ABSTRACT
Expanding serotype coverage of pneumococcal conjugate vaccines (PCVs) to target prevailing disease-causing serotypes could further reduce disease burden. To address this need, 2 different PCVs have been investigated: a 20-valent PCV (PCV20; includes the 13 serotypes in the 13-valent PCV [PCV13] plus 7 additional serotypes [8, 10A, 11A, 12F, 15B, 22F, 33F]) and a complementary 7-valent PCV (cPCV7; contains only the 7 additional serotypes). This phase 1b, randomized, controlled, double-blind study evaluated PCV20 and cPCV7 safety and immunogenicity in healthy Japanese adults 18–49 years of age residing in the United States for ≤5 years. Participants (n = 104) were randomized equally to receive a single dose of PCV20, cPCV7, or PCV13. Immunogenicity was assessed at baseline and 1 month after vaccination using serotype-specific opsonophagocytic activity (OPA) titers and serotype-specific immunoglobulin G (IgG) concentrations. Prompted local reactions and systemic events; adverse events (AEs); and serious AEs and newly diagnosed chronic disease were assessed 14 days, through 1 month, and upto 6 months following vaccination, respectively. OPA immune responses were robust for all 20 serotypes in the PCV20 group and for the 7 serotypes in the cPCV7 group 1 month after vaccination. IgG immune response showed similar trends. Injection site pain and muscle pain were the most common local reaction and systemic event; the majority were mild or moderate in severity. Few AEs and no severe AEs, serious AEs, or safety-related withdrawals were reported. Taken together, administration of PCV20 or cPCV7 in Japanese adults was well tolerated and induced robust serotype-specific functional immune responses. NCT03642847.
Introduction
Streptococcus pneumoniae infection causes invasive pneumococcal disease (IPD), which includes meningitis, bacteremia, and community-acquired pneumonia (CAP), as well as common noninvasive diseases that cause a health burden, such as acute otitis media and sinusitis.Citation1,Citation2 The global burden of pneumococcal disease is substantial, especially in young children and adults ≥65 years of age; in 2016, approximately 197 million episodes of lower respiratory infections caused by S pneumoniae and 1.2 million associated deaths occurred worldwide.Citation3,Citation4 In 2017, the incidence of IPD in Japanese adults ≥65 years of age was 5.38 per 100,000, with 8.3% of all 1891 cases resulting in death.Citation5 Since IPD is only a small portion of pneumococcal disease (approximately 10%–25% of pneumococcal CAP may be bacteremicCitation6,Citation7), the true burden is expected to be much greater.
Although more than 95 pneumococcal serotypes have been identified, the majority of IPD is caused by a limited number of these serotypes.Citation4,Citation8 Pneumococcal vaccines targeting multiple serotypes are available: these include the 23-valent PCV (PPSV23; Pneumovax® 23, Merck and Co., Inc., Whitehouse Station, NJ), which contains unconjugated polysaccharides, as well as pneumococcal conjugate vaccines (PCVs) such as the 13-valent pneumococcal conjugate vaccine (PCV13; Prevenar 13®, Pfizer Inc, New York, NY).Citation4 In contrast to PPSV23, PCVs generally induce T-cell–dependent immunity, which generates robust functional antibody responses associated with long-term protection.Citation9,Citation10 In addition, PCVs are efficacious against nonbacteremic vaccine-type CAP in adultsCitation11 and evidence from several countries, including Japan, has established PCV effectiveness against disease in both vaccinated and unvaccinated populations.Citation5,Citation12-14
In November 2013, PCV13 was recommended for routine vaccination of children in Japan, including those ≤5 years of age who are at increased risk of pneumococcal disease.Citation5 In addition, PPSV23 is also routinely recommended for adults ≥65 years of age in Japan who are at increased risk of pneumococcal disease; PCV13 may also be used in adults.Citation5 Despite routine PPSV23 recommendations, 66% of IPD cases among adults in Japan occurring between April 2013 and March 2015 were caused by the PPSV23 serotypes, with 22% of cases attributed to serotypes contained in PPSV23 but not PCV13.Citation15 Thus, increasing serotype coverage with expanded-valent PCVs would address a continued unmet need.Citation13,Citation16-21
A 20-valent PCV (PCV20) in development contains the serotypes present in PCV13 as well as 7 additional serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F) to further expand serotype coverage of disease-causing serotypes beyond PCV13. The serotypes targeted by PCV20 were selected based on their prevalence and wide geographic distribution among the residual pneumococcal disease-causing serotypes, as well as for their antibiotic resistance and disease severity characteristics.Citation13,Citation16-18,Citation20,Citation22-24 The same 7 additional serotypes in PCV20 are also contained in PPSV23;Citation25 however, PCV20 is anticipated to further reduce the global burden of pneumococcal disease by providing a robust response and protection against these 7 additional serotypes than PPSV23, while continuing to protect against the 13 serotypes in PCV13. The complementary 7-valent PCV (cPCV7) is a similar formulation to PCV20, but does not include the PCV13 serotypes. cPCV7 is being investigated as a potential alternative approach to expand coverage when used in complement with PCV13. A phase 1/2 study of cPCV7 in healthy adults 50–85 years of age (NCT03313050) has been completed.
