ABSTRACT
Background: Currently, children aged 6–9 years have the highest incidence rate of mumps in China. Although China has introduced a two-dose schedule of measles-mumps-rubella vaccine into routine immunization (at 8 months and 18 months), the incidence rate of mumps in high-risk populations might not decrease due to waning immunity. Here we report a mumps outbreak supporting this hypothesis.
Methods: The descriptive epidemiological method was used to summarize the overall characteristics of the course of the outbreak. We conducted a retrospective cohort study to evaluate the vaccine effectiveness (VE) of mumps-containing vaccine (MuCV).
Results: A total of 78 cases were identified during the outbreak and the estimated vaccination coverage was 84.7%. Of 454 vaccinated students, 335 (73.8%) had received one-dose MuCV, 93 (20.5%) two-dose, and 26 (5.7%) three-dose. The VEs for both the one-dose (−17.0%, 95%CI: −120.3–38.2%) and two-dose groups (−10.0%, 95%CI: −138.0–48.8%) were not performed well, whereas the VE for the three-dose group was 100%. However, we found that the overall VE was 74.2% (95% CI: 9.7–92.6%) for students vaccinated within 5 years. We also observed that there was a broadly linear increase in mumps infection risk in both one-dose and two-dose group when the time since last dose vaccination was more than 5 years.
Conclusions: The overall VE for both one-dose and two-dose MuCV was discouraging, but it appeared to be moderately effective within 5 years after vaccination. Further surveillance and seroepidemiological data are needed to understand the impact of the new vaccination strategy on mumps in China.
Introduction
Mumps is a common childhood infectious disease caused by mumps virus and clinically characterized by pain and swelling of the parotid gland, fever, and headache.Citation1 Although mumps is usually benign and spontaneously resolves, serious complications such as meningitis, encephalitis, orchitis, and deafness can occur, especially in adolescents and young adults.Citation1,Citation2 Like other vaccine-preventable diseases, the introduction of vaccination is considered the best way to prevent mumps infection. The most commonly used mumps vaccine strains worldwide include the Jeryl Lynn, Leningrad-Zagreb, and Urabe, whereas Hoshino and Rubini strains have had limited use due to their poor immunogenicity.Citation3 Depending on the specific vaccine strain used, the median vaccine effectiveness (VE) against mumps for one-dose and two-dose vaccination was 78% (49% to 92%) and 88% (66% to 95%),Citation3,Citation4 respectively. Subsequently, the widespread use of mumps vaccine has substantially reduced morbidity and the number of severe complications due to mumps.Citation2,Citation5 However, outbreaks have been reported in countries with high vaccine coverage, highlighting that the effectiveness of the two doses of measles-mumps-rubella vaccine (MMR) may not be as high as expected.Citation6–9 Hence, prevention and control of the mumps epidemic have become a new public health problem.
In China, the mumps vaccine is manufactured using the S79 strain, which is derived through further attenuation from the US Jeryl Lynn strain.Citation10 Although the policy for a single-dose MMR targeting children aged 18 months was introduced into the Expanded Program on Immunization (EPI) in 2007, the incidence of mumps has remained the highest among children and adolescents in China.Citation11,Citation12 The single-dose MMR vaccine schedule has a limited effect on controlling mumps, suggesting a two-dose MMR schedule is highly needed in China.Citation10,Citation13–15 Thus, to control the epidemic of mumps, China has introduced an extra dose of MMR into routine immunization since June 1, 2020. The current immunization schedule of two doses of MMR is given at the age of 8 months and 18 months. However, few countries recommend the administration of the first MMR dose before 12 months.Citation16 In contrast, the United States (US) Centers for Disease Control and Prevention (CDC) recommends mumps-containing vaccine (MuCV) given before 12 months of age should not be counted as part of the series. Children vaccinated with MuCV before 12 months of age should be revaccinated with two doses of MMR, the first of which should be administered when the child is at least 12 months of age.Citation17 The reason for early vaccination in China might be based on efforts to reduce measles transmission.Citation16 Although this optimized immunization strategy for mumps in China is inspiring, whether it will result in a high prevalence of mumps in adolescents and young adults due to waning immunity and lead to more disease burden has not been fully evaluated.
