ABSTRACT
In June 2019, the Advisory Committee on Immunization Practices (ACIP) changed the recommendation for routine 13-valent pneumococcal conjugate vaccine (PCV13) use in immunocompetent adults aged ≥65 years, including those with select chronic medical conditions (CMC). ACIP now recommends PCV13 for this group of adults based on shared clinical decision-making. Because adults with CMC continue to be at increased risk for pneumococcal disease, we assessed the cost-effectiveness of administering PCV13 in series with the recommended 23-valent pneumococcal polysaccharide vaccine (PPSV23) for adults aged ≥19 years with CMC.
We used a probabilistic model following a cohort of 19-year-old adults. We used Monte Carlo simulation to estimate the impact on program, medical, and non-medical costs (in 2017 U.S. dollars [$], societal perspective), and pneumococcal disease burden when administering PCV13 in series with PPSV23. We used PCV13 efficacy and post-licensure vaccine effectiveness (VE) data to estimate VE against PCV13 type disease (separately for disease by serotype 3 [ST3], the most common PCV13 type, and all other PCV13 serotypes). We considered a range of estimates for sensitivity analyses. Analyses were performed in 2019.
In the base case, assuming no PCV13 effectiveness against ST3 disease, adding a dose of PCV13 upon CMC diagnosis cost $689,299 per QALY gained. This declined to $79,416 per QALY if VE against ST3 was estimated to be equivalent to other PCV13-types.
Administering PCV13 in series with the recommended PPSV23 for adults with CMC was not cost saving. Results were sensitive to estimated PCV13 VE against ST3 disease.
Introduction
In the United States, approximately 30,000 cases of invasive pneumococcal disease (IPD) such as pneumococcal meningitis and bacteremia occur annually.Citation1 People who are at the extremes of age (<2 years, ≥65 years)Citation2 and those with select underlying medical conditions are at increased risk of pneumococcal disease.Citation3 Introduction of pneumococcal conjugate vaccines into the routine infant immunization program in the United States led to substantial decline in IPD caused by vaccine serotypes, not only among vaccinated children, but also in unvaccinated older individuals through indirect effects.Citation4,Citation5 Since 2012, U.S. adults aged ≥19 years with immunocompromising conditions, cerebrospinal fluid leaks, or cochlear implants have been recommended to receive 13-valent pneumococcal conjugate vaccine (PCV13) in series with 23-valent polysaccharide vaccine (PPSV23).Citation6 A previous cost-effectiveness analysis showed that such intervention would be cost saving.Citation7 Adults aged ≥19 years with chronic medical conditions (CMC), such as chronic heart, lung, liver disease, diabetes mellitus, alcoholism, and cigarette smoking, are recommended to receive PPSV23 only. In 2014, PCV13 was recommended in series with PPSV23 for adults aged ≥65 years (including those with CMC).Citation8 In June 2019, the Advisory Committee on Immunization Practices (ACIP) voted to remove the recommendation for routine PCV13 use in immunocompetent adults aged ≥65 years in light of additional years of data showing the impact of herd effects from childhood immunization; a cost-effectiveness analysis showed that continuing the recommendation in a setting of observed to date indirect effects from PCV13 use in children would cost 561,682 USD per quality-adjusted life year (QALY) gained, compared to 62,065 USD per QALY estimated in 2013 due to declines in remaining vaccine-preventable disease burden in adults.Citation9 However, ACIP recognized that some adults aged ≥65 years who are at increased risk for exposure to PCV13 serotypes or at increased risk for pneumococcal disease as a result of underlying medical conditions may benefit from PCV13 vaccination, and recommended PCV13 use based on shared clinical decision making.Citation10 Adults with underlying conditions continue to be at increased risk for pneumococcal disease: a recent study reported that adults with CMC can have 2.3–15.4 times the incidence of IPD compared to adults without indication for pneumococcal vaccination.Citation3 ACIP does not currently recommend PCV13 to adults with CMC, and PPSV23 alone is recommended for routine use in adults aged ≥19 years with CMC. Thus, we sought to evaluate the cost-effectiveness of implementing PCV13 in series with PPSV23 for all adults aged ≥19 years with CMC.
Methods
Model
In 2019, we developed a probabilistic model to estimate the public health impact and the cost-effectiveness of administering a dose of PCV13 at the diagnosis of CMC (chronic heart disease, chronic lung disease, diabetes, alcohol abuse, or chronic liver disease) to a hypothetical cohort of 19 year old adults in the United States. We used Monte Carlo simulation via spreadsheet-based softwareCitation11 to estimate the impact of administering PCV13 in series with PPSV23 on program costs, medical costs, non-medical costs, and disease burden.