In a first-in-human study in healthy adults 18–49 years of age and a phase 2 study in healthy adults 60–64 years of age, PCV20 was well tolerated and induced functional immune responses against all 20 vaccine serotypes.Citation26,Citation27 To expand the available data for PCV20 and cPCV7 and to support clinical development of PCV20 in Japan, this phase 1b, randomized, controlled, double-blind study evaluated the safety and immunogenicity of PCV20 and cPCV7 in Japanese adults residing in the United States.
Methods
Study design and participants
This phase 1b study (NCT03642847) was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with all International Council for Harmonisation Good Clinical Practice Guidelines. All participants provided consent for inclusion in the study before any study-specific activity was performed.
Key inclusion criteria were Japanese men and women 18–49 years of age (ie, of Japanese ethnicity who were born in Japan and with both parents and 4 grandparents who were born in Japan) who have not lived outside of Japan for >5 years in total. Exclusion criteria were current or past history of clinically significant hematologic, renal, endocrine, pulmonary, gastrointestinal, cardiovascular, hepatic, psychiatric, neurologic, immunologic, metabolic, urologic, or dermatologic disease, or any other major disease/condition, previous pneumococcal vaccination or microbiologically proven IPD, or receipt of immunosuppressive therapy or known/suspected immunodeficiency or immunosuppressive conditions.
This study had a 3-arm, parallel design and enrolled participants who were randomized at each center in a 1:1:1 ratio to receive PCV20, cPCV7, or PCV13. Each participant was randomized to a vaccine group using an interactive response technology system (interactive Web-based response) following a computer-generated randomization allocation schedule. Study investigators and participants were blinded to vaccine group and remained blinded until the last participant completed the follow-up visit at 6 months after vaccination. A single 0.5-mL dose of PCV20, cPCV7, or PCV13 was administered intramuscularly into the deltoid muscle. Each dose of PCV20 and PCV13 contains 2.2 µg of each saccharide within its formulation except for 6B, which contains 4.4 µg. cPCV7 contains 2.2 µg of each saccharide.
In this study, PCV13 served as a control for safety assessments of PCV20 and cPCV7 and to provide context for the descriptive immunogenicity assessments of the 13 serotypes in common with PCV20. Participants had blood drawn on the day of vaccination and approximately 1 month after vaccination.
Assessments
Participants were asked to monitor and record local reactions, systemic events, fever, and antipyretic/pain medication use each evening for 14 days following vaccination using an electronic diary. Local reactions included redness, swelling, and pain at the injection site, and systemic events included headache, fatigue, muscle pain, and joint pain. Participants were provided a digital thermometer and asked to record oral temperature; a fever was defined as an oral temperature ≥38.0°C. Adverse events (AEs) were collected from consent through 1 month after vaccination. Serious AEs (SAEs) and newly diagnosed chronic medical conditions (NDCMCs) were collected up to 6 months after vaccination. Acute reactions occurring within the first 30 minutes after vaccination were documented as an AE or SAE as appropriate.