From October 2018 to January 2019, a large mumps outbreak with a high attack rate (AR) occurred in a nine-year coherent school (including both primary and junior high school students) in Lu’an, Anhui Province. The preliminary investigation showed that students who had been vaccinated with MuCV seemed to have higher AR than those without vaccination (13.7% vs. 12.7%), sparking local public health authority concerns on the VE for mumps and the potential of public vaccine hesitancy in the future. Hence, we conducted a thorough and emergent field investigation to identify the factors that contributed to this highly concerning outbreak.
Materials and methods
Outbreak setting
This outbreak occurred in a nine-year coherent school in the rural area of Lu’an, China with 617 students and 77 teachers. Among 617 students, 378 (62.3%) were elementary school students and 239 (38.7%) were junior high school students. The school has only two buildings (No. 1 Building and No. 2 Building) and a small canteen for students. The classrooms for grades 1 to 7 were in the No. 1 Building, while grades 8 to 9 were in the No. 2 Building.
Study design
This was a two-step study. In the first stage of the study, we aimed to summarize the overall characteristics of the course of the outbreak by the descriptive epidemiological method. After completing a face-to-face epidemiological investigation in this school, we designed a structured questionnaire to collect crucial information on demographics (e.g., gender, grade, and residential address), vaccine status, history of mumps infection, clinical characteristics, and vaccine type received. To evaluate the VEs of MuCV, we conducted a retrospective cohort study in the second stage of the study. We used the formula: VE = (ARU – ARV)/ARU × 100% to evaluate VEs and 95% confidence intervals (CIs) according to our previous work,Citation6,Citation18 where ARU and ARV referred to the AR of unvaccinated students and vaccinated students, respectively. Subgroup analyses by doses (one dose, two doses, and three doses) and time since last dose vaccination (< 5 years, ≥ 5 years) were performed to evaluate VEs among different vaccination status populations. Those students with a mumps history before the outbreak or an unknown vaccination history were excluded from the cohort.
Definition and case identification
We defined a case of mumps as any person with acute onset of unilateral or bilateral parotid gland swelling lasting more than 2 days and without other apparent cause among students and teachers during the outbreak period. We identified mumps cases by one of the following situations: (1) cases diagnosed by a doctor and reported to the National Notifiable Communicable Disease Report System (NNDRS), (2) medical records from the hospitals near the school, or (3) self-reported cases (students with affirmative answers in the questionnaire). To ensure the accuracy and consistency of the self-reported cases, we interviewed the students’ parents by telephone to further confirm the case status, including date of illness onset and clinical symptoms. All cases were clinically diagnosed or self-reported.
Vaccination status ascertainment
Data on the vaccination status information, such as timing of vaccination and type of vaccine, were reviewed and confirmed by EPI staff from the Lu’an CDC. The information was mainly collected from immunization records provided by students. As for students who did not provide records, we searched in the Anhui Immunization Information Management System (AIIMS) by the child’s name, parents’ names, and birth dates to obtain vaccination information. We consulted local vaccination clinic workers to review data from 15-year, archived vaccination cards for older students who did not provide records and were also not included in the AIIMS, as they were born before 2005.
Statistical analysis
Data from the questionnaires were entered into Epidata 3.1 software. Data cleaning and descriptive epidemiologic data analysis were performed using Microsoft Excel 2016. Possible differences in the characteristics of different groups were tested by chi-square test, t-test, and Kruskal-Wallis test where appropriate. The cumulative risk of mumps infection over time was estimated by Kaplan-Meier method and the difference among vaccination doses was tested by log-rank test. All statistical analyses were performed using IBM SPSS Statistics 20.0. Two-sided p-values were reported to be statistically significant at < 0.05.
