ABSTRACT
In India, the high neonatal and infant mortality rate is due in part to an increasing number of preterm and low birth weight (LBW) infants. Given the immaturity of immune system, these infants are at an increased risk of hospitalization and mortality from vaccine-preventable diseases (VPDs). In this narrative review, we screened the scientific literature for data on the risk of VPDs, vaccination delay and factors related to it in Indian preterm and LBW infants. Although routine childhood vaccinations are recommended regardless of gestational age or birth weight, vaccination is often delayed. It exposes these infants to a higher risk of infections, their associated complications, and death. After-birth complications, lack of awareness of recommendations, vaccine efficacy and effectiveness and concerns related to safety are some of the common barriers to vaccination. Awareness campaigns might help substantiate the need for (and value of) vaccination in preterm and LBW infants.
PLAIN LANGUAGE SUMMARY
What is the context?
In India, the high neonatal mortality rate is due in part to an increasing number of pretern and low birth weight intants.
Affected infants have a poorly developed inmune system and are more susceptible to contracting vaccine-preventable diseases.
The Indian Academy of Pediatrics recommends vaccination according to the same schedule used for full term infants, following chronological (not gestational) age.
Delays in vaccinations increase the risk of preventable infections.
What is new?
Our review of the scientific literature shows that, in India:
infections have more serious conseuences in preterm and low birth weight infants
delays to vaccinate affected infants are common, mostly due to safety and effectiveness concerns from parents and healthcare pracitionrs.
What is the impact?
Improving mternal nutritional status and immunization, and perinatal care could help reduce the number of preterm and low birth weight infants.
Combining maternal immunization with vaccination of affected infants can confer safe and effective protection.
Awareness campaigns for parents and healthcare practitioners could address the issue of vaccination delay in pretern and low birth weight infants in India.
Introduction
Preterm birth and low birth weight (LBW) in newborns is a source of significant global public health concern.Citation1 Although LBW often characterizes preterm babies, the two terms cannot be used interchangeably.Citation2 World Health Organization (WHO) defines preterm newborn as birth before 37 weeks of gestation and further categorizes it into extremely preterm (<28 weeks), very preterm (28–32 weeks) and moderate to late preterm (32–37 weeks).Citation3 LBW is defined as weight at birth of <2,500 g and is categorized into very LBW (<1,500 g) and extremely LBW (<1,000 g).Citation4
Preterm birth can be either spontaneous or induced (e.g. elective cesarean or other non-medical reasons).Citation5,Citation6 Correspondingly, LBW could be associated with preterm birth, or could be due to restricted fetal growth, or a combination of both.Citation7 Risk factors for prematurity and LBW include undernutrition, genetics, infections, underlying comorbidities (e.g., diabetes), maternal history of multiple pregnancies, chronic maternal stress induced by infections and inflammation, socioeconomic factors, and lifestyle choices of the mother (e.g., smoking).Citation6,Citation8
Preterm and LBW infants are at a higher risk of infections and death compared to full-term and normal birth weight infants.Citation9 The major risk factors are perinatal infections, prolonged hospitalization after birth, iatrogenic complications of lifesaving therapies, low levels of circulating maternal antibodies, and an immature immune system.Citation9 Specifically, the immaturity of the immune system is known to increase with decreasing gestational age and birth weight.Citation10–12 Perinatal infections could be fatal and are associated with long-term sequelae that can lead to impaired neuro-developmental functioning, inhibited growth, chronic diseases and long-term physical health consequences.Citation6,Citation10–12
Increasing numbers of preterm and LBW newborns every year could add to the disease burden on healthcare systems and individual families, depending on the setting.Citation1,Citation6,Citation8,Citation13 According to the WHO, more than 10% of infants (i.e., ~15 million infants per year) were born preterm and 15%–20% of infants (i.e., >20 million infants per year) were born with LBW in 2014–2015.Citation1,Citation2,Citation14 Preterm birth directly contributes to neonatal mortality, accounting for nearly 1 million deaths every year,Citation1 while LBW is a major predictor of mortality and morbidity in preterm children.Citation1 Highest levels of neonatal mortality and morbidity are reported in low- and middle-income countries, with Africa and Asia being responsible for the majority of this public health burden.Citation15,Citation16 In 2018, approximately 50% of all deaths under 5 years of age were reported from just five countries: Democratic Republic of the Congo, Ethiopia, India, Nigeria, and Pakistan. Among these countries, about 33% of deaths were reported in Nigeria and India alone.Citation17
The Indian context
In 2017, India recorded approximately one million deaths (20% of the global) among children under 5 years of age.Citation18 Of these, 0.57 million were neonatal deaths in which the reported causes were preterm birth (27.7%), encephalopathy due to birth asphyxia and trauma (14.5%), lower respiratory infections (11.0%), congenital birth defects (8.6%), sepsis and other infections (6.1%), hemolytic disease and jaundice (3.2%), diarrheal diseases (2.7%), tetanus (0.7%), other disorders (22.0%), and other causes (3.5%).Citation18 This situation is alarming as India accounts for 23.4% of the global preterm births.Citation14 Estimates of LBW infants are notable: during 2013–2014, amongst approximately 19 million newborns,Citation19 68.7% were weighed at birth and among these, 18.6% were LBW (i.e., approximately 2.43 million births).Citation20
The majority of deaths in children under 5 years of age and morbidity associated with infectious diseases can be averted by timely interventions including adequate nutrition, clean water, appropriate maternal care during pregnancy and immunization of the mother and infant.Citation17 The WHO and the Advisory Committee on Vaccines and Immunization Practices of the Indian Academy of Pediatrics (IAP) recommend that all infants receive immunization, regardless of any restrictions based on gestational age or birth weight, with the qualified exception of the hepatitis B vaccine as the birth dose is not counted toward the full schedule due to a reduced immune response.Citation21–23 provides an overview of the recommended vaccines in children ≤12 months of age.
Table 1. Vaccination recommendations and overview of availability of immunogenicity and safety of recommended vaccinations for preterm and LBW infants ≤12 months of age
Rationale of the review
Despite the existence of vaccination recommendations, several studies in high-income countries have reported either a significant delay or a complete lack of immunization in preterm infants.Citation47–50 The situation is unlikely to be different in India, as a high level of vaccine-preventable disease (VPD) burden in infants or children persists.Citation51 Within this context, there is a need to better understand the factors and barriers related to the absence or delay in vaccination among preterm and LBW infants. This information could help bridge existing knowledge gaps in the scientific community, specifically among healthcare providers (HCPs) who are perceived as the most trusted advisors and influencers of vaccination decisions.Citation52
A recent publication summarizing practical issues surrounding vaccination in preterm infants lends support to the implementation of existing vaccination recommendations for preterm and LBW infants in India.Citation53 However, information on the extent of vaccination delay in preterm and LBW infants has not been previously summarized. In this review, we outline the rationale for immunization and highlight the risks of VPDs in preterm and LBW infants. We also provide an overview of recommended vaccinations, with a focus on whether efficacy/effectiveness and safety data are available in these populations. Lastly, we present the caveats linked to different vaccination strategies that could be utilized to mitigate the burden of VPDs in preterm and LBW infants in India. elaborates on the findings in a form that could be shared with patients by HCPs.