Blood samples for immunogenicity assessments were collected before and 1 month after vaccination. Opsonophagocytic activity (OPA) titers and concentrations of anticapsular immunoglobulin G (IgG) for the 20 pneumococcal serotypes in PCV20 (ie, 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, and 33F) were determined. The assays used to measure OPA titers and IgG concentrations for each of the 20 serotypes have been described previously.Citation26
Objectives and endpoints
The primary objective was to describe the safety of PCV20 and cPCV7, which included local reactions, systemic events, AEs, SAEs, and NDCMCs. The secondary objective was to describe the immunogenicity of PCV20 and cPCV7 as measured by pneumococcal serotype-specific OPA geometric mean titers (GMTs) to each of the 20 serotypes at 1 month after vaccination. Other endpoints included IgG geometric mean concentrations (GMCs) at 1 month after vaccination and the geometric mean fold-rises (GMFRs) in OPA titers and IgG concentrations from before to 1 month after vaccination.
Analyses
The study sample size of 33 adults per vaccine group to characterize safety and immunogenicity with PCV20 and cPCV7 was not based on any formal statistical hypothesis testing. All analyses for the safety and immunogenicity data were descriptive in nature. Safety endpoints were analyzed with the safety population, which included any participant who received 1 dose of PCV20, cPCV7, or PCV13 with safety follow-up after vaccination. Percentages with 2-sided Clopper-Pearson 95% CIs were provided by vaccine group for all primary safety endpoints (ie, percentages of participants reporting local reactions, systemic events, or AEs).
Immunogenicity results were analyzed based on the evaluable immunogenicity population, which included any participant who received randomized vaccine, had blood collection between 27–49 days after vaccination, had a valid OPA titer or IgG concentration determined for ≥1 serotype at the 1 month after vaccination time point, and did not have any other major protocol violations. Serotype-specific OPA geometric mean titers and the 2-sided 95% CIs from each vaccine group were calculated based on the assay results in natural log scale and then exponentiating the results following the Student’s t distribution. OPA GMFRs and the 2-sided 95% CIs from before vaccination to 1 month after vaccination were calculated using the same method as for the OPA GMTs and the 95% CIs, but performed on the ratios of OPA titers at 1 month after to before vaccination. Serotype-specific pneumococcal anticapsular IgG GMCs and GMFRs were calculated similarly as OPA GMTs and GMFRs. OPA titers or IgG concentrations below the lower limit of quantitation (LLOQ) were set to 0.5 × LLOQ for analyses.
Results
Participants
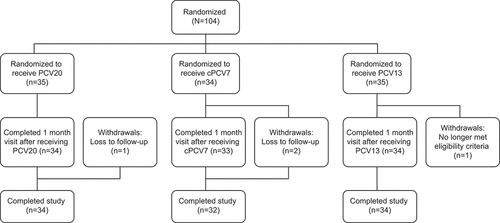
The study was conducted from August 29, 2018, through March 29, 2019, at 3 study sites in the United States. A total of 104 participants were randomized (PCV20, n = 35; cPCV7, n = 34; PCV13, n = 35; ). Overall, 103 participants received vaccination; 1 participant was randomized to the PCV13 group, but did not receive vaccine as the participant was found not to meet eligibility criteria. A total of 100 participants (96.2%) were vaccinated and completed the study (3 participants received vaccine but were lost to follow-up). The safety population included 103 participants (PCV20, n = 35; cPCV7, n = 34; PCV13, n = 34). A total of 101 participants were included in the evaluable immunogenicity population (3 participants, 1 in each group, did not have a valid assay result for ≥1 serotype at 1 month after the vaccination time point). Demographic characteristics were similar for the vaccine groups; 59.2% of participants were women and the mean (SD) age was 31.3 (10.1) years ().
Table 1. Participant Demographics (Safety Population)
Safety
Overall, 77.1% (n = 27), 76.5% (n = 26), and 79.4% (n = 27) of participants in the PCV20, cPCV7, and PCV13 groups experienced local reactions, respectively ()). The most frequently reported local reaction for each group was pain at the injection site (PCV20: 77.1%, n = 27; cPCV7: 76.5%, n = 26; PCV13: 79.4%, n = 27); most local reactions were mild or moderate in severity. The median onset of local reactions across vaccine groups was 1.0–3.0 days and generally of short duration.
