ABSTRACT
The immune system is often called a double-edged sword, due to the inextricable link between cancer immunity and allergy/autoimmunity. Intriguingly, a growing number of cases have been reported in which PD-1 blockade triggers the exacerbation of tuberculosis (TB), an organ-invasive granulomatous disease caused by bacterial infection. As a result, the exacerbation of TB is now considered a severe adverse effect of nivolumab and pembrolizumab. In this letter, we report the strong expression of PD-L1 in epithelioid granulomatous lesions in tuberculosis, sarcoidosis, Crohn’s disease, and foreign body granuloma. In addition, we discussed the exacerbation of tuberculosis after anti-PD-1 antibody-based cancer immunotherapy.
To the editor:
The immune system is often called a double-edged sword, due to the inextricable link between cancer immunity and allergy/autoimmunity. Although cancer immunotherapy based on immune checkpoint inhibitors (ICIs) has been hugely successful, these drugs often cause severe immune-related adverse events because they nonspecifically enhance the immune response. Intriguingly, a growing number of cases have been reported in which PD-1 blockade triggers the exacerbation of tuberculosis (TB), an organ-invasive granulomatous disease caused by bacterial infection. As a result, the exacerbation of TB is now considered a severe adverse effect of nivolumab and pembrolizumab.Citation1 However, the underlying mechanism of TB exacerbation due to anti-PD-1 inhibitors, which should boost immunity, is not yet understood. We anticipated that investigating the expression of PD-L1 in TB lesions would contribute to the understanding of PD-1 blockade-induced TB.
In this study, we performed immunohistochemical analysis with the E1L3N anti-PD-L1 monoclonal antibody in order to analyze PD-L1 expression in 15 epithelioid granulomatous lesions (3 cases of TB, 5 cases of sarcoidosis, 3 cases of Crohn’s disease, and 4 cases of foreign body granuloma). None of the patients received ICI treatment. All types of epithelioid cells, including Langhans giant cells and foreign body-type multinucleated giant cells, strongly expressed PD-L1 at their cell borders (). Thus, in the setting of pathological diagnosis, PD-L1 is useful for identifying epithelioid granulomatous lesions, which is sometimes difficult by morphological observation alone.
Figure 1. High levels of PD-L1 are expressed in epithelioid granulomatous lesions in tuberculosis, sarcoidosis, Crohn’s disease, and foreign body granuloma. Immunohistochemical staining for PD-L1 was performed using clone E1L3N (Cell signaling technology, Danvers, MA). Bar = 100 μm
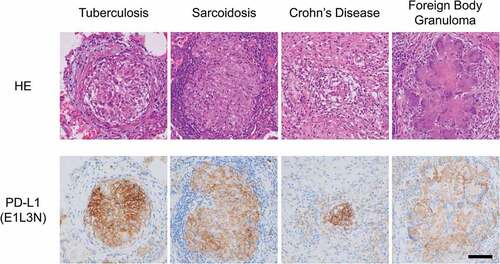
Although TB, sarcoidosis, and Crohn’s disease are all invasive granulomatous diseases, the pathophysiology of TB is distinct in terms of its infectious rather than autoimmune etiology. Anti-TNFα agents often induce or exaggerate TB while suppressing various types of harmful autoinflammatory conditions, including Crohn’s disease and steroid-resistant sarcoidosis. In contrast, PD-1 blockade is reported to commonly exacerbate TB and sarcoidosis or sarcoid-like reaction, an autoimmune disorder of unknown cause that is also characterized by granuloma formation.Citation2 Epithelioid granuloma containing multinucleated giant cells originates from macrophages, which express PD-L1 upon IFNγ or TNFα stimulation. Taken together with our current results, these cytokines may commonly underlie the different types of granuloma formation. In addition, we can speculate that ICI-induced immune activation would promote granuloma formation through macrophage activation. Although no cases have been reported, ICIs may also exacerbate Crohn’s disease, another autoimmune disease involving granuloma formation.
A previous study showed that PD-1-deficient mice were unable to inhibit TB proliferation and that PD-1-deficient mice were sensitive to TB.Citation3 This is consistent with human epidemiology. In that study, the vulnerability to TB in PD-1-deficient mice was attributed to excessive inflammation caused by the PD-1 deficiency. A possible explanation of the vulnerability to TB caused by PD-1 inhibition in both humans and mice is that anti-PD-1-mediated immune activation increases and promotes subclinical anti-TB inflammation. This is compatible with the increased sarcoid granuloma reaction induced by PD-1 inhibition. However, uncontrolled bacterial proliferation cannot be explained by PD-1 blockade-mediated immune enhancement, although excessive inflammation may increase severity or prove fatal in individual cases.