Characteristics of the immune system of preterm and LBW infants
Neonates predominantly rely on their first line of defense (physical barrier) and then innate immune response rather than adaptive immune response. At birth, both immune defense mechanisms are immature.Citation54 This immune system immaturity is amplified in infants born preterm and in those with LBW, due to several deficiencies ().
Table 2. Characteristics of the immune response in preterm and LBW infantsCitation10,Citation54–Citation56.
Defense against pathogens consists of physical barriers, such as keratinized skin and mucous membranes lining the respiratory and gastrointestinal tracts, and chemical barriers containing various enzymes and other substances that elicit a direct antimicrobial action or inhibit microbial adherence to body surfaces.Citation55 Compared to full-term infants, this barrier is undeveloped in preterm and LBW infants, making it susceptible to ruptures and therefore serving as an inefficient defense barrier.Citation55 Furthermore, antimicrobial peptides-producing flora are reduced in number within the mucosal barrier of the respiratory and gastrointestinal tracts, thus facilitating the penetration of pathogens and increasing the risk of infection.Citation57
When pathogens cross the first line of defense, the innate immune response is triggered through several pathways. This innate immune response is partial in preterm and LBW infants due to availability of smaller number of neutrophils compared to full-term and normal birth weight infants. Neutrophils generate oxygen radicals that facilitate intracellular killing of pathogens and can also perform phagocytosis.Citation55,Citation56,Citation58 Similarly, a smaller pool of monocytes is available in preterm and LBW infants. Monocytes are capable of phagocytosis, secretion of cytokines or chemokines and antigen presentation, and regulate the activation of B-cells and T-cells, which are integral parts of the adaptive immune response.Citation55,Citation56 Consequently, preterm and LBW infants are at a high risk of infection ().
Intrauterine inflammation, which may cause premature immune activation and cytokine production, directly contributes to preterm birth,Citation56 and may lead to immune tolerance and reduced immune function in preterm and LBW newborns. Furthermore, medical interventions at the time of delivery can impact immune development and function. For example, antenatal corticosteroid treatment to prevent newborn respiratory disease is associated with reductions in lymphocyte proliferation, cytokine production and an increased risk of infection.Citation56
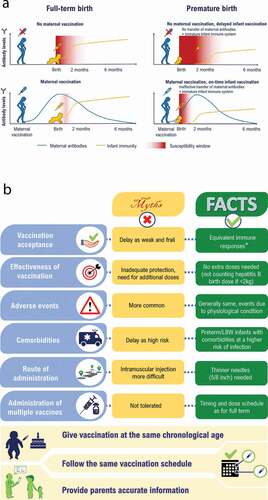
Soluble proteins such as immunoglobulins (Ig) and peptides facilitate phagocytosis and elicit antimicrobial properties. The production of soluble proteins by the fetus is limited and thus adaptive immunity is mostly provided through maternal antibodies. Maternal IgG antibodies are transferred to the fetus starting at approximately 17 weeks of gestation, with cord-blood IgG levels similar to maternal titers after 32 weeks of gestation and up to 2-fold higher at term birth.Citation59,Citation60 Due to this, preterm infants have low levels of circulating maternal IgG as a function of gestational age at birth. This leads to a higher susceptibility of infants to contract infections, including those that can be prevented by vaccinations.Citation59,Citation61
VPDs in preterm and LBW infants
Newborns usually contract infections either in the perinatal or the postpartum period. Exposure to infections is especially critical in preterm and LBW infants because of their immature immune system and inadequate levels of maternal antibodies.Citation54–56,Citation59 This aspect is depicted in for reference. Data on the risk of VPDs among preterm and LBW infants in India are lacking, therefore we report information from other relevant countries (). In comparison to full-term and normal birth weight infants, preterm and LBW infants are at an increased risk of hospitalization and mortality from VPDs such as diphtheria,Citation62 influenza,Citation68 invasive pneumococcal disease,Citation39 bacterial meningitis,Citation66 pertussis,Citation64,Citation65,Citation69 bacterial and viral pneumonia,Citation66 rotavirus gastroenteritisCitation67 and tetanus.Citation63 Importantly, the literature suggests that an increased risk of infection positively correlates with the degree of prematurity and LBW.Citation66,Citation70 Specifically, infection of the very and extremely LBW infants with opportunistic and aggressive multidrug-resistant pathogens often results in death.Citation70
Table 3. Risk of vaccine-preventable disease in preterm and LBW infants
Figure 2. Vaccination in preterm and LBW infants in India (A) Window of susceptibility to disease (B) Barriers to vaccination due to knowledge gaps among HCPs and parents ± ‡.
Vaccination programs and timing in preterm and LBW infants
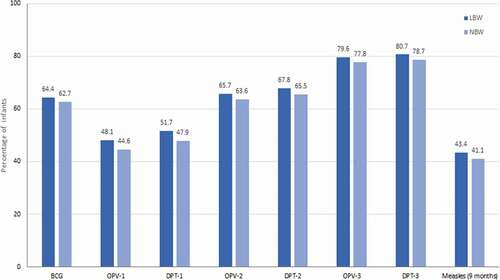
Published literature suggests that vaccination in preterm and LBW infants is delayed despite the existence of recommendations.Citation47–50 Due to this, the risk of complications and mortality from preventable infections is multiplied as the susceptibility window to infections is increased from the time of birth.Citation69 Vaccination delay or refusal of vaccines for preterm and LBW infants appears to be a prevalent issue in India as documented from several studies.Citation71–73 In these studies, delays in timely vaccination for each vaccine was defined as administration of the vaccine dose after 28 days of the minimum recommended age, meaning that vaccination was categorized as delayed if given on day 29 or later for Bacillus Calmette-Guerin (BCG), 71 days or later (after 10 completed weeks) for diphtheria-pertussis-tetanus (DPT)-first dose (DPT-1) and for DPT–third dose (DPT-3), when the infant was vaccinated at >18 weeks of age.Citation71–73 For measles, delayed vaccination was defined as having received the vaccine after 4 weeks of recommended/due-time, i.e. after 9 completed months of age (measles is recommended at 9 months of age).Citation74 In the first prospective study, almost half of the infants <33.5 weeks of gestational age (very preterm) and weighing <1,500 g (very LBW) were without immunization, while 62.5% of the remaining infants had a documented delay in immunization.Citation72 In the second study, data from the National Family and Health Survey-4 revealed that LBW infants with a birth weight <2,000 g had higher odds of a delay in receiving the BCG vaccine (adjusted odds ratio [aOR] 2.33, 95% confidence interval [CI] 1.89, 2.89) and the DPT-1 (aOR 1.53, 95% CI 1.26, 1.86) and the first dose of the measles vaccine (aOR 1.36, 95% CI 1.11, 1.67).Citation71 In a third study, in which 10,644 LBW infants (<2,500 g) were enrolled and followed until 12 months of age, a significantly lower immunization uptake was documented both in terms of the proportion of infants immunized and of the timing of vaccine administration (). About 3 out of 10 LBW infants were fully immunized by the age of 1 year (i.e., had received the BCG vaccine, three doses of the DPT vaccine, the oral polio vaccine, and the measles vaccine).Citation73 There was a delay in the time of administration of the vaccines compared to the recommended timing. The median delay (interquartile range) for the BCG vaccine was 41 (19–75), and for the three doses of the DPT vaccine (DPT–1, DPT–2 and DPT–3) was 30 (12–63), 46 (23–89) and 62 (34–112) days, respectively.Citation73 For the measles vaccine, the median delay from the recommended timing was 24 (9–46) days.Citation73
Figure 3. Immunization delay among LBW infants±‡.