We tracked new disease incidence and deaths due to IPD and non-bacteremic pneumococcal pneumonia (NBPP) by single year of age through life expectancy or until age 100 years (Figure S1). We assumed that IPD results in hospitalization or death, and NBPP results in either outpatient visit, hospitalization, or death. We estimated hospitalizations due to IPD as well as inpatient and outpatient encounters due to NBPP by age group (age 19–64 years, ≥65 years). We modeled the effects of replacing the currently recommended schedule of administering PPSV23 alone at CMC diagnosis with a schedule of administering PCV13 at CMC diagnosis followed by PPSV23 a year later. Adults with CMC receive only one lifetime dose of PCV13, but if PPSV23 was administered before age 65 years, a second dose is administered at age 65 years or later so that at least 5 years has lapsed since the last PPSV23 dose.Citation12 Adults who developed CMC after age 65 years would receive PPSV23 at age 65 years and a dose of PCV13 at the time of CMC diagnosis. We used the societal perspective for this analysis.
Study population
We used a cohort of 4,256,608 19-year-olds in 2017 in the United States. We stratified the population by single year of age and followed the population from age 19 through life expectancy or until age 100 years.Citation13 We applied age-based incidence rates for individual CMC obtained from a variety of sources to the study cohort to estimate the size of CMC population by age. Incidence rates for adults with chronic heart disease and chronic liver disease were estimated from prevalence rates from the National Health and Nutrition Examination Survey,Citation14,Citation15 and incidence rates for chronic lung disease, diabetes, and alcohol abuse were obtained or estimated from Centers for Disease Control and Prevention (CDC) surveillance systems.Citation16,Citation17
Model parameters
Disease burden estimates, vaccine effectiveness and coverage, and cost per case used as model inputs are summarized in . For multivariate sensitivity analyses, we draw from lognormal distributions for cost parameters, pert distributions for vaccine coverage and effectiveness parameters, and normal distributions for all other model inputs.
Table 1. Model input parameters for U.S. adults with chronic medical conditions, by age group
Disease parameters
Pneumococcal disease was classified by serotype (ST) groups based on available information on direct PCV13 or PPSV23 effects among adults or indirect effects on adult disease from the childhood PCV13 program. Due to lack of further population-level impact of PCV13 on disease caused by ST3 and 19 F,Citation32–35 ST 3 and 19 F were tracked separately from other PCV13 types (1, 4, 5, 6A, 6B, 7 F, 9 V, 14, 18 C, 19A, and 23 F) for indirect effects, and ST 3 was tracked separately for direct effects (see “Vaccine effectiveness and coverage” below). ST6C was included as part of PCV13 types given the cross-reaction with ST6A.Citation36,Citation37 STs unique to PPSV23 included the 11 STs contained in PPSV23 but not in PCV13 (ST2, 8, 9 N, 10A, 11A, 12 F, 15B, 17 F, 20, 22 F, 33 F). In 2016–2017, the proportion of ST3 IPD alone constituted 14.7% of all IPD in adults aged 19–64 years with CMC, and 16.2% in adults aged ≥65 years with CMC ().
The number of ST-group-specific IPD cases among adults 19–64 years old and ≥65 years old with any of the CMC conditions were obtained from CDC’s Active Bacterial Core surveillance (ABCs) during 2016–2017 (CDC unpublished data). To calculate IPD incidence, we used ABCs case counts as numerators and age-group specific population estimates for persons with CMC obtained from the 2016–2017 National Health Interview Survey (NHIS) as denominators.Citation38 Case fatality ratios were also obtained from ABCs by age group ().
For NBPP, we first obtained age-group specific incidence rates for all-cause pneumonia (including healthy, with CMC, and immunocompromised) from literature.Citation18–20,Citation22 We then estimated age-group-specific all-cause pneumonia incidence for adults with CMC using incidence ratios comparing all-cause pneumonia rates among adults with CMC to that among healthy adults from the literature,Citation21 and corresponding population denominators from NHIS. We used data from a multi-site observational study of adults hospitalized with pneumonia to estimate the proportion of all-cause pneumonia due to PCV13 ST.Citation23 The proportion of all-cause pneumonia caused by ST unique to PPSV23, ST 3 and 19 F, was estimated by applying the ST distribution for IPD based on ABCs data. Case fatality ratios for inpatient NBPP were obtained from the 2014 National Inpatient Sample.Citation39 We assumed that there were no deaths associated with outpatient NBPP.
We assumed that PCV13 type disease (including ST6C, excluding ST3 and ST19F) had an annual disease reduction of 4.1% through indirect effects from the childhood PCV13 program.Citation32 We assumed that there were no further declines of pneumococcal diseases caused by ST3 and ST19F through indirect effects. Given that pneumococcal carriage in children is considered to be the main source of transmission of pneumococci to adults,Citation40 and given the low vaccine-type pneumococcal carriage in adults post PCV introduction in children,Citation41–43 we assumed that vaccinating adults with PCV13 would not result in additional indirect effects. In addition, serotype replacement has not been observed to date in the United States post PCV13-introduction in children.Citation44,Citation45 Therefore, we did not consider serotype replacement in our model.