Results
Outbreak description
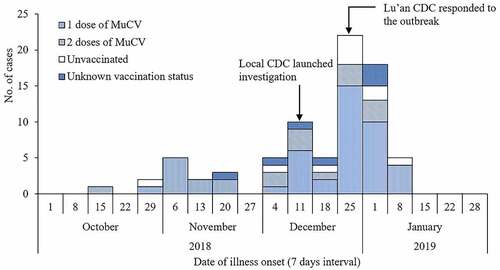
Between October 20, 2018, and January 13, 2019, Lu’an CDC identified 78 outbreak-related cases, of which all were students and 44 (56.4%) were male. Of 78 outbreak-related cases, 71 (91.0%) were clinically diagnosed and seven (9.0%) were self-reported. The overall AR was 11.2% (78/694) during the outbreak. The index case was a male 6th-grade student who regularly had lunch in the school canteen and reported having two doses of MuCV vaccination before the outbreak. He developed parotitis on October 20, 2018. Two weeks later, one of his classmates and two of his friends living in the same village, who had close contact with him, developed mumps. As shown in , the outbreak lasted almost four months, and the onset of illness peaked from December 25, 2018, to January 7, 2019. Clinical symptoms included parotid gland swelling (100%), fever (30.8%), and headache (20.5%). Four (5.2%) cases experienced complications, including encephalitis (two cases) and deafness (two cases).
Vaccination status elaboration
The questionnaires were returned by 568 of 617 students with a response rate of 92.1% and information on vaccination status was available for 536 (94.4%) students. As shown in , the estimated overall vaccination coverage (VC) was 84.7% (454/536), and VCs varied among different grades (59.6% to 100%). Of 454 vaccinated students, 335 (73.8%) had received one-dose of MuCV, 93 (20.5%) two-dose, and 26 (5.7%) three-dose. In the entire cohort of 568 students, the overall mumps AR was 13.7%, ranging from 0 to 29.0% among different grades. Compared with grade 1–3, VCs in the other grades was lower, and ARs was higher. Interestingly, grades with a moderate level of VCs (grade 4–7) had the highest ARs. Therefore, we divided nine grades into three groups (G1: grade 1–3; G2: grade 4–7; and G3: grade 8–9) and calculated the mean interval time (MIT) since the last dose of MuCV vaccination. We found the MIT for G1, G2, and G3 was 5.5 ± 1.3 years, 7.9 ± 2.0 years, and 10.7 ± 2.7 years, respectively. When we restricted our analysis to the 78 outbreak-related cases, 61 (78.2%) had received at least one dose of MuCV and 10 (12.8%) had not been immunized. Of 61 cases who had received MuCV, the MIT since the last dose vaccination was 8.8 ± 2.4 years before the outbreak.