Barriers to vaccination in preterm and LBW infants
Overall in India, several barriers to infant vaccination according to the recommended schedule have been documented. Vaccine hesitancy was a common barrier across different age groups.Citation52,Citation75,Citation76 Several factors were identified as causes of vaccine hesitancy in India: these relate to immunization effectiveness, safety/adverse events, provider belief, attitudes of parents, religious/socioeconomic factors, and policy guidelines regarding vaccination.Citation52,Citation75 These factors become even more complex in preterm and LBW infants.Citation53,Citation71–73 Factors of delayed vaccination in preterm and LBW infants were identified in two studies.Citation71,Citation73 Choudhary et al. and Upadhyay et al. both reported that Islamic religion and young maternal age (<20 years of age) were associated with lower odds of full immunization and higher odds of delayed vaccination for DPT–1. Female sex of the infant, birth weight <2,000 g, delivery by unskilled personnel, higher number of children and a lack of awareness about vaccination risks/benefits among mothers were also associated with lower odds of full immunization. In contrast, a high level of maternal education was strongly associated with improved vaccination status of the infant.Citation71,Citation73 Across studies, the main reason for a delay in vaccination was the general lack of awareness among HCPs and parents about vaccination benefits and concerns about possible adverse events due to vaccination in preterm and LBW infants.Citation71–73,Citation76 To this, we suggest the use of vaccines with published efficacy and safety data in the preterm and LBW infant population ().
Despite the availability of evidence and clear guidelines related to vaccination in India,Citation21,Citation22 there are wide knowledge gaps among HCPs and parents regarding the safety and efficacy of vaccines.Citation53 Further details can be seen in . Several factors were found to influence the attitude of HCPs toward vaccination for preterm and LBW infants. These include the perception of limited vaccine effectiveness, the risk of vaccination-induced serious adverse events and contraindication following postnatal steroid administration.Citation56 HCPs further perceive that birth weight, current weight, or the level of prematurity should determine the initiation of immunization.Citation50 The lack of clear vaccination recommendations from HCPs ultimately guides the decision of parents or caregivers of the infant to reject vaccinations.Citation77 Even if there are clear recommendations, a low education level and awareness of the parent or caregiver could delay vaccination or lead to refusal.Citation72,Citation76,Citation77 In India, a lack of education for girls and young women, who are socially viewed as the primary caregiver, could undermine immunization efforts.Citation76 Other factors such as home births in IndiaCitation71 and the cost of vaccinationCitation73 also tend to qualify as impediments to the vaccination of preterm and LBW infants.
Strategies to mitigate the burden of VPDs in preterm and LBW infants
Successful treatment of infections in preterm and LBW infants relies on early recognition and diagnosis, which is known to be challenging.Citation70 While the majority of infants will have some risk factors, there are several presenting symptoms that are nonspecific.Citation70 Therefore, it has become imperative to prevent the burden of infectious diseases in preterm and LBW infants. This can be achieved using a two-fold strategy targeting both mothers and their newborn infants.
It is vital to reduce the risks of infection in premature infants through prevention of infections in expectant mothers. It is essential to implement a comprehensive strategy comprising multiple elements such as improving maternal nutritional status, diagnosing and treating pregnancy-related conditions, and providing adequate maternal and perinatal care. The prevention of infections through immunization activities, which are known to be effective in circumventing the risks associated with VPDs, should also be encouraged.Citation24 Several immunization strategies have been suggested for the protection of newborn infants, the features of which are further discussed.
Indirect immunization strategies
The use of indirect immunization strategies such as maternal immunization and cocooning have been suggested as relevant strategies to alleviate the burden of VPDs in infants (e.g. tetanus, pertussis, influenza etc.).Citation24,Citation63–65,Citation78 Vaccination during pregnancy (maternal immunization) can provide protection against VPD for the mother, the developing fetus and the newborn through maternal antibodies transfer via the placenta and subsequently the breast milk.Citation79 An example is neonatal tetanus, which tends to occur during the first 3–14 days of life and which carries a case fatality rate of 100% in newborns. Through immunization efforts, maternal and neonatal tetanus have been eliminated from India.Citation80 Pertussis and influenza are other preventable diseases with potentially severe consequences (such as apnea, pneumonia and seizures in newborns) that can be averted through maternal immunization.Citation78,Citation81 Maternal immunization provides clear benefits. It is worth noting that the uptake of maternal immunization can however be slow.Citation82,Citation83 Common reasons include issues of confidence (i.e., fear of adverse pregnancy outcomes, lack of awareness, failure of the HCP to recommend vaccination and convenience/access [including cost]) and vaccine efficacy, driven possibly by the timing of vaccination.Citation82–84
Recent studies have suggested that antigen‐specific cord-blood antibody titers are greater following maternal immunization with the tetanus, diphtheria, and acellular pertussis vaccine in the second, rather than the third trimester.Citation85,Citation86 For influenza vaccination, researchers have shown that seasonal influenza vaccination should be given at any stage of pregnancy, with the caveat that it takes 2 weeks after vaccination for the mother to be protected against influenza.Citation87–90 Public health authorities have also revised their recommendations, with a few of them even recommending vaccinations as early as possible during pregnancy.Citation89,Citation90 Further research efforts to establish the appropriate timing of vaccinations during pregnancy could strengthen the use of maternal immunization in preventive neonatology.Citation84
Other indirect immunization strategies such as cocooning could be considered when maternal immunization is missed or delayed. The IAP recommendation states that immunizing individuals who have regular contacts with a newborn might help reduce the risk of infection in newborns.Citation78 However, there is little evidence to support the use of this strategy in protecting the extremely preterm and LBW infants. Additionally, cost and logistical barriers could further limit the widespread implementation of this strategy.Citation91,Citation92
Direct immunization of preterm and LBW infants
In preterm and LBW infants, implementing the same vaccination schedule as set forth for full-term and normal birth weight infants appears crucial, as can be seen in the vaccination recommendations (). Specific guidance regarding the implementation of vaccination when the infant is in the neonatal intensive care unit (NICU) is not explicitly mentioned in the guidelines; there is limited evidence to suggest that vaccination could be considered in the NICU if the infant is stable or after discharge from the NICU in the ward.Citation93 also provides an overview of the main references that provide immunogenicity/efficacy, effectiveness and safety data for the recommended vaccinations specific to the preterm and LBW infant population. This evidence base supports the vaccination of infants regardless of prematurity level or birth weight at the recommended chronological age according to the vaccine-specific prescribing information.