Vaccine effectiveness and coverage
Vaccine effectiveness (VE) was assumed to be higher for adults with CMC aged 19–64 years compared to adults aged ≥65 years. For adults with CMC aged ≥65 years, the VE estimate of PCV13 against PCV13-type (including ST6C, excluding ST3) IPD was based on the results of a U.S. case-control study.Citation25 VE estimate of PCV13 against PCV13-type NBPP came from a post-hoc analysis of the Community-Acquired Pneumonia Immunization Trial in Adults (CAPITA).Citation26 Given that no impact of PCV13 on ST3 IPD was observed at the population level since PCV13 introduction for adults in 2014Citation18 and PCV13 was not effective against ST3 IPD in a U.S. post-licensure study,Citation25 we assumed zero as the point estimate and lower bound for PCV13 VE against both ST3 IPD and NBPP. For VE estimate of PPSV23 against IPD, we assumed the base case to be the same as PCV13, and used the point estimates of a meta-analysis for the upper and lower range.Citation27 Our base case assumed that PPSV23 was not effective against NBPP based on results of several meta-analyses ().Citation27–29 For adults aged 19–64 years with CMC, VE estimates of PCV13 against PCV13-type IPD and NBPP were based on the CAPITA data.Citation24 PPSV23 VE against PPSV23-type IPD was obtained from a meta-analysis.Citation27
Given the uncertainties around the estimates for PCV13 VE against ST3 pneumococcal disease and a range of estimates obtained from different post-licensure studies, we performed one-way sensitivity analyses where PCV13 VE against ST3 disease was estimated to be as high as VE against all-PCV13-type disease (75% for IPD and 45% for NBPP for 19–64 year olds,Citation24 67% for IPD and 32.5% for adults aged ≥65 yearsCitation26).
We assumed no waning of immunity for 5 years after PCV13 receipt;Citation46 we assumed that the VE waned by 10% every 5 years thereafter until age 70. We assumed linear declines within 5-year increments. From age 70 to 85, we assumed a linear decline from the remaining VE to zero.Citation47 Because VE was assumed to be zero for those aged ≥85 years, we assumed that adults who develop CMC at age ≥85 years will not receive PCV13. Consistent with previous analyses,Citation9 we assumed that after receipt of PPSV23, there was a linear decline to 50% from the original VE by year 5, then to 30% by year 10, then to 0% by year 15. If the patient is revaccinated at or after age 65 years (5 years after the last PPSV23 vaccination), then we assumed that the full VE (67%) against PPSV23-type IPD was achieved in year 1 and followed the same waning pattern over the next 15 years. Regardless of the order of sequence in which PCV13 and PPSV23 were given, we applied the VE estimate that was higher for STs that were common between PCV13 and PPSV23.
We obtained PPSV23 coverage estimates from 2017 NHIS data for adults aged 19–64 years at increased risk for pneumococcal diseaseCitation30 and 2014 NHIS data for adults aged ≥65 years. We used 2014 coverage estimates for older adults since 2015–2017 NHIS does not distinguish between PCV13 and PPSV23 administration.Citation31 For adults aged 19–64 years, we assumed that PCV13 coverage will be as high as PPSV23 coverage. For adults aged ≥65 years, we used coverage data from Medical Claims as of June 2017 for lower bound,Citation48 and IQVIA claims data as of September 2017 for the upper bound.Citation49,Citation50 We used the midpoint of these estimates as the point estimate for PCV13 coverage.
Health utility and medical and non-medical costs
We applied the same assumptions for health utility and medical and non-medical costs as in our previous study.Citation9 Briefly, we used QALY decrements to account for differences in disease severity across pneumococcal disease (e.g., IPD, NBPP, disease treated as inpatient and outpatient). We used 0.00865 for IPD, 0.006 for inpatient NBPP, and 0.004 for outpatient NBPP.Citation51 Each of these QALY decrements were scaled by age-specific baseline QALY values for adults with CMC ranging from 0.51 for those aged ≥86 years to 0.72 for adults aged 19 years. Cost per episode of pneumococcal disease was calculated from Centers for Medicare and Medicaid (CMS) Medicare claims from 2000 to 2015 (). We used 95% of the average wholesale price for PCV13 ($192.64) and PPSV23 ($98.85) as reported by Medicare Part B (December 2017) (). We used 26.61 USD for the cost of vaccine administration, estimated from the Medicare reimbursement for immunization administration (HCPCS code 90471) in 2017 averaged across all Medicare Administrative Contractors. We estimated patient time and travel cost at 30.12 USD.Citation52 We inflated costs to 2017$ using the Personal Consumption Expenditure deflator for health accounts for medical costs and the Consumer Price Index for non-medical costs,Citation53 and discounted all future outcomes and costs by 3% per year.