Another possible explanation of the vulnerability to TB in the setting of PD-1 blockade is that it is somehow beneficial for the growth of Mycobacterium tuberculosis. In TB patients, M. tuberculosis-specific peripheral T lymphocytes release abundant IFNγ, which is used to diagnose TB infection. In contrast, IFNγ can increase PD-L1 expression in macrophages and tumor cells. Therefore, M. tuberculosis-specific T cell-derived IFNγ would increase PD-L1 expression in epithelioid cells and Langhans giant cells. PD-L1-expressing epithelioid cells present M. tuberculosis-derived peptide via HLA class I molecules. In the setting of PD-1/PD-L1 axis inhibition, when CD8-positive cytotoxic lymphocytes (CTLs) recognize a complex of mycobacterial peptides and HLA class I molecules on epithelioid cells under the stimulation of appropriate costimulatory molecules and an optimal cytokine environment, CTLs would kill the epithelioid cells and Langhans giant cells. An early study has reported that the viability of M. bovis bacillus Calmette-Guérin, a model of M. tuberculosis, released from dead macrophages is dependent on the type of cell death, with more viable bacteria released in the case of necrosis.Citation4
On the other hand, CTL-induced apoptosis of infected macrophages is considered to lower the viability of M. tuberculosis, thereby controlling infection. In addition, blockage of the PD-1/PD-L1 pathway promotes the susceptibility of macrophages to M. tuberculosis-specific CTL-induced cell death.Citation5 This would suggest that inhibition of PD-1/PD-L1 should be beneficial for TB treatment. However, the reverse is true: it has become clear that PD-1 blockade exacerbates TB in the clinical setting. What is wrong with the mechanism above?
The type of cell death induced by CTLs is considered to be apoptosis. Yet, it is unclear whether the macrophages infected with TB are killed by cytotoxic T cells due to pure apoptosis with no dissemination of viable bacteria. Indeed, a recent report showed that tumor cell destruction by CTLs is not pure classic apoptosis, but rather immunogenic cell death. Abundant PD-L1 expression in epithelioid granulomas indicates that these lesions suppress the immune reaction via the PD-1/PD-L1 axis. The physiological function of PD-L1 expression in antigen-presenting cells (APCs) would involve the self-defense mechanism of these cells. APCs present antigenic peptides to T cells. APCs that do not possess T cell-inhibitory machinery via the PD-1/PD-L1 axis would be eliminated by self-immunity. Inhibition of the PD-1/PD-L1 pathway may result in the release of viable bacterial cells as a result of the CTL-mediated death of M. tuberculosis-infected epithelioid cells, disturbing anti-TB immunity and exacerbating the disease.
In conclusion, we have shown that epithelioid granulomatous lesions express high levels of PD-L1. Cancer immunotherapy with PD-1/PD-L1 blockade embossed additional pathophysiological role of this axis in TB. Further study is needed to understand PD-1/PD-L1 blockade induced systemic immune-reactions.
Author contributions
Tku analyzed the clinical and pathological data. TKu, YH, and TTo wrote and edited the manuscript. TTs, TKa, KM and TH conceived the study and assisted in the preparation of the manuscript. All authors read and approved the final manuscript.
Disclosure of potential conflicts of interest
With approval of the institutional review board (322-149: Clinicopathological investigation of epithelioid granulomatous lesions), the formalin-fixed, paraffin-embedded material archives of Sapporo Medical University Hospital. Informed consent was obtained through an opt-out on the website according to the guidelines of the Declaration of Helsinki. The authors declare that they have no competing interests.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (A) from the Japan Society for the Promotion of Science (JSPS; grant number 17H01540) and the Project for Cancer Research and Therapeutic Evolution (P-CREATE) from the Japan Agency for Medical Research and Development (AMED) to T. Torigoe.
References
- Barber DL, Sakai S, Kudchadkar RR, Fling SP, Day TA, Vergara JA, Ashkin D, Cheng JH, Lundgren LM, Raabe VN, et al. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci Transl Med. 2019;11:11. doi:10.1126/scitranslmed.aat2702.
- Chorti E, Kanaki T, Zimmer L, Hadaschik E, Ugurel S, Gratsias E, Roesch A, Bonella F, Wessendorf TE, Wälscher J, et al. Drug-induced sarcoidosis-like reaction in adjuvant immunotherapy: increased rate and mimicker of metastasis. Eur J Cancer. 2020;131:18–26. doi:10.1016/j.ejca.2020.02.024.
- Lazar-Molnar E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, Porcelli SA, Almo SC, Nathenson SG, Jacobs WR, et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A. 2010;107(30):13402–07. doi:10.1073/pnas.1007394107.
- Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med. 1994;180:1499–509. doi:10.1084/jem.180.4.1499.
- Suarez GV, CDC MG, Vecchione MB, Trifone CA, Marín Franco JL, Genoula M, Moraña EJ, Balboa L, Quiroga MF. PD-1/PD-L1 pathway modulates macrophage susceptibility to mycobacterium tuberculosis specific CD8(+) T cell induced death. Sci Rep. 2019;9:187. doi:10.1038/s41598-018-36403-2.