Across the different vaccinations, the degree of immune response may vary in terms of geometric mean titers in preterm infants, but protective and durable responses are achieved in most cases.Citation94,Citation95 Studies have shown that, following administration of vaccines, preterm and LBW infants mount an immune response directly proportional to their gestational age and birth weight.Citation96 Importantly, vaccines display a good safety profile even when given in combination, without compromising the immune response; this could potentially alleviate concerns of parents or HCPs with respect to safety.Citation97 In addition, vaccinations recommended for use in healthy infants and children have shown good levels of efficacy, safety, and effectiveness regardless of prematurity or birth weight ().
Among the combination vaccines available, the diphtheria, tetanus, pertussis, hepatitis B, inactivated polio vaccine and Hemophilus influenzae type b (DTPa-HBV-IPV/Hib), given alone or with other pediatric vaccines, has a clinically acceptable safety and immunogenicity profile in preterm (>24 weeks) and LBW (as low as 700 g) infants as in full-term infants, although HBV and Hib vaccine responses appeared lower in preterm and LBW infants.Citation37 The occurrence of post-immunization cardiorespiratory events is influenced by the severity of underlying neonatal conditions, but most tend to resolve spontaneously or require minimal intervention.Citation37 These data make a strong case for the vaccination of preterm and LBW infants according to the schedule proposed for full-term and normal birth weight infants (i.e., chronological age). However, monitoring of the preterm/LBW infant up to 72 hours after vaccination is recommended.Citation98 Notably, additional doses of HBV should be administered in infants receiving the first dose during the first days of life if they weigh less than 2,000 g because of a reduced immune response; for preterm infants born to hepatitis B Ag-positive mothers, both Ig and HBV should be given within 12 hours.Citation24,Citation31,Citation99 The timeliness of vaccination and completion of the primary vaccination series at chronological age rather than gestational age appears crucial to provide the earliest possible protection in preterm and LBW infants.Citation95 Importantly, we suggest the use of vaccines that have been tested in the preterm and LBW infant population and have robust efficacy and a clinically acceptable safety profile.
Discussion
The considerations presented in this review have both clinical and public health implications for India. In recent decades, India has seen a significant improvement in neonatal and infant health after the introduction of several initiatives by the Government of India (GOI).Citation51 India’s National Health Policy 2017 set a target of 16 deaths per 1,000 live births for neonatal mortality by 2025,Citation100 and the GOI has also set a target of less than 10 neonatal deaths per 1,000 live births by 2030 under the India Newborn Action Plan.Citation101 Within this context, prematurity and LBW in neonates deserve special attention, as a significant number of children born in India are born preterm or have LBW.Citation14,Citation19 Although a systematic literature search was not included in this review, which is a limitation, it reaches its objective of raising awareness on the importance of reducing the incidence of VPDs in preterm and LBW infants in India through immunization.
Published evidence from studies conducted outside India indeed shows that prematurity and LBW can predispose the infant, given their immunocompromised status, to a high risk of VPDs.Citation54,Citation56,Citation69,Citation96,Citation102 Reducing the incidence of VPDs in this vulnerable population after birth is the need of the hour. This can be achieved through timely immunization of the mother and newborn. Maternal immunization should be encouraged and there is a large evidence base supporting the safety and effectiveness of immunization during pregnancy.Citation84,Citation103 Similarly, vaccines in preterm and LBW infants are equally safe, immunogenic and effective as compared to full-term and normal birth weight infants.Citation94–96 Generating more evidence on the timing of maternal immunization, as well as identifying and addressing barriers to vaccination uptake, are key challenges to overcome.Citation84,Citation88
In India, healthcare institutions advocate that preterm and LBW infants are vaccinated following the same schedule as that of their counterparts who are born full-term with normal birth weights, apart from the hepatitis B vaccine wherein an additional dose is required.Citation21–23 Notwithstanding these recommendations, studies from India show that preterm and LBW infants are vaccinated with a significant delay,Citation71,Citation73,Citation76 driven by the clinical judgment of the treating HCP whose recommendation is instrumental in ensuring vaccination. Delays due to true contraindications (e.g., severe combined immunodeficiency disease) are justified, but avoiding risks related to ‘small for gestational age’ or birthweight are often cited as the reason behind vaccination delays. LBW appears to be a strong indicator of vaccination delay. Given that being born preterm is a leading cause of LBW, gestational age could also be recognized as a predictor of vaccination delay.Citation50 Data specific to vaccination delays in premature infants from India are lacking and are needed to shape the national vaccination policy. In addition, information assessing the relationship between vaccination delay and disease occurrence should be generated through large-scale observational studies. Further studies estimating vaccination coverage in preterm and LBW infants might provide insights on the scale of the problem and the underlying reasons for vaccination delay.
Delayed vaccination increases the susceptibility window to VPDs and their complications.Citation50 There are several barriers in achieving timely vaccination of preterm and LBW infants in India. Among these, HCP and parent knowledge, perceptions and attitudes to vaccination stand out. The role of HCPs in facilitating immunization uptake is well-documented hence training HCPs to discuss the risks versus benefits of vaccinations with parents, on scientifically validated grounds, seems highly relevant.Citation50,Citation77 To achieve this, HCPs must regularly acquire up-to-date information on vaccinations in preterm and LBW infants. Besides efficacy and safety, parents tend to worry about the number of vaccinations.Citation53 Targeted education and awareness initiatives for HCPs and health literacy interventions for parents, with focus on the importance, effectiveness and safety of vaccinations could help bridge immunization gaps in the vulnerable preterm and LBW infant population. In addition, the use of combination vaccines should be encouraged, as it addresses parents’ fears of multiple injections and increases the acceptance and compliance with the vaccination schedule.Citation97
Conclusion
Routine childhood vaccinations can help reduce or eliminate the burden of VPDs and should be given to preterm and LBW babies, regardless of prematurity or birth weight. It is crucial that HCPs are made aware that preterm and LBW infants could be faced with detrimental health effects if vaccinations are not administered in a timely manner. Inappropriate delays in vaccinating this fragile population should be minimized by ensuring that vaccination discussions are encouraged with families and caregivers at the point of care. These steps should be closely integrated within neonatal and other overall infant health management strategies to increase vaccination compliance and improve health in the fragile population of preterm and LBW infants.