Multivariate sensitivity analysis
In addition to the one-way sensitivity analyses mentioned above, we conducted multivariate sensitivity analysis using ranges for model inputs indicated in using previously described methods.Citation9
Results
Under base-case assumptions (), adding a dose of PCV13 for adults with CMC resulted in 54 fewer IPD cases, 319 fewer hospitalized NBPP cases, 565 fewer non-hospitalized NBPP cases, 4 fewer IPD deaths, and 10 fewer NBPP deaths over a lifetime of a single cohort (). This is predicted to save a total of 174 discounted QALY in this cohort over the entire follow-up period with a total discounted cost of 120 USD million, resulting in a cost of 689,299 USD per QALY (5th percentile: 165,232 USD per QALY; 95th percentile: 686,856 USD per QALY). In the one-way sensitivity analyses, the cost per QALY declined to 79,416 USD when we assumed that PCV13 VE against ST3 disease was equivalent to the VE estimates for other PCV13 STs. The decline was more substantial if we assumed higher VE estimates against ST3 NBPP ($93,184 per QALY gained) compared to ST3 IPD ($431,419 per QALY gained) ().
Table 2. Impact of adding PCV13 at diagnosis of chronic medical conditions, base case
Table 3. Impact of adding PCV13 at diagnosis of chronic medical conditions, one-way sensitivity analyses
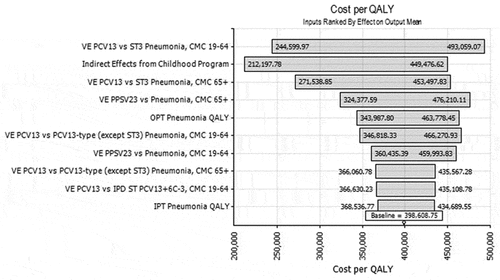
A multivariate sensitivity analyses on all model parameters showed that PCV13 VE against ST3 NBPP in adults aged 19–64 years old had the greatest impact on cost per QALY: when parameter values were in the top 10% of those drawn from the range in , the mean cost per QALY was 244,600 USD; the cost per QALY was 493,059 USD when parameter values were in the bottom 10% (). Indirect effects from the childhood PCV13 program and PCV13 VE against ST3 NBPP in adults aged ≥65 years had the next largest impact on the cost-effectiveness ratio. As the ACIP does not use a cost-effectiveness threshold, Figure S2 shows the cumulative density of cost per QALY estimates returned by the model across the full range of thresholds. In 99.39% of simulations the cost was more than 100,000 USD per QALY, in 89.96% of simulations the cost was more than 200,000 USD per QALY, and 50% of simulations had costs higher than 350,000 USD per QALY.
Figure 1. Tornado diagram of model sensitivity to outputs
Discussion
Our results showed that adding a dose of PCV13 to the currently recommended PPSV23 for adults with CMC will provide limited public health impact against preventing pneumococcal disease under base case assumptions for our model inputs. The estimated impact on disease outcomes was smaller, with a larger cost per QALY gained than what was recently estimated for vaccinating all immunocompetent adults (including those with CMC conditions) aged ≥65 years.Citation9 This is likely because although most of the disease burden occurs in older adults, the transition to vaccination of persons with CMC happens earlier in life when disease risk may be lower.
As shown by the tornado diagram ur results were sensitive to the assumptions around PCV13 effectiveness against ST3 disease, particularly NBPP. In our base case, we assumed that PCV13 had no effectiveness against ST3 disease. Post-licensure studies report varying estimates of PCV13 effectiveness against ST3 disease. A recent meta-analysis based on three studies estimated PCV13 VE against ST3 hospitalized CAP 52.5% (95% CI: 6.2–75.9%), which is higher than the VE estimated from a randomized-controlled study for all vaccine-type pneumococcal pneumonia.Citation54,Citation55 However, immunogenicity studies in infantsCitation56–60 and adultsCitation55 have generally shown a lower immunologic response to ST3 compared to the immune response to other PCV13 ST. In addition, population-level surveillance data have not demonstrated a PCV13 impact on ST3 disease in the U.S. after PCV13 introduction among adults aged ≥65 years. Surveillance for hospitalized pneumonia among adults in 21 U.S. hospitals showed that although the proportion of overall PCV13-type pneumonia decreased 2 years after PCV13 introduction for adults, the proportion of ST3 pneumonia increased in adults ≥65 years.Citation23 ABCs data showed that during 2014–2018, ST3 IPD incidence increased during a period when PCV13 coverage reached nearly 50% while IPD caused by other PCV13 STs decreased among adults aged ≥65 years (ABCs unpublished data),Citation32 for whom routine PCV13 vaccination was recommended in 2014. Given these data, the base case assumption in our model of no PCV13 effectiveness against ST3 disease was consistent with lack of population-level impact on ST3 disease in the U.S.