Table 1. The distribution of vaccination coverage and attack rate among different grades
Vaccine effectiveness evaluation
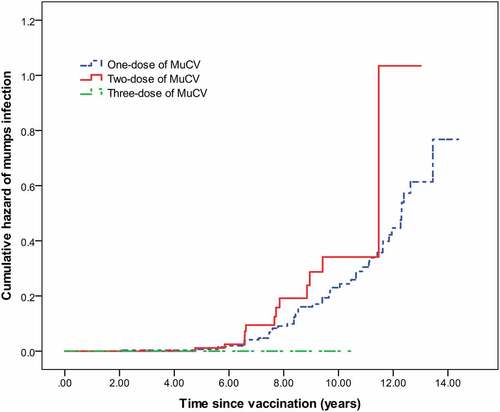
A total of 523 students were enrolled in our cohort to evaluate the VEs after excluding 45 students with previous mumps history or unknown vaccination history. The cohort included 71 cases and 452 non-cases. The overall VE and VEs by different subgroups are shown in . Unfortunately, we found that the overall AR in vaccinated students was higher than that of unvaccinated students (13.7% vs. 12.7%, p> .05), indicating the overall VE was insufficient to protect mumps infection during the outbreak. Subgroup analyses by doses revealed that the VEs for both the one-dose group (−9.0%, 95%CI: −102.7–41.9%) and two-dose group (−17.0%, 95%CI: −120.3–38.2%) were the least protective, whereas the VE for the three-dose group was 100%. Subgroup analyses by time since last dose vaccination found that the overall VE was 74.2% (95% CI: 9.7–92.6%) for students vaccinated within 5 years and −30.0% (95% CI: −143.2–30.3%) for students vaccinated more than 5 years prior. We observed that MIT since the last dose vaccination of the mumps case group was longer than that of the non-case group (8.76 ± 2.9 years vs. 7.44 ± 2.67 years, p< .01). In addition, we compared the MIT among one-dose (7.7 ± 2.9 years), two-dose (7.5 ± 2.1 years), and three-dose group (7.1 ± 1.4 years) and found no significant difference among the three groups (χ2 = 0.205, p= .903). The Kaplan-Meier curves showed no significant difference in the cumulative risk of mumps infection over time for the three groups (log-rank test: χ2 = 3.135, p= .209). For the 26 students who received three-dose of MuCV, no cases of mumps were reported. However, we observed a broadly linear increase in mumps infection risk both in one-dose and two-dose groups when the time since last dose vaccination was more than 5 years ().
Table 2. Estimated VEs for MuCV during an outbreak in Lu’an, China, 2018–2019
Discussion
We reported on a large mumps outbreak with a high attack rate in a nine-year coherent school. Based on a thorough field investigation, we found that the VC of MuCV was only 84.5% among school students, suggesting that this school had achieved a moderate level of herd immunity, which could not prevent the outbreak. Moreover, we demonstrated that the overall VE for both one-dose and two-dose MuCV was discouraging but appeared to be moderate effective within 5 years after vaccination.Surprisingly, although the mean time interval of the three-dose group was more than 7 years, which was comparable with that of the one-dose and two-dose group, none of the students who had received three-dose MuCV developed mumps during the outbreak.
The World Health Organization (WHO) has recommended that the prevention of mumps requires sustained, high levels of immunization coverage and more than one vaccine dose.Citation16 The herd immunity threshold for block mumps transmission has been estimated at 86–93%, while the critical vaccination coverage was estimated as 90–98% when the assumed VE was 95%.Citation19 However, in this outbreak, we found that both the VC and VE for mumps were not satisfactory, which likely contributed to the outbreak. Furthermore, epidemiological investigation found that the intensity of exposure (e.g., the small canteen may have increased exposure risk) and delays in the recognition of mumps cases (9.0% of cases were not clinically diagnosed) may have also contributed to the outbreak.