Disclosure of potential conflicts of interest
Santosh Soans declares no financial and non-financial relationships and activities and no conflicts of interest. Attila Mihalyi, Valerie Berlaimont and Shafi Kolhapure are employees of the GSK group of companies, hold shares in the GSK group of companies and declare no other financial and non-financial relationships and activities. Resham Dash and Ashish Agrawal are employees of the GSK group of companies and declare no non-financial conflicts of interest.
Contributorship
All authors participated in the design of this narrative review, interpretation of the results; and the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Acknowledgments
The authors thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Benjamin Lemaire coordinated the manuscript development and editorial support. Amrita Ostawal (Arete Communication UG) provided medical writing support.
Additional information
Funding
References
- World Health Organization. WHA global nutrition targets 2025: low birth weight policy brief. [accessed 2020 May 10]. https://www.who.int/nutrition/topics/globaltargets_lowbirthweight_policybrief.pdf.
- Blencowe H, Krasevec J, de Onis M, Black RE, An X, Stevens GA, Borghi E, Hayashi C, Estevez D, Cegolon L, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Global Health. 2019;7(7):e849–e60. doi:10.1016/S2214-109X(18)30565-5.
- World Health Organization. Preterm birth. 2018 [accessed 2020 May 09]. https://www.who.int/news-room/fact-sheets/detail/preterm-birth.
- World Health Organization. Newborns with low birth weight (%). [accessed 2020 May 09]. https://www.who.int/whosis/whostat2006NewbornsLowBirthWeight.pdf.
- Goldenberg RL, Gravett MG, Iams J, Papageorghiou AT, Waller SA, Kramer M, Culhane J, Barros F, Conde-Agudelo A, Bhutta ZA, et al. The preterm birth syndrome: issues to consider in creating a classification system. Am J Obstet Gynecol. 2012;206(2):113–18. doi:10.1016/j.ajog.2011.10.865.
- Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A-B, Kinney M, Lawn J. Born Too Soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(Suppl 1):S2. doi:10.1186/1742-4755-10-S1-S2.
- Cutland CL, Lackritz EM, Mallett-Moore T, Bardaji A, Chandrasekaran R, Lahariya C, Nisar MI, Tapia MD, Pathirana J, Kochhar S, et al. Low birth weight: case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2017;35(48):6492–500. doi:10.1016/j.vaccine.2017.01.049.
- Rogers LK, Velten M. Maternal inflammation, growth retardation, and preterm birth: insights into adult cardiovascular disease. Life Sci. 2011;89(13–14):417–21. doi:10.1016/j.lfs.2011.07.017.
- Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: When? Where? Why? Lancet. 2005;365(9462):891–900. doi:10.1016/S0140-6736(05)71048-5.
- Collins A, Weitkamp JH, Wynn JL. Why are preterm newborns at increased risk of infection? Arch Dis Child Fetal Neonatal Ed. 2018;103:F391–4. doi:10.1136/archdischild-2017-313595.
- Miller JE, Hammond GC, Strunk T, Moore HC, Leonard H, Carter KW, Bhutta Z, Stanley F, de Klerk N, Burgner DP, et al. Association of gestational age and growth measures at birth with infection-related admissions to hospital throughout childhood: a population-based, data-linkage study from Western Australia. Lancet Infect Dis. 2016;16(8):952–61. doi:10.1016/S1473-3099(16)00150-X.
- Ray KN, Lorch SA. Hospitalization of early preterm, late preterm, and term infants during the first year of life by gestational age. Hosp Pediatr. 2013;3(3):194–203. doi:10.1542/hpeds.2012-0063.
- UNICEF/WHO. Low birthweight (LBW) estimates. 2019. [accessed 2020 May 18]. https://data.unicef.org/topic/nutrition/low-birthweight/.
- Chawanpaiboon S, Vogel JP, Moller A-B, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Global Health. 2019;7(1):e37–46. doi:10.1016/S2214-109X(18)30451-0.
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look P. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88(1):31–38. doi:10.2471/BLT.08.062554.
- United Nations Children’s Fund and World Health Organization. Low Birthweight: country, regional and global estimates. New York (USA): UNICEF; 2004 [accessed 2020 April 30]. https://www.unicef.org/publications/files/low_birthweight_from_EY.pdf.
- World Health Organization. Children: reducing mortality. Geneva (Switzerland): World Health Organization; 2019 accessed 2020 April 30. https://www.who.int/news-room/fact-sheets/detail/children-reducing-mortality.
- Dandona R, Kumar GA, Henry NJ, Joshua V, Ramji S, Gupta SS, Agrawal D, Kumar R, Lodha R, Mathai M, et al. Subnational mapping of under-5 and neonatal mortality trends in India: the Global Burden of Disease Study 2000–2017. Lancet. 2020;395(10237):1640–58. doi:10.1016/S0140-6736(20)30471-2.
- The World Bank. World Development Indicators. Birth rate, crude (per 1,000 people) - India. [accessed 2020 May 10. https://data.worldbank.org/indicator/SP.DYN.CBRT.IN?locations=IN.
- Ministry of Women and Child Development GoI. Rapid survey on children. New Delhi: India fact sheet (2013–14). [accessed 2020 July 21\]. https://wcd.nic.in/sites/default/files/State%20RSOC.pdf.
- Balasubramanian S, Shah A, Pemde HK, Chatterjee P, Shivananda S, Guduru VK, Soans S, Shastri D, Kumar R. Indian academy of pediatrics (IAP) advisory committee on vaccines and immunization practices (ACVIP) recommended immunization schedule (2018–19) and update on immunization for children aged 0 through 18 years. Indian Pediatr. 2018;55(12):1066–74. doi:10.1007/s13312-018-1444-8.
- Universal Immunisation Programme. National Health Portal of India. [accessed 2020 10 May. https://www.nhp.gov.in/universal-immunisation-programme_pg.
- World Health Organization. Hepatitis B vaccines: WHO position paper, July 2017 - Recommendations. Vaccine. 2019;37(2):223–25. doi:10.1016/j.vaccine.2017.07.046.
- World Health Organization. Summary of WHO position papers - recommendations for routine immunization. 2019 [accessed 2020 10 May]. https://www.who.int/immunization/policy/Immunization_routine_table1.pdf?ua.
- ECDC. United Kingdom and Germany: comparison of recommended vaccinations. 2020 [accessed 2020 August 03]. https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByCountries?SelectedCountry1Id=171&SelectedCountry2Id=6&IncludeChildAgeGroup=true&IncludeChildAgeGroup=false&IncludeAdultAgeGroup=false.
- CDC. Recommended child and adolescent immunization schedule for ages 18 years or younger. 2020 [accessed 2020 August 03]. https://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf.
- CDC Special Situations: general Best Practice Guidelines for Immunization: best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). [accessed 2020 August 03]. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/special-situations.html#ref-11.