Figure 2. Reactogenicity Events Including (a) Local Reactions and (b) Systemic Events Occurring Up to 14 Days After Vaccination. The single report of fever (38.8°C; 1 participant in the PCV20 group) is not included. Number of participants: PCV20, n = 35; cPCV7, n = 34; PCV13, n = 34. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; PCV13 = 13-valent pneumococcal conjugate vaccine; PCV20 = 20-valent pneumococcal conjugate vaccine
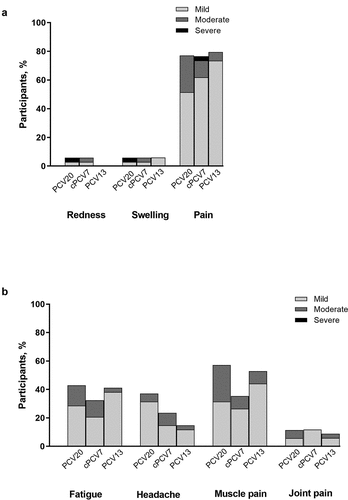
Systemic events were experienced by 77.1% (n = 27), 41.2% (n = 14), and 64.7% (n = 22) of participants in the PCV20, cPCV7, and PCV13 groups, respectively ()). The most frequent systemic events reported across all vaccine groups were muscle pain, fatigue, and headache. The majority of systemic events in each vaccine group were mild or moderate in severity. One participant in the PCV20 group experienced a fever of 38.8°C on the day of vaccination that resolved by the next day; no other fevers were reported.
At 1 month after vaccination, the percentages of participants reporting any AE were similar across study groups (PCV20: 5.7%, n = 2; cPCV7: 5.9%, n = 2; PCV13: 8.8%, n = 3). Three AEs were considered related to vaccination (injection site erythema and injection site swelling in the PCV20 group; fatigue in the cPCV7 group). No severe AEs, SAEs, safety-related withdrawals, or NDCMCs were reported, and no deaths occurred during the study.
Immunogenicity
Before vaccination, OPA GMTs for all 20 serotypes were generally low and similar across the vaccine groups (; Supplemental Table 1). At 1 month after vaccination, OPA GMTs were higher than before vaccination for the vaccine serotypes across all groups. One month after PCV20 administration, OPA GMFRs for the PCV20 serotypes ranged from 6.1-fold (serotypes 3 and 11A) to 150.7-fold (serotype 8). At 1 month after cPCV7 administration, OPA GMFRs for the cPCV7 serotypes ranged from 6.8-fold (serotype 11A) to 192.7-fold (serotype 15B). At 1 month after PCV13 administration, OPA GMFRs for the PCV13 serotypes ranged from 9.2-fold (serotype 3) to 177.6-fold (serotype 4).
Figure 3. Pneumococcal OPA GMTs and GMFRs With 2-Sided 95% CIs for Each Serotype. Assay results below the LLOQ were set to 0.5 × LLOQ in the analysis. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMFRs = geometric mean fold-rises from before vaccination to 1 month after vaccination; GMTs = geometric mean titers; LLOQ = lower limit of quantitation; OPA = opsonophagocytic activity; PCV13 = 13-valent pneumococcal conjugate vaccine; PCV20 = 20-valent pneumococcal conjugate vaccine
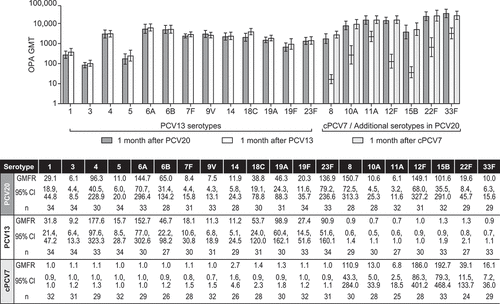
The majority of participants in the PCV20 group achieved ≥4-fold rises in OPA titers for all PCV20 serotypes from before vaccination to 1 month after PCV20 administration, with frequency ranging from 54.8% (serotype 7F) to 97.0% (serotype 23F) (Supplemental Table 2). The percentage of participants in the cPCV7 group achieving ≥4-fold rises in OPA titers for the cPCV7 serotypes ranged from 40.0% (serotype 11A) to 96.4% (serotype 12F). For the PCV13 group, the percentage of participants achieving ≥4-fold rises in OPA titers for the PCV13 serotypes ranged from 55.2% (serotype 14) to 100% (serotype 1). These same trends were observed in the IgG responses (Supplemental Figure 1; Supplemental Tables 3, 4).
Discussion
This phase 1b study showed that PCV20 and cPCV7 were safe and well tolerated among healthy Japanese adults, with a safety profile that was similar to PCV13. In addition, PCV20 and cPCV7 elicited robust serotype-specific functional OPA immune responses.