Because the AR in the vaccinated students was higher than that of unvaccinated students and nearly 80% of cases had been vaccinated before the outbreak, parents of the vaccinated students had received some misleading information, such as whether the vaccines were fake or ineffective. Fortunately, we observed that vaccine-induced protection was not as bad as had been thought. Our subgroup analyses showed that the overall VE for one-dose of MuCV within 5 years was 74.2%, which was consistent with previous investigations.Citation20,Citation21 Besides, we noted that students who had been vaccinated more than 5 years prior were found to be at increased risk of mumps infection. Our study had a limited sample size and we therefore could not calculate the increased odds of mumps infection for each year. Nonetheless, we found a broadly linear increase in mumps infection risk in both the one-dose and two-dose groups in students who had been vaccinated more than 5 years prior, suggesting the VE of MuCV wanes over time. Our results were coherent with previous outbreak investigations performed in US,Citation9 France,Citation7 and England,Citation22 and similar results were also observed in some seroepidemiological studies in China.Citation15,Citation23 At present, the waning immunity of mumps vaccine with different vaccine strains such as Jeryl Lynn, Leningrad Zagreb, and Urabe, has been observed in other outbreak investigations.Citation8,Citation10,Citation24 Fortunately, waning immunity was not observed in the three-dose group in our study, suggesting that a third-dose intervention may be an appropriate control measure in highly vaccinated populations that could limit the scale of the outbreak.Citation7,Citation8
The implementation of a two-dose immunization strategy for mumps control in China is promising. However, we have concerns about the new strategy, such as the short time interval between MMR doses and the administration of the first dose at an earlier age. The optimal immunization schedule maximizes the time of protection against disease infection by minimizing the time between the interference of maternal antibodies and vaccination.Citation25 Regarding the time interval between MMR doses, the optimal age for the second dose of MMR administration varies widely among countries. In France, a second MMR dose is administered at age 3–6 years.Citation7 In the US, the two doses of MMR are administered at 12–15 months and 4–6 years of age,Citation26 while in China, the two doses of MMR are given at 8 months and 18 months of age. However, completing two doses of MMR within a short time interval may lead to a higher risk of mumps infection in adolescents due to waning immunity.Citation9,Citation15 Based on our results, we assume protection with MMR wanes over time and is insufficient after 5 years post-vaccination. Because children aged 6–9 years have the highest incidence rate of mumps in China,Citation12 the incidence rate in high-risk populations might not decrease. As an alternative, administration of the second MMR dose for preschool children seems more feasible. Notably, the earlier age of first-dose administration may also lead to lower mumps geometric antibody concentration,Citation27 which warrants further study due to the lack of evidence found in our survey.
This mumps outbreak was unique for several reasons. First, students in the vaccination group seemed to be more likely to be infected, which led to misunderstanding among the parents of the students and even local public health workers and might cause vaccine hesitation in the local area. Therefore, it is necessary to conduct a thorough investigation to eliminate these adverse effects. Second, mumps transmission was intense, far exceeding the protection afforded by the vaccine among intense-exposure students. For example, students in grades with a moderate level of vaccine coverage had the highest attack rate compared with others. This can be explained by higher intense exposure, and that the initial case and secondary cases infected by him were distributed in those grades.
This study also had some limitations. First, our case definition relied largely on clinical symptoms and allowed for self-report of symptoms among students, thus we may have underestimated or overestimated the VE for the mumps vaccine. Second, some of the variables which are of great interest for policymakers included in the subgroup analysis were unstable and limited by the small number of samples. Third, the mumps virus strain of this outbreak has not been characterized or sequenced; hence, we did not discuss the effect on the mismatch of the vaccine virus strain with the circulating outbreak strains. Despite these limitations, our study provides additional evidence for public health policymakers regarding recommendations for feasible strategies for mumps control.
Conclusions
In conclusion, this outbreak was attributed to low vaccine coverage and insufficient vaccine effectiveness of MuCV due to the waning of immunity over time. Additionally, high-intensity exposure in school was a crucial factor in the high rate of transmission. The benefits of the new immunization strategy for mumps in China are still unclear. Further surveillance data and seroepidemiological studies are needed to understand the impact of the new vaccination strategy on mumps infection in China.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed. The findings and conclusions expressed in this publication are those of the authors and do not necessarily represent the official position of the Lu’an Center for Disease Control and Prevention.
Authors’ contributions
Danni Wang, Wei Qin, Jian Wang, and Tingyue Nie participated in the data analysis and drafted the manuscript. Wei Qin critically reviewed and supervised the development of the paper. Wei Qin, Fan Pan, Yao Wang, and Jian Wang performed the field epidemiological investigation and participated in the immunization record review. Tingyue Nie was responsible for data visualization. All the authors reviewed and edited the final manuscript.