- Government of Canada. Recommended immunization schedules: Canadian immunization guide. 2020 [accessed 2020 August 03]. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-1-key-immunization-information/page-13-recommended-immunization-schedules.html#p1c12a5.
- Australian Technical Advisory Group on Immunisation. Australian immunisation handbook. 2018 [accessed 2020 August 03]. https://immunisationhandbook.health.gov.au/vaccination-for-special-risk-groups/vaccination-for-preterm-infants.
- World Health Organization. BCG vaccine: WHO position paper, February 2018 – recommendations. Vaccine. 2018;36(24):3408–10. doi:10.1016/j.vaccine.2018.03.009.
- Lau Y-L, Tam AY, Ng KW, Tsoi NS, Lam B, Lam P, Yeung CY. Response of preterm infants to hepatitis B vaccine. J Pediatr. 1992;121(6):962–65. doi:10.1016/S0022-3476(05)80352-X.
- Belloni C, Chirico G, Pistorio A, Orsolini P, Tinelli C, Rondini G. Immunogenicity of hepatitis B vaccine in term and preterm infants. Acta Paediatr. 2007;87(3):336–38. doi:10.1111/j.1651-2227.1998.tb01448.x.
- Thayyil-Sudhan S, Singh M, Broor S, Xess I, Paul VK, Deorari AK. Is zero dose oral polio vaccine effective in preterm babies? Ann Trop Paediatr. 1998;18(4):321–24. doi:10.1080/02724936.1998.11747967.
- D’Angio CT, Maniscalco WM, Pichichero ME. Immunologic response of extremely premature infants to tetanus, Haemophilus influenzae, and polio immunizations. Pediatrics. 1995;96:18–22.
- Slack MH, Cade S, Schapira D, Thwaites RJ, Crowley-Luke A, Southern J, Borrow R, Miller E. DT5aP-Hib-IPV and MCC vaccines: preterm infants‘ response to accelerated immunisation. Arch Dis Child. 2005;90(4):338–41. doi:10.1136/adc.2004.052720.
- Klein NP, Gans HA, Sung P, Yasukawa LL, Johnson J, Sarafanov A, Chumakov K, Hansen J, Black S, Dekker C, et al. Preterm infants‘ T cell responses to inactivated poliovirus vaccine. J Infect Dis. 2010;201(2):214–22. doi:10.1086/649590.
- Omenaca F, Vazquez L, Garcia-Corbeira P, Mesaros N, Hanssens L, Dolhain J, Gómez IP, Liese J, Knuf M. Immunization of preterm infants with GSK’s hexavalent combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type b conjugate vaccine: A review of safety and immunogenicity. Vaccine. 2018;36(7):986–96. doi:10.1016/j.vaccine.2018.01.005.
- Vazquez L, Garcia F, Ruttimann R, Coconier G, Jacquet J-M, Schuerman L. Immunogenicity and reactogenicity of DTPa-HBV-IPV/Hib vaccine as primary and booster vaccination in low-birth-weight premature infants. Acta Paediatr. 2008;97(9):1243–49. doi:10.1111/j.1651-2227.2008.00884.x.
- Shinefield H, Black S, Ray P, Fireman B, Schwalbe J, Lewis E. Efficacy, immunogenicity and safety of heptavalent pneumococcal conjugate vaccine in low birth weight and preterm infants. Pediatr Infect Dis J. 2002;21(3):182–86. doi:10.1097/00006454-200203000-00003.
- Nieminen H, Rinta-Kokko H, Jokinen J, Puumalainen T, Moreira M, Borys D, Schuerman L, Palmu AA. Effectiveness of the 10-valent pneumococcal conjugate vaccine among girls, boys, preterm and low-birth-weight infants – results from a randomized, double-blind vaccine trial. Vaccine. 2019;37(28):3715–21. doi:10.1016/j.vaccine.2019.05.033.
- Martinón-Torres F, Czajka H, Center KJ, Wysocki J, Majda-Stanislawska E, Omeñaca F, Bernaola Iturbe E, Blazquez Gamero D, Concheiro-Guisan A, Gimenez-Sanchez F, et al. 13-valent pneumococcal conjugate vaccine (PCV13) in preterm versus term infants. Pediatrics. 2015;135(4):e876–86. doi:10.1542/peds.2014-2941.
- Esposito S, Pugni L, Mosca F, Principi N. Rotarix® and RotaTeq® administration to preterm infants in the neonatal intensive care unit: review of available evidence. Vaccine. 2018;36(36):5430–34. doi:10.1016/j.vaccine.2017.10.013.
- Van der Wielen M, Van Damme P. Pentavalent human-bovine (WC3) reassortant rotavirus vaccine in special populations: a review of data from the Rotavirus Efficacy and Safety Trial. Eur J Clin Microbiol Infect Dis. 2008;27(7):495–501. doi:10.1007/s10096-008-0479-5.
- Omenaca F, Sarlangue J, Szenborn L, Nogueira M, Suryakiran PV, Smolenov IV, Han HH. Safety, reactogenicity and immunogenicity of the human rotavirus vaccine in preterm European Infants: a randomized phase IIIb study. Pediatr Infect Dis J. 2012;31(5):487–93. doi:10.1097/INF.0b013e3182490a2c.
- Roue J-M, Nowak E, Le Gal G, Lemaitre T, Oger E, Poulhazan E, Giroux J-D, Garenne A, Gagneur A, et al. Impact of rotavirus vaccine on premature infants. Clin Vaccine Immunol. 2014;21(10):1404–09. doi:10.1128/CVI.00265-14.
- DʼAngio CT, Heyne RJ, Duara S, Holmes LC, OʼShea TM, Wang H, Wang D, Sánchez PJ, Welliver RC, Ryan RM, et al. Immunogenicity of trivalent influenza vaccine in extremely low-birth-weight, premature versus term infants. Pediatr Infect Dis J. 2011;30(7):570–74. doi:10.1097/INF.0b013e31820c1fdf.
- Batra JS, Eriksen EM, Zangwill KM, Lee M, Marcy SM, Ward JI. Evaluation of vaccine coverage for low birth weight infants during the first year of life in a large managed care population. Pediatrics. 2009;123(3):951–58. doi:10.1542/peds.2008-0231.
- Woestenberg PJ, van Lier A, van der Maas NA, Drijfhout IH, Oomen PJ, de Melker HE. Delayed start of diphtheria, tetanus, acellular pertussis and inactivated polio vaccination in preterm and low birth weight infants in the Netherlands. Pediatr Infect Dis J. 2014;33(2):190–98. doi:10.1097/INF.0000000000000106.
- Hofstetter AM, Jacobson EN, deHart MP, Englund JA. Early Childhood Vaccination Status of Preterm Infants. Pediatrics. 2019;144(3):e20183520. doi:10.1542/peds.2018-3520.
- Sisson H, Gardiner E, Watson R. Vaccination timeliness in preterm infants: an integrative review of the literature. J Clin Nurs. 2017;26(23–24):4094–104. doi:10.1111/jocn.13916.