PCVs induce T-cell–dependent immune responses and are associated with long-term protection against pneumococcal disease.Citation28 Moreover, PCVs have shown efficacy against nonbacteremic pneumonia and otitis media and can reduce nasopharyngeal colonization, providing herd immunity.Citation29,Citation30 In contrast, PPSV23 elicits T-cell−independent immune responses that provide short-lived protection; PPSV23 also does not reduce vaccine-serotype nasopharyngeal carriage and the impact of PPSV23 on nonbacteremic pneumonia remains questionable.Citation28,Citation31,Citation32 Therefore, PCV20 has the potential to improve protection against pneumococcal disease compared with PPSV23.
Although the introduction and continued use of PCVs have led to significant global progress toward preventing pneumococcal disease, combating the additional serotypes that continue to cause disease remains an unmet need.Citation20 In the 3 years following the introduction of childhood PCV7 in Japan in 2010, a 57% reduction in all IPD incidence was observed among children <5 years of age.Citation12 Following replacement of PCV7 with PCV13 for routine childhood immunization, a 97% decrease in the incidence of childhood IPD caused by PCV13 serotypes was observed by 2017; however, non-PCV13 serotypes continue to cause IPD, including more than 20% of IPD cases in adults in Japan caused by serotypes contained only in PPSV23.Citation5,Citation15 Notably, PPSV23 is the only pneumococcal vaccine routinely recommended for individuals ≥65 years of age in Japan;Citation5 this suggests that the known limitations of PPSV23, such as poor vaccine effectiveness especially against pneumonia, short-lived duration of protection, and quality of immune responses induced by unconjugated polysaccharide, might not be optimal for pneumococcal disease protection.Citation5,Citation28,Citation33 Thus, broadening serotype coverage of PCVs to include those prevailing serotypes responsible for disease is essential to both directly and indirectly protect against pneumococcal disease.
A previous first-in-human study found PCV20 to be well tolerated and to induce immune responses against all 20 vaccine serotypes in healthy adults 18–49 years of age.Citation26 Additionally, a phase 2 study in US adults 60–64 years of age showed the safety profile of PCV20 was consistent with PCV13, and PCV20 elicited robust OPA responses to all vaccine serotypes that were somewhat lower to those elicited by PCV13 and generally higher than those for PPSV23 for the serotypes in common.Citation27 The phase 2 data supported phase 3 clinical development of PCV20 in adults and demonstrate the potential for PCV20 to provide expanded protection against pneumococcal disease. Following the findings of that phase 2 study, PCV20 received breakthrough therapy designation by the United States Food and Drug Administration for adults 18 years and older.Citation34 The results of the current PCV20 study showed tolerability and immunogenicity findings in Japanese adults are similar to the previous PCV20 phase 1 and 2 studies, providing support for the continued development of PCV20 in this population. Additional safety and immunogenicity data with PCV20 are anticipated from adult phase 3 studies, including a pivotal study assessing the noninferiority of immune responses between PCV20 and the licensed PCV13 and PPSV23 (NCT03828617, NCT03760146, and NCT03835975). The current study also showed that functional immune responses were elicited to all 7 vaccine serotypes after a single dose of cPCV7, and the vaccine was well tolerated with few AEs reported in healthy Japanese adults.
This is the first study to assess the safety and immunogenicity of PCV20 and cPCV7 in Japanese adults 18–49 years of age and is strengthened by its randomized design. Potential limitations of the study are the small size, which fits the purpose of the study but which may limit the breadth of conclusions drawn, and the ability to generalize to other age or racial groups. However, the results in this small study suggest that the safety and immunogenicity profile of PCV20 in a Japanese population is similar to those in prior PCV20 studies in non-Japanese populations.
In conclusion, PCV20 and cPCV7 were well tolerated and induced serotype-specific functional OPA immune responses in Japanese adults. These results help support clinical development of PCV20 in Japan.
Disclosure of Potential Conflicts of Interest
David Fitz-Patrick has received research grants from Pfizer. Mariano Young, Daniel A. Scott, Ingrid L. Scully, Gary Baugher, Yahong Peng, Kathrin U. Jansen, William Gruber, and Wendy Watson are employees of Pfizer and may hold stock or stock options.