Our study is subject to several limitations. First, data on VE of PPSV23 against vaccine-type pneumococcal pneumonia are inconsistent, leading to uncertainties of this estimate; many studies, including meta-analyses, have demonstrated no effectiveness. As in our previous studies among older adults,Citation9,Citation61 assuming effectiveness of PPSV23 against pneumococcal pneumonia 45% (equivalent to PCV13 VECitation24) would have resulted in higher cost per QALY gained for PCV13. Second, we assumed that PCV13 coverage in adults aged 19–64 years will be the same as PPSV23 coverage, which is much lower than that among adults aged ≥65 years. However, our sensitivity analysis showed that PCV13 coverage did not have significant impact on the outcome of the model. Third, we have limited data on the effectiveness or duration of protection of the vaccines in people with CMC, especially in younger adults. Additionally, people with CMC consist of groups with varying degree of risk against pneumococcal disease.Citation3 We attempted to address the uncertainty through multivariate sensitivity analyses, which showed that the results were most sensitive to the assumption of PCV13 VE against ST3 pneumonia. Most of the studies we referenced targeted older adults; therefore, it is possible that we underestimated VE or duration of protection from PCV13 in younger adults with CMC. Lastly, there are limited data on utility decrements to use for pneumococcal diseases. We used values from published reports;Citation51 however, higher utility decrements have been use in other models, which, if applied, would have reduced the cost per QALY gained for our base case.Citation62
In summary, our study showed that the strategy to administer PCV13 to adults aged ≥19 years with CMC is not economically favorable and is expected to lead to limited public health benefits due to reductions in disease burden through pediatric PCV13 use and small residual vaccine-preventable disease burden among adults with CMC. The strategy with PCV13 for adults with CMC had higher costs per QALY gained than recommending routine immunization for all immunocompetent adults aged ≥65 years.Citation9 Our results were sensitive to the assumptions around PCV13 effectiveness against ST3 disease, particularly NBPP. Higher-valency pneumococcal conjugate vaccines covering broader range of serotypes causing disease among adults are expected to be licensed in the near future,Citation63,Citation64 and will provide opportunities to reduce remaining pneumococcal disease burden in adults with CMC including ST3 disease if these new vaccines consistently demonstrate population-level impact against ST3 disease.
Supplemental Material
Download MS Word (168.8 KB)Acknowledgments
Dr. Stoecker’s work on this project was funded by an Intergovernmental Personnel Agreement with the Centers for Disease Control and Prevention (CDC).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Centers for Disease Control and Prevention. Bact Facts Interactive Beta v8.2. Active Bacterial Core surveillance (ABCs).
- Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, Damaske B, Stefonek K, Barnes B, Patterson J, et al. Epidemiology of Invasive Streptococcus pneumoniae Infections in the United States, 1995-1998 Opportunities for Prevention in the Conjugate Vaccine Era. JAMA. 2001;285(13):1729–35. doi:10.1001/jama.285.13.1729.
- Ahmed SS, Pondo T, Xing W, McGee L, Farley M, Schaffner W, Thomas A, Reingold A, Harrison L, Lynfield R, et al. Early Impact of 13-valent Pneumococcal Conjugate Vaccine Use on Invasive Pneumococcal Disease among Adults with and without Underlying Medical Conditions-United States. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 2019.
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, et al. Decline in Invasive Pneumococcal Disease after the Introduction of Protein–Polysaccharide Conjugate Vaccine. N Engl J Med. 2003;348(18):1737–46. doi:10.1056/NEJMoa022823.
- Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, Farley MM, Harrison LH, Schaffner W, Reingold A, Bennett NM, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294(16):2043–51. doi:10.1001/jama.294.16.2043.
- Centers for Disease Control & Prevention. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine for Adults with Immunocompromising Conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbidity and mortality weekly report 2012; 61:816–19.
- Cho BH, Stoecker C, Link-Gelles R, Moore MR Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011–21. doi:10.1016/j.vaccine.2013.10.024.
- Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, Hadler S, Pilishvili T.. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged >/=65 Years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbidity and mortality weekly report 2014; 63:822–25.
- Stoecker C, Kobayashi M, Matanock A, Cho BH, Pilishvili T Cost-effectiveness of continuing pneumococcal conjugate vaccination at age 65 in the context of indirect effects from the childhood immunization program. Vaccine. 2019;38(7):1770–77. doi:10.1016/j.vaccine.2019.12.029.
- Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged >/=65 Years: updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morbidity and mortality weekly report 2019; 68:1069–75.
- Palisade Corporation. @Risk 5.7. Newfield, NY2011.
- Centers for Disease Control and Prevention. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR 2010; 59:1102–06.
- Arias E, Xu J Unites States Life Tables, 2017. National Vital Statistics Reports, 2019.
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017; 135:e146–e603.
- Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, Volk ML. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2015;49(8):690–96. doi:10.1097/MCG.0000000000000208.
- Rycroft CE, Heyes H, Lanza L, Becker K. Epidemiology of chronic obstructive pulmonary disease: a literature review. Int J Chron Obstruct Pulmon Dis. 2012;7:457–94. doi:10.2147/COPD.S32330.
- Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of Alcohol Dependence Among US Adult Drinkers, 2009–2011. Prev Chronic Dis. 2014;11:E206. doi:10.5888/pcd11.140329.
- Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N Engl J Med. 2015;373(5):415–27. doi:10.1056/NEJMoa1500245.
- Hayes BH, Haberling DL, Kennedy JL, Varma JK, Fry AM, Vora NM. Burden of Pneumonia-Associated Hospitalizations: united States, 2001-2014. Chest. 2018;153(2):427–37. doi:10.1016/j.chest.2017.09.041.
- Ramirez JA, Wiemken TL, Peyrani P, Arnold FW, Kelley R, Mattingly WA, Nakamatsu R, Pena S, Guinn BE, Furmanek SP, et al. Adults Hospitalized With Pneumonia in the United States: incidence, Epidemiology, and Mortality. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2017;65(11):1806–12. doi:10.1093/cid/cix647.
- Weycker D, Farkouh RA, Strutton DR, Edelsberg J, Shea KM, Pelton SI. Rates and costs of invasive pneumococcal disease and pneumonia in persons with underlying medical conditions. BMC Health Serv Res. 2016;16(1):182. doi:10.1186/s12913-016-1432-4.
- Nelson JC, Jackson M, Yua O, Whitney CG, Bounds L, Bittner R, Zavitkovsky A, Jackson LA. Impact of the Introduction of Pneumococcal Conjugate Vaccine on Rates of Community Acquired Pneumonia in Children and Adults. Vaccine. 2008;26(38):4947–54. doi:10.1016/j.vaccine.2008.07.016.
- Isturiz RE, Ramirez J, Self WH, Grijalva CG, Counselman FL, Volturo G, Ostrosky-Zeichner L, Peyrani P, Wunderink RG, Sherwin R, et al. Pneumococcal epidemiology among us adults hospitalized for community-acquired pneumonia. Vaccine. 2019;37(25):3352–61. doi:10.1016/j.vaccine.2019.04.087.
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AMM, Sanders EAM, Verheij TJM, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–25. doi:10.1056/NEJMoa1408544.
- Pilishvili T, Almendares O, Nanduri S, Warnock R, Wu X, McKean S, Kelman J, Farley M, Schaffner W, Thomas A, et al. Case-Control Study to Evaluate Pneumococcal Vaccines Effectiveness against Invasive Pneumococcal Disease (IPD) Among U.S. Medicare Beneficiaries ≥65 Years Old. ISPPD Abstract 0682 2018.
- Suaya JA, Jiang Q, Scott DA, Gruber WC, Webber C, Schmoele-Thoma B, Hall-Murray CK, Jodar L, Isturiz RE. Post Hoc Analysis of the Efficacy of the 13-Valent Pneumococcal Conjugate Vaccine against Vaccine-type Community-acquired Pneumonia in at-risk Older Adults. Vaccine. 2018;36(11):1477–83. doi:10.1016/j.vaccine.2018.01.049.
- Falkenhorst G, Remschmidt C, Harder T, Hummers-Pradier E, Wichmann O, Bogdan C, Ho PL. Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23) against Pneumococcal Disease in the Elderly: systematic Review and Meta-Analysis. PloS One. 2017;12(1):e0169368. doi:10.1371/journal.pone.0169368.
- Schiffner-Rohe J, Witt A, Hemmerling J, von Eiff C, Leverkus F-W, Chalmers JD. Efficacy of PPV23 in Preventing Pneumococcal Pneumonia in Adults at Increased Risk – A Systematic Review and Meta-Analysis. PloS One. 2016;11(1):e0146338. doi:10.1371/journal.pone.0146338.
- Tin Tin Htar M, Stuurman AL, Ferreira G, Alicino C, Bollaerts K, Paganino C, Reinert RR, Schmitt H-J, Trucchi C, Vestraeten T, et al. Effectiveness of pneumococcal vaccines in preventing pneumonia in adults, a systematic review and meta-analyses of observational studies. PloS One. 2017;12(5):e0177985. doi:10.1371/journal.pone.0177985.