- Sankar MJ, Neogi SB, Sharma J, Chauhan M, Srivastava R, Prabhakar PK, Khera A, Kumar R, Zodpey S, Paul VK, et al. State of newborn health in India. J Perinatol. 2016;36(S3):S3–8. doi:10.1038/jp.2016.183.
- Agrawal A, Kolhapure S, Di Pasquale A, Rai J, Mathur A. Vaccine Hesitancy as a Challenge or Vaccine Confidence as an Opportunity for Childhood Immunisation in India. Infectious Diseases and Therapy. 2020;9(3):421–32. doi:10.1007/s40121-020-00302-9.
- Sahoo T, Gulla K, Verma S. Vaccination in preterm infants: an Indian prospective. Indian J Child Health. 2020:1–7. doi:10.32677/IJCH.2020.v07.i01.001.
- Durandy A. Ontogeny of the Immune System. Transfus Med Hemother. 2003;30(5):222–27. doi:10.1159/000074287.
- Mussi-Pinhata MM, Rego MA. Immunological peculiarities of extremely preterm infants: a challenge for the prevention of nosocomial sepsis. J Pediatr (Rio J). 2005;81:S59–68. doi:10.1590/S0021-75572005000200008.
- Melville JM, Moss TJ. The immune consequences of preterm birth. Front Neurosci. 2013;7:79. doi:10.3389/fnins.2013.00079.
- Sampah MES, Hackam DJ. Dysregulated Mucosal Immunity and Associated Pathogeneses in Preterm Neonates. Front Immunol. 2020;11:899. doi:10.3389/fimmu.2020.00899.
- Carr R, Huizinga TW. Low soluble FcRIII receptor demonstrates reduced neutrophil reserves in preterm neonates. Arch Dis Child Fetal Neonatal Ed. 2000;83(2):F160–F. doi:10.1136/fn.83.2.F160.
- van den Berg JP, Westerbeek EAM, van der Klis FRM, Berbers GAM, van Elburg RM. Transplacental transport of IgG antibodies to preterm infants: a review of the literature. Early Hum Dev. 2011;87(2):67–72. doi:10.1016/j.earlhumdev.2010.11.003.
- Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36(5):248–55. doi:10.1111/j.1600-0897.1996.tb00172.x.
- Heininger U, Riffelmann M, Leineweber B, Wirsing von Koenig CH. Maternally derived antibodies against Bordetella pertussis antigens pertussis toxin and filamentous hemagglutinin in preterm and full term newborns. Pediatr Infect Dis J. 2009;28(5):443–45. doi:10.1097/INF.0b013e318193ead7.
- Clarke KEN, MacNeil A, Hadler S, Scott C, Tiwari TSP, Cherian T. Global Epidemiology of Diphtheria, 2000–20171. Emerg Infect Dis. 2019;25(10):1834–42. doi:10.3201/eid2510.190271.
- Lambo JA, Anokye EA. Prognostic factors for mortality in neonatal tetanus: a systematic review and meta-analysis. Int J Infect Dis. 2013;17(12):e1100–10. doi:10.1016/j.ijid.2013.05.016.
- Riise ØR, Laake I, Vestrheim D, Flem E, Moster D, Riise Bergsaker MA, Storsæter J. Risk of pertussis in relation to degree of prematurity in children less than 2 years of age. Pediatr Infect Dis J. 2017;36(5):e151–6. doi:10.1097/INF.0000000000001545.
- Marshall H, Clarke M, Rasiah K, Richmond P, Buttery J, Reynolds G, Andrews R, Nissen M, Wood N, McIntyre P, et al. Predictors of disease severity in children hospitalized for pertussis during an epidemic. Pediatr Infect Dis J. 2015;34(4):339–45. doi:10.1097/INF.0000000000000577.
- Hviid A, Melbye M. The impact of birth weight on infectious disease hospitalization in childhood. Am J Epidemiol. 2007;165(7):756–61. doi:10.1093/aje/kwk064.
- Newman RD, Grupp-Phelan J, Shay DK, Davis RL. Perinatal risk factors for infant hospitalization with viral gastroenteritis. Pediatrics. 1999;103(1):E3. doi:10.1542/peds.103.1.e3.
- Garcia MN, Philpott DC, Murray KO, Ontiveros A, Revell PA, Chandramohan L, MUNOZ FM. Clinical predictors of disease severity during the 2009–2010 A(HIN1) influenza virus pandemic in a paediatric population. Epidemiol Infect. 2015;143(14):2939–49. doi:10.1017/S0950268815000114.
- Langkamp DL, Davis JP. Increased risk of reported pertussis and hospitalization associated with pertussis in low birth weight children. J Pediatr. 1996;128(5):654–59. doi:10.1016/S0022-3476(96)80131-4.
- Plano LRW. The changing spectrum of neonatal infectious disease. J Perinatol. 2010;30(S1):S16–20. doi:10.1038/jp.2010.92.
- Choudhary TS, Reddy NS, Apte A, Sinha B, Roy S, Nair NP, Sindhu KN, Patil R, Upadhyay RP, Chowdhury R, et al. Delayed vaccination and its predictors among children under 2 years in India: insights from the national family health survey–4. Vaccine. 2019;37(17):2331–39. doi:10.1016/j.vaccine.2019.03.039.
- Gandhi P, Dande V. A study to assess the knowledge and practice of initiation of immunisation in mothers of low birth weight Neonatal Intensive Care Unit (NICU) graduates in a tertiary care centre. Int J Contemp Pediatr. 2018;5(2):278. doi:10.18203/2349-3291.ijcp20175929.
- Upadhyay RP, Chowdhury R, Mazumder S, Taneja S, Sinha B, Martines J, Bahl R, Bhandari N, Bhan MK. Immunization practices in low birth weight infants from rural Haryana, India: findings from secondary data analysis. J Glob Health. 2017;7(2):020415. doi:10.7189/jogh.07.020415.
- Ministry of Health and Family Welfare WHOI. Immunization handbook for medical officers reprint 2017. New Delhi: World Health Organization; 2017.
- Gurnani V, Haldar P, Aggarwal MK, Das MK, Chauhan A, Murray J, Arora NK, Jhalani M, Sudan P. Improving vaccination coverage in India: lessons from Intensified Mission Indradhanush, a cross-sectoral systems strengthening strategy. BMJ. 2018;363:k4782. doi:10.1136/bmj.k4782.
- Vikram K, Vanneman R, Desai S. Linkages between maternal education and childhood immunization in India. Soc Sci Med. 2012;75(2):331–39. doi:10.1016/j.socscimed.2012.02.043.
- Yaqub O, Castle-Clarke S, Sevdalis N, Chataway J. Attitudes to vaccination: A critical review. Soc Sci Med. 2014;112:1–11. doi:10.1016/j.socscimed.2014.04.018.