Data sharing statement
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Supplemental Material
Download MS Word (143.5 KB)Acknowledgments
Editorial/medical writing support was provided by Tricia Newell, PhD, and Emily Stackpole, PhD, of ICON plc (North Wales, PA, USA), and was funded by Pfizer Inc.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- World Health Organization. 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec. 2008;83(42):373–84.
- Wald ER. Acute otitis media and acute bacterial sinusitis. Clin Infect Dis. 2011;52 Suppl 4(Suppl4):S277–283. doi:10.1093/cid/cir042.
- Collaborators GBDLRI. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210. doi:10.1016/S1473-3099(18)30310-4.
- World Health Organization. Pneumococcal vaccines WHO position paper–2012. Wkly Epidemiol Rec. 2012;87(14):129–44.
- National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division. Pneumococcal infections in 2017. Japan: Ministry of Health, Labour and Welfare of Japan; 2018.
- Said MA, Johnson HL, Nonyane BA, Deloria-Knoll M, O’Brien KL, AGEDD Adult Pneumococcal Burden Study Team, Andreo F, Beovic B, Blanco S, Boersma WG, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8(4):e60273. doi:10.1371/journal.pone.0060273.
- McLaughlin JM, Jiang Q, Isturiz RE, Sings HL, Swerdlow DL, Gessner BD, Carrico RM, Peyrani P, Wiemken TL, Mattingly WA, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against hospitalization for community-acquired pneumonia in older US adults: a test-negative design. Clin Infect Dis. 2018;67(10):1498–506. doi:10.1093/cid/ciy312.
- Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev. 2015;28(3):871–99. doi:10.1128/CMR.00024-15.
- Shiramoto M, Irie S, Juergens C, Yamaji M, Tamai S, Aizawa M, Belanger T, Gruber WC, Scott DA, Schmoele-Thoma B. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine when administered to healthy Japanese adults aged ≥50 years: an open-label trial. Hum Vaccin Immunother. 2014;10(7):1850–58. doi:10.4161/hv.28633.
- Frenck RW Jr., Fiquet A, Gurtman A, van Cleeff M, Davis M, Rubino J, Smith W, Sundaraiyer V, Sidhu M, Emini EA, et al. Immunogenicity and safety of a second administration of 13-valent pneumococcal conjugate vaccine 5 years after initial vaccination in adults 50 years and older. Vaccine. 2016;34(30):3454–62. doi:10.1016/j.vaccine.2016.04.093.
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–25. doi:10.1056/NEJMoa1408544.
- Suga S, Chang B, Asada K, Akeda H, Nishi J, Okada K, Wakiguchi H, Maeda A, Oda M, Ishiwada N, et al. Nationwide population-based surveillance of invasive pneumococcal disease in Japanese children: effects of the seven-valent pneumococcal conjugate vaccine. Vaccine. 2015;33:6054–60. doi:10.1016/j.vaccine.2015.07.069.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–09. doi:10.1016/S1473-3099(14)71081-3.
- Myint TT, Madhava H, Balmer P, Christopoulou D, Attal S, Menegas D, Sprenger R, Bonnet E. The impact of 7-valent pneumococcal conjugate vaccine on invasive pneumococcal disease: a literature review. Adv Ther. 2013;30(2):127–51. doi:10.1007/s12325-013-0007-6.
- Fukusumi M, Chang B, Tanabe Y, Oshima K, Maruyama T, Watanabe H, Kuronuma K, Kasahara K, Takeda H, Nishi J, et al. Invasive pneumococcal disease among adults in Japan, April 2013 to March 2015: disease characteristics and serotype distribution. BMC Infect Dis. 2017;17(1):2. doi:10.1186/s12879-016-2113-y.
- Hausdorff WP, Hanage WP. Interim results of an ecological experiment - conjugate vaccination against the pneumococcus and serotype replacement. Hum Vaccin Immunother. 2016;12(2):358–74. doi:10.1080/21645515.2015.1118593.
- Metcalf BJ, Gertz RE Jr., Gladstone RA, Walker H, Sherwood LK, Jackson D, Li Z, Law C, Hawkins PA, Chochua S, et al. Strain features and distributions in pneumococci from children with invasive disease before and after 13-valent conjugate vaccine implementation in the USA. Clin Microbiol Infect. 2016;22(1):60.e69-60.e29. doi:10.1016/j.cmi.2015.08.027.