- Hung M-C, Williams WW, Lu P-J, Woods LO, Koppaka R, Lidley MC Vaccination Coverage among Adults in the United States, National Health Interview Survey, 2017, 2018.
- Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, et al. Surveillance of Vaccination Coverage Among Adult Populations - United States, 2014. Morbidity and mortality weekly report Surveillance summaries (Washington, DC: 2002) 2016; 65:1–36.
- Pilishvili T, Gierke R, Xing W, Farley M, Schaffner W, Thomas A, Reingold A, Harrison L, Lynfield R, McGuire S, et al. Impact of 13-valent Pneumococcal Conjugate Vaccine (PCV13) use on Invasive Pneumococcal Disease (IPD) among Adults in the United States. ISPPD Abstract 0696. Melbourne, Australia., 2018.
- van der Linden M, Imöhl M, Perniciaro S, Melo-Cristino J. Limited indirect effects of an infant pneumococcal vaccination program in an aging population. PloS One. 2019;14(8):e0220453. doi:10.1371/journal.pone.0220453.
- Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, Andrews NJ, Miller E, Ramsay ME. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–51. doi:10.1016/S1473-3099(18)30052-5.
- Pilishvili T, Gierke R, Farley MM, Schaffner W, Thomas A, Reinert RR, Harrison LH, Holtzman C, McGuire G, Petit S, et al. Changes in Invasive Pneumococcal Disease (IPD) among Children Following 6 years of 13-valent Pneumococcal Conjugate Vaccine (PCV13) use in the United States. ISPPD Abstract 0643. Melbourne, Australia., 2018.
- Dagan R, Patterson S, Juergens C, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America. 2013;57(7):952–62. doi:10.1093/cid/cit428.
- Grant LR, O’Brien SE, Burbidge P, Haston M, Zancolli M, Cowell L, Johnson M, Weatherholtz RC, Reid R, Santosham M, et al. Comparative immunogenicity of 7 and 13-valent pneumococcal conjugate vaccines and the development of functional antibodies to cross-reactive serotypes. PloS One. 2013;8(9):e74906. doi:10.1371/journal.pone.0074906.
- National Center for Health Statistics. National Health Interview Survey.
- HCUP Databases. Healthcare Cost and Utilization Project (HCUP). Rockville(MD): Agency for Healthcare Research and Quality; 2014 [accessed 2019 Aug 29]. http://www.hcup-us.ahrq.gov/nisoverview.jsp
- Wyllie AL, Rümke LW, Arp K, Bosch A, Bruin JP, Rots NY, Wijmenga-Monsuur AJ, Sanders EAM, Trzciński K. Molecular surveillance on Streptococcus pneumoniae carriage in non-elderly adults; little evidence for pneumococcal circulation independent from the reservoir in children. Sci Rep. 2016;6(1):34888. doi:10.1038/srep34888.
- Milucky J, Carvalho MDG, Rouphael N, Bennett NM, Talbot HK, Harrison LH, Farley MM, Walston J, Pimenta F, Lessa FC, et al. Streptococcus pneumoniae colonization after introduction of 13-valent pneumococcal conjugate vaccine for US adults 65 years of age and older, 2015–2016. Vaccine. 2019;37(8):1094–100. doi:10.1016/j.vaccine.2018.12.075.
- Bruce MG, Singleton R, Bulkow L, Rudolph K, Zulz T, Gounder P, Hurlburt D, Bruden D, Hennessy T. Impact of the 13-valent pneumococcal conjugate vaccine (pcv13) on invasive pneumococcal disease and carriage in Alaska. Vaccine. 2015;33(38):4813–19. doi:10.1016/j.vaccine.2015.07.080.
- Hammitt LL, Bruden DL, Butler JC, Baggett HC, Hurlburt DA, Reasonover A, Hennessy T. Indirect Effect of Conjugate Vaccine on Adult Carriage of Streptococcus pneumoniae: an Explanation of Trends in Invasive Pneumococcal Disease. J Infect Dis. 2006;193(11):1487–94. doi:10.1086/503805.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–09. doi:10.1016/S1473-3099(14)71081-3.
- Lewnard JA, Hanage WP. Making sense of differences in pneumococcal serotype replacement. Lancet Infect Dis. 2019;19(6):e213–e20. doi:10.1016/S1473-3099(18)30660-1.
- Patterson S, Webber C, Patton M, Drews W, Huijts SM, Bolkenbaas M, Gruber WC, Scott DA, Bonten MJM. A post hoc assessment of duration of protection in CAPiTA (Community Acquired Pneumonia immunization Trial in Adults). Trials Vaccinology. 2016;5:92–96. doi:10.1016/j.trivac.2016.04.004.
