, Arredondo-García J, Dietze R, Perroud A, Cortés M, Bonaparte M, Wang H, Pagnon A, et al. Immune response persistence and safety of a booster dose of tetravalent dengue vaccine in adolescents and adults who previously completed the three-dose schedule 4–5 years earlier in latin america: a randomized placebo-controlled trial. Pediatr Infect Dis J. 2020; 39(10): 961–968 doi:10.1097/INF.0000000000002830.
- Khodadadi L, Cheng Q, Radbruch A, Hiepe F. The Maintenance of Memory Plasma Cells. Front Immunol. 2019;10:721. doi:10.3389/fimmu.2019.00721
- Fink K. Origin and Function of Circulating Plasmablasts during Acute Viral Infections. Front Immunol. 2012;3:78. doi:10.3389/fimmu.2012.00078
- Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–94. doi:10.1038/nri2508.
- Appanna R, Kg S, Xu MH, Toh Y-X, Velumani S, Carbajo D, Lee CY, Zuest R, Balakrishnan T, Xu W, et al. Plasmablasts during acute dengue infection represent a small subset of a broader virus-specific memory B cell pool. EBioMedicine. 2016;12:178–88. doi:10.1016/j.ebiom.2016.09.003.
- Dayan GH, Galán-Herrera JF, Forrat R, Zambrano B, Bouckenooghe A, Harenberg A, Guy B, Lang J. Assessment of bivalent and tetravalent dengue vaccine formulations in flavivirus-naïve adults in Mexico. Hum Vaccin Immunother. 2014;10:2853–63. doi:10.4161/21645515.2014.972131.
- Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–28. doi:10.1038/nm.f.1774.
- Boyd A, Almeida JR, Darrah PA, Sauce D, Seder RA, Appay V, Gorochov G, Larsen M. Pathogen-specific T cell polyfunctionality is a correlate of T cell efficacy and immune protection. PloS One. 2015;10:e0128714. doi:10.1371/journal.pone.0128714.