- Tomczyk S, Lynfield R, Schaffner W, Reingold A, Miller L, Petit S, Holtzman C, Zansky SM, Thomas A, Baumbach J, et al. Prevention of antibiotic-nonsusceptible invasive pneumococcal disease with the 13-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2016;62(9):1119–25. doi:10.1093/cid/ciw067.
- Vadlamudi NK, Chen A, Marra F. Impact of 13-pneumococcal conjugate vaccine among adults: a systematic review and meta-analysis. Clin Infect Dis. 2018. doi:10.1093/cid/ciy872.
- Wantuch PL, Avci FY. Invasive pneumococcal disease in relation to vaccine type serotypes. Hum Vaccin Immunother. 2019;15(4):874–75. doi:10.1080/21645515.2018.1564444.
- Isturiz RE, Ramirez J, Self WH, Grijalva CG, Counselman FL, Volturo G, Ostrosky-Zeichner L, Peyrani P, Wunderink RG, Sherwin R, et al. Pneumococcal epidemiology among US adults hospitalized for community-acquired pneumonia. Vaccine. 2019;37(25):3352–61. doi:10.1016/j.vaccine.2019.04.087.
- Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLoS One. 2017;12(5):e0177113. doi:10.1371/journal.pone.0177113.
- Cui YA, Patel H, O’Neil WM, Li S, Saddier P. Pneumococcal serotype distribution: a snapshot of recent data in pediatric and adult populations around the world. Hum Vaccin Immunother. 2017;13(6):1–13. doi:10.1080/21645515.2016.1277300.
- Yahiaoui RY, Bootsma HJ, Den Heijer CDJ, Pluister GN, John Paget W, Spreeuwenberg P, Trzcinski K, Stobberingh EE. Distribution of serotypes and patterns of antimicrobial resistance among commensal Streptococcus pneumoniae in nine European countries. BMC Infect Dis. 2018;18(1):440. doi:10.1186/s12879-018-3341-0.
- Pneumovax® 23 (pneumococcal vaccine polyvalent). Full prescribing information. Whitehouse Station (NJ): Merck & Co., Inc.; 2015.
- Thompson A, Lamberth E, Severs J, Scully I, Tarabar S, Ginis J, Jansen KU, Gruber WC, Scott DA, Watson W. Phase1 trial of a 20-valent pneumococcal conjugate vaccine in healthy adults. Vaccine. 2019;37(42):6201–07. doi:10.1016/j.vaccine.2019.08.048.
- Hurley D, Griffin C, Young M, Scott DA, Pride MW, Scully IL, Ginis J, Severs J, Jansen KU, Gruber WC, et al. Safety, tolerability, and immunogenicity of a 20-Valent Pneumococcal Conjugate Vaccine (PCV20) in adults 60 to 64 years of age. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa1045.
- Clutterbuck EA, Lazarus R, Yu LM, Bowman J, Bateman EA, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. 2012;205(9):1408–16. doi:10.1093/infdis/jis212.
- Sings HL. Pneumococcal conjugate vaccine use in adults - addressing an unmet medical need for non-bacteremic pneumococcal pneumonia. Vaccine. 2017;35:5406–17. doi:10.1016/j.vaccine.2017.05.075.
- Yildirim I, Shea KM, Pelton SI. Pneumococcal disease in the era of pneumococcal conjugate vaccine. Infect Dis Clin North Am. 2015;29(4):679–97. doi:10.1016/j.idc.2015.07.009.
- Daniels CC, Rogers PD, Shelton CM. A review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. J Pediatr Pharmacol Ther. 2016;21(1):27–35. doi:10.5863/1551-6776-21.1.27.
- Schuchat A. Pneumococcal prevention gets older and wiser. JAMA Intern Med. 2015;175(12):1897–98. doi:10.1001/jamainternmed.2015.6133.
- Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422. doi:10.1002/14651858.CD000422.pub3.
- Pfizer Inc. Pfizer initiates phase 3 program for 20-valent pneumococcal conjugate vaccine for the prevention of invasive disease and pneumonia in adults aged 18 years and older. Business Wire. [accessed 2020 July 5]. https://www.businesswire.com/news/home/20181214005069/en/.