- Vashishtha V, Bansal C, Gupta S. Pertussis vaccines: position paper of Indian Academy of Pediatrics (IAP). Indian Pediatr. 2013;50(11):1001–09. doi:10.1007/s13312-013-0274-y.
- Rasmussen SA, Watson AK, Kennedy ED, Broder KR, Jamieson DJ. Vaccines and pregnancy: past, present, and future. Semin Fetal Neonatal Med. 2014;19(3):161–69. doi:10.1016/j.siny.2013.11.014.
- Cousins S. India is declared free of maternal and neonatal tetanus. BMJ: British Medical Journal. 2015;350:h2975. doi:10.1136/bmj.h2975.
- Singh M, Tanvir T, Nagoji D, Madan A, Gattem S, Singh H. Influenza vaccine: a viable option to protect pregnant women and infants from seasonal flu: a retrospective hospital-based study in India. Int J Clin Pract. 2019;73(7):e13361. doi:10.1111/ijcp.13361.
- Wilson RJ, Paterson P, Jarrett C, Larson HJ. Understanding factors influencing vaccination acceptance during pregnancy globally: A literature review. Vaccine. 2015;33(47):6420–29. doi:10.1016/j.vaccine.2015.08.046.
- Sobanjo-ter Meulen A, Duclos P, McIntyre P, Lewis KDC, Van Damme P, O’Brien KL, Klugman KP. Assessing the evidence for maternal pertussis immunization: a report from the bill & melinda gates foundation symposium on pertussis infant disease burden in low- and lower-middle-income countries. Clin Infect Dis. 2016;63(suppl 4):S123–33. doi:10.1093/cid/ciw530.
- Bergin N, Murtagh J, Philip RK. Maternal vaccination as an essential component of life-course immunization and its contribution to preventive neonatology. Int J Environ Res Public Health. 2018;15(5):847. doi:10.3390/ijerph15050847.
- Eberhardt CS, Blanchard-Rohner G, Lemaître B, Boukrid M, Combescure C, Othenin-Girard V, Chilin A, Petre J, de Tejada BM, Siegrist C-A, et al. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin Infect Dis. 2016;62(7):829–36. doi:10.1093/cid/ciw027.
- Eberhardt CS, Blanchard-Rohner G, Lemaître B, Combescure C, Othenin-Girard V, Chilin A, Petre J, Martinez de Tejada B, Siegrist C-A. Pertussis antibody transfer to preterm neonates after second- versus third-trimester maternal immunization. Clin Infect Dis. 2017;64(8):1129–32. doi:10.1093/cid/cix046.
- Blanchard-Rohner G, Meier S, Bel M, Combescure C, Othenin-Girard V, Swali RA, Martinez de Tejada B, Siegrist C-A. Influenza vaccination given at least 2 weeks before delivery to pregnant women facilitates transmission of seroprotective influenza-specific antibodies to the newborn. Pediatr Infect Dis J. 2013;32(12):1374–80. doi:10.1097/01.inf.0000437066.40840.c4.
- Zhong Z, Haltalli M, Holder B, Rice T, Donaldson B, O’Driscoll M, Le-Doare K, Kampmann B, Tregoning JS. The impact of timing of maternal influenza immunization on infant antibody levels at birth. Clin Exp Immunol. 2019;195(2):139–52. doi:10.1111/cei.13234.
- World Health Organization. Maternal and neonatal immunization field guide for Latin America and the Caribbean. Washington (D.C): PAHO; 2017 [accessed 2020 July 20]. https://iris.paho.org/bitstream/handle/10665.2/34150/9789275119501-eng.pdf?sequence=1%26isAllowed=y.
- Melbourne Vaccine Education Centre. Maternal vaccination during pregnancy. 2020 [accessed 2020 July 20]. https://mvec.mcri.edu.au/immunisation-references/maternal-vaccination-during-pregnancy/.
- Agrawal A, Singh S, Kolhapure S, Kandeil W, Pai R, Singhal T. Neonatal pertussis, an under-recognized health burden and rationale for maternal immunization: a systematic review of South and South-East Asian Countries. Infectious Diseases and Therapy. 2019;8(2):139–53. doi:10.1007/s40121-019-0245-2.
- Libster R, Edwards KM. How can we best prevent pertussis in infants? Clin Infect Dis. 2012;54(1):85–87. doi:10.1093/cid/cir780.
- Bhave S, Bhise S, Chavan SC, Naik SS, Pusapati RV, Bavdekar A, Pandit A. Hepatitis B vaccination in premature and low birth weight (LBW) babies. Indian Pediatr. 2002;39:625–31.
- Baxter D. Vaccine responsiveness in premature infants. Hum Vaccin. 2010;6(6):506–11. doi:10.4161/hv.6.6.12083.
- Saari TN. Immunization of preterm and low birth weight infants. American Academy of Pediatrics Committee on Infectious Diseases Pediatrics. 2003;112:193–98.
- Bonhoeffer J, Siegrist C-A, Heath PT. Immunisation of premature infants. Arch Dis Child. 2006;91(11):929–35. doi:10.1136/adc.2005.086306.
- Chiappini E, Petrolini C, Caffarelli C, Calvani M, Cardinale F, Duse M, Licari A, Manti S, Martelli A, Minasi D, et al. Hexavalent vaccines in preterm infants: an update by Italian Society of Pediatric Allergy and Immunology jointly with the Italian Society of Neonatology. Italian Journal of Pediatrics. 2019;45(1):145. doi:10.1186/s13052-019-0742-7.
- Omeñaca F, Garcia-Sicilia J, García-Corbeira P, Boceta R, Romero A, Lopez G, Dal-Ré R. Response of preterm newborns to immunization with a hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B Virus-inactivated polio and haemophilus influenzae type B vaccine: first experiences and solutions to a serious and sensitive issue. Pediatrics. 2005;116(6):1292–98. doi:10.1542/peds.2004-2336.
- Waitz M, Hopfner R, Hummler HD, Heininger U. Hepatitis B postexposure prophylaxis in preterm and low-birth-weight infants. AJP Rep. 2015;5(1):e67–72. doi:10.1055/s-0035-1547329.
- Ministry of Health and Family Welfare GoI. National health policy 2017. New Delhi; 2017 [accessed 2020 May 10]. https://main.mohfw.gov.in/sites/default/files/9147562941489753121.pdf.
- Ministry of Health and Family Welfare GoI. INAP: India newborn action plan. New Delhi; 2014 [accessed 2020 May 10]. https://www.newbornwhocc.org/INAP_Final.pdf.
- Healy C. Immunization strategies to protect preterm infants. Neoreviews. 2010;11(8):e409–e418. doi:10.1542/neo.11-8-e409.
- Giles ML, Krishnaswamy S, Wallace EM. Maternal immunisation: What have been the gains? Where are the gaps? What does the future hold? F1000Research. 2018;7:1733. doi:10.12688/f1000research.15475.1.