Ethical considerations
This mumps outbreak investigation was a part of the public health response and ethical clearance was not required. We obtained written informed consent from parents before enrolling patients during this investigation.
Acknowledgments
We sincerely acknowledge the contributions of the public health workers from the Yu’an district CDC. We also would like to thank the staff from this school for their assistance in the outbreak investigation. Many thanks to Dr. Dashuang Li for helping us collect students’ immunization records.
References
- Hviid A, Rubin S, Mühlemann K. Mumps. Lancet. 2008;371(9616):932–44. doi:10.1016/S0140-6736(08)60419-5.
- Su SB, Chang HL, Chen AK. Current status of mumps virus infection: epidemiology, pathogenesis, and vaccine. Int J Environ Res Public Health. 2020:17. doi:10.3390/ijerph17051686.
- Lam E, Rosen JB, Zucker JR. Mumps: an update on outbreaks, vaccine efficacy, and genomic diversity. Clin Microbiol Rev. 2020:33. doi:10.1128/CMR.00151-19.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS. Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62:1–34.
- Beleni AI, Borgmann S. Mumps in the vaccination age: global epidemiology and the situation in Germany. Int J Environ Res Public Health. 2018:15. doi:10.3390/ijerph15081618.
- Fields VS, Safi H, Waters C, Dillaha J, Capelle L, Riklon S, Wheeler JG, Haselow DT. Mumps in a highly vaccinated Marshallese community in Arkansas, USA: an outbreak report. Lancet Infect Dis. 2019;19(2):185–92. doi:10.1016/S1473-3099(18)30607-8.
- Vygen S, Fischer A, Meurice L, Mounchetrou Njoya I, Gregoris M, Ndiaye B, Ghenassia A, Poujol I, Stahl JP, Antona D, et al. Waning immunity against mumps in vaccinated young adults, France 2013. Euro Surveill. 2016;21(10):30156. doi:10.2807/1560-7917.ES.2016.21.10.30156.
- Cardemil CV, Dahl RM, James L, Wannemuehler K, Gary HE, Shah M, Marin M, Riley J, Feikin DR, Patel M, et al. Effectiveness of a third dose of MMR vaccine for mumps outbreak control. N Engl J Med. 2017;377(10):947–56. doi:10.1056/NEJMoa1703309.
- Cortese MM, Jordan HT, Curns AT, Quinlan PA, Ens KA, Denning PM, Dayan GH. Mumps vaccine performance among university students during a mumps outbreak. Clin Infect Dis. 2008;46(8):1172–80. doi:10.1086/529141.
- Qin W, Wang Y, Yang T, Xu XK, Meng XM, Zhao CJ, Li SY, Xie SY, Li KC, Su H. Outbreak of mumps in a student population with high vaccination coverage in China: time for two-dose vaccination. Hum Vaccin Immunother. 2019;15(9):2106–11. doi:10.1080/21645515.2019.1581526.
- Dong Y, Wang L, Burgner DP, Miller JE, Song Y, Ren X, Li Z, Xing Y, Ma J, Sawyer SM, et al. Infectious diseases in children and adolescents in China: analysis of national surveillance data from 2008 to 2017. BMJ. 2020;369:m1043. doi:10.1136/bmj.m1043.
- Jiang RJ, Yin QZ, Xu MJ, Zhao ZM, Deng Y, Che YC. Epidemiological characteristics of mumps in mainland China from 2004 to 2018 and key population for prevention and control. Zhongguo Dang Dai Er Ke Za Zhi. 2019;21:441–44.
- Sun X, Tang F, Hu Y, Deng X, Wang Z, Zhou M, Liu Y. High risk of mumps infection in children who received one dose of mumps-containing vaccine: waning immunity to mumps in children aged 2-5 years from kindergartens in Jiangsu Province, China. Hum Vaccin Immunother. 2020;16(7):1738–42. doi:10.1080/21645515.2019.1708162.