- van Werkhoven CH, Huijts SM, Bolkenbaas M, Grobbee DE, Bonten MJ. The Impact of Age on the Efficacy of 13-valent Pneumococcal Conjugate Vaccine in Elderly. Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America. 2015;61(12):1835–38. doi:10.1093/cid/civ686.
- Black CL, Williams WW, Warnock R, Pilishvili T, Kim D, Kelman JA Pneumococcal Vaccination Among Medicare Beneficiaries Occurring After the Advisory Committee on Immunization Practices Recommendation for Routine Use Of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine for Adults Aged >/=65 Years. MMWR Morbidity and mortality weekly report 2017; 66:728–33.
- Finland M, Barnes MW. Changes in occurrence of capsular serotypes of Streptococcus pneumoniae at Boston City Hospital during selected years between 1935 and 1974. J Clin Microbiol. 1977;5:154–66.
- Pfizer. Inc. internal sales data for PCV13. 2016.
- Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31–32):4203–14. doi:10.1016/j.vaccine.2004.05.003.
- Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Influenza vaccination health impact and cost effectiveness among adults aged 50 to 64 and 65 and older. Am J Prev Med. 2006;31(1):72–79. doi:10.1016/j.amepre.2006.03.008.
- Bureau of Labor Statistics. Consumer Price Index Archived News Releases.
- McLaughlin JM, Jiang Q, Gessner BD, Swerdlow DL, Sings HL, Isturiz RE, Jodar L. Pneumococcal conjugate vaccine against serotype 3 pneumococcal pneumonia in adults: A systematic review and pooled analysis. Vaccine. 2019;37(43):6310–16. doi:10.1016/j.vaccine.2019.08.059.
- van Deursen AMM, van Houten MA, Webber C, Patton M, Scott DA, Patterson S, Sidhu M, Drews W, Gruber WC, Emini EA, et al. Immunogenicity of the 13-Valent Pneumococcal Conjugate Vaccine in Older Adults With and Without Comorbidities in the Community-Acquired Pneumonia Immunization Trial in Adults (CAPiTA). Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 2017; 65:787–95.
- Kim DS, Shin SH, Lee HJ, Hong YJ, Lee SY, Choi KM, Oh CE, Kim KH, Juergens C, Patterson S, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given to korean children receiving routine pediatric vaccines. Pediatr Infect Dis J. 2013;32:266–73.
- Yeh SH, Gurtman A, Hurley DC, Block SL, Schwartz RH, Patterson S, Jansen KU, Love J, Gruber WC, Emini EA, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in infants and toddlers. Pediatrics. 2010;126(3):e493–505. doi:10.1542/peds.2009-3027.
- Weckx LY, Thompson A, Berezin EN, de Faria SM, da Cunha CA, Pride M, Patterson S, Gruber WC, Emini EA, Scott DA, et al. A phase 3, randomized, double-blind trial comparing the safety and immunogenicity of the 7-valent and 13-valent pneumococcal conjugate vaccines, given with routine pediatric vaccinations, in healthy infants in Brazil. Vaccine. 2012;30(52):7566–72. doi:10.1016/j.vaccine.2012.10.040.
- Kieninger DM, Kueper K, Steul K, Juergens C, Ahlers N, Baker S, Jansen KU, Devlin C, Gruber WC, Emini EA, et al. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine. 2010;28(25):4192–203. doi:10.1016/j.vaccine.2010.04.008.
- Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, Slack M, Ladhani SN, Miller E, Goldblatt D, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–46. doi:10.1016/S1473-3099(14)70822-9.
- Stoecker C, Kim L, Gierke R, Pilishvili T. Incremental Cost-Effectiveness of 13-valent Pneumococcal Conjugate Vaccine for Adults Age 50 Years and Older in the United States. J Gen Intern Med. 2016;31(8):901–08. doi:10.1007/s11606-016-3651-0.
- Wateska AR, Nowalk MP, Lin CJ, Harrison LH, Schaffner W, Zimmerman RK, Smith KJ. Pneumococcal Vaccination in Adults Aged ≥65 Years: cost-Effectiveness and Health Impact in U.S. Populations. American Journal of Preventive Medicine. 2020;58(4):487–95. doi:10.1016/j.amepre.2019.10.022.
- Merck & Co. Inc. Merck Announces Results from Phase 2 Trial of Investigational 15-valent Pneumococcal Conjugate Vaccine (V114) in Infants. May 8, 2019.
- Pfizer Inc. Pfizer Announces Top-Line Results from Phase 3 Study of 20-Valent Pneumococcal Conjugate Vaccine in Pneumococcal Vaccine-Naive Adults Aged 18 Years or Older. March 18, 2020.