- Ma C, Liu Y, Tang J, Jia H, Qin W, Su Y, Wang H, Hao L. Assessment of mumps-containing vaccine effectiveness during an outbreak: importance to introduce the 2-dose schedule for China. Hum Vaccin Immunother. 2018;14(6):1392–97. doi:10.1080/21645515.2018.1428508.
- Liu Y, Liu Z, Deng X, Hu Y, Wang Z, Lu P, Guo H, Sun X, Xu Y, Tang F, et al. Waning immunity of one-dose measles-mumps-rubella vaccine to mumps in children from kindergarten to early school age: a prospective study. Expert Rev Vaccines. 2018;17(5):445–52. doi:10.1080/14760584.2018.1445529.
- WHO. Mumps virus vaccines. Wkly Epidemiol Rec. 2007; 82: 51–60.
- Centers for Disease Control and Prevention (CDC). Epidemiology and prevention of vaccine-preventable diseases. 13thed; 2015. Chapter 15: Mumps. https://www.cdc.gov/vaccines/pubs/pinkbook/mumps.html
- Qin W, Xu XK, Wang Y, Meng XM, Yang CW, Xia F, Su H. Clinical characteristics and risk factors associated with breakthrough varicella during varicella outbreaks. Hum Vaccin Immunother. 2020:1–6. doi:10.1080/21645515.2019.1704574.
- Plans-Rubió P. Evaluation of the establishment of herd immunity in the population by means of serological surveys and vaccination coverage. Hum Vaccin Immunother. 2012;8(2):184–88. doi:10.4161/hv.18444.
- Deeks SL, Lim GH, Simpson MA, Gagné L, Gubbay J, Kristjanson E, Fung C, Crowcroft NS. An assessment of mumps vaccine effectiveness by dose during an outbreak in Canada. CMAJ. 2011;183(9):1014–20. doi:10.1503/cmaj.101371.
- Castilla J, García Cenoz M, Arriazu M, Fernández-Alonso M, Martínez-Artola V, Etxeberria J, Irisarri F, Barricarte A. Effectiveness of Jeryl Lynn-containing vaccine in Spanish children. Vaccine. 2009;27(15):2089–93. doi:10.1016/j.vaccine.2009.02.001.
- Cohen C, White JM, Savage EJ, Glynn JR, Choi Y, Andrews N, Brown D, Ramsay ME. Vaccine effectiveness estimates, 2004-2005 mumps outbreak, England. Emerg Infect Dis. 2007;13(1):12–17. doi:10.3201/eid1301.060649.
- Pang H, Zhou Y, Zhao W, Jiang Q. Seroprevalence and determinants associated with mumps antibodies after 20 years of MMR vaccination in urban area of Shanghai, China. Int J Environ Res Public Health. 2018:15. doi:10.3390/ijerph15102089.
- Schwarz NG, Bernard H, Melnic A, Bucov V, Caterinciuc N, An der Heiden M, Andrews N, Pebody R, Aidyralieva C, Hahné S. Mumps outbreak in the Republic of Moldova, 2007-2008. Pediatr Infect Dis J. 2010;29(8):703–06. doi:10.1097/INF.0b013e3181d743df.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization — recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011;60:1–64.
- Marin M, Marlow M, Moore KL, Patel M. Recommendation of the advisory committee on immunization practices for use of a third dose of mumps virus-containing vaccine in persons at increased risk for mumps during an outbreak. MMWR Morb Mortal Wkly Rep. 2018;67(1):33–38. doi:10.15585/mmwr.mm6701a7.
- Ceyhan M, Kanra G, Erdem G, Kanra B. Immunogenicity and efficacy of one dose measles-mumps-rubella (MMR) vaccine at twelve months of age as compared to monovalent measles vaccination at nine months followed by MMR revaccination at fifteen months of age. Vaccine. 2001;19(31):4473–78. doi:10.1016/s0264-410x(01)00207-9.