ABSTRACT
In renal-cell carcinoma (RCC), tumor-reactive T-cell responses can occur spontaneously or in response to systemic immunotherapy with cytokines and immune checkpoint inhibitors. Cancer vaccines and engineered T-cell therapies are designed to selectively augment tumor antigen-specific CD8+ T-cell responses with the goal to elicit tumor regression and avoid toxicities associated with nonspecific immunotherapies. In this review, we provide an overview of the central role of T-cell immunity in the treatment of advanced RCC. Clinical outcomes for antigen-targeted vaccines or other T-cell-engaging therapies for RCC are summarized and evaluated, and emerging new strategies to enhance the effectiveness of antigen-specific therapy for RCC are discussed.
Introduction
Renal-cell carcinoma (RCC) accounts for 90% of malignant kidney neoplasms in adults and is the eighth most common cancer in the United States.Citation1Although nephrectomy for localized tumors can be curative, ~30% of patients develop metastatic disease.Citation2 RCC is resistant to cytotoxic chemotherapiesCitation3 and despite the development of targeted molecular therapies in the mid 2000’s including vascular endothelial growth factor (VEGF) receptor-selective tyrosine kinase inhibitors (TKIs) and mammalian target of rapamycin (mTOR) inhibitors, primary or acquired tumor resistance is common and metastatic RCC (mRCC) is generally considered incurable.
However, among common epithelial cancers, mRCC is uniquely sensitive to systemic immunotherapy. By the early 1990’s, systemic cytokine therapies with interferon-alpha (IFN-α) or interleukin-2 (IL-2) were the standard frontline treatment options for mRCC. Although cytokines were largely replaced by molecularly targeted drugs, recent phase III studies of immune checkpoint blocking antibodies targeting programmed cell death protein-1 (PD-1) or programmed cell death ligand-1 (PD-L1) have shown a positive benefit in comparison to targeted therapy. Immunotherapy is now re-established as the most common initial treatment for advanced RCC. Nevertheless, outcomes are heterogeneous ranging from durable tumor regression to primary refractory disease. Complete radiographic responses remain uncommon, occurring in 3–9% of patients receiving frontline immune checkpoint inhibitors.Citation4–6
The antitumor effects produced by both cytokine and immune checkpoint blocking agents are thought to be mediated by tumor-reactive T cells. The long history of clinically applied immunotherapy for advanced RCC has encouraged the development and testing of numerous antigen-targeted T cell-mediated treatment options for mRCC with the goal of selectively augmenting anti-tumor activity and avoiding toxicity. This review provides an overview of the central role of T-cell immunity in the treatment of advanced RCC. Clinical outcomes for antigen-specific vaccines or other T-cell-engaging therapies for RCC are evaluated, and emerging new strategies to enhance the effectiveness of antigen-targeted therapy for RCC are discussed.
Early evidence for RCC-specific T-cell immunity
Since the first report in 1928,Citation7 the phenomenon of spontaneous tumor regression has been more frequently associated with mRCC than most other cancer types. The frequency of spontaneous regression in RCC patients has been estimated at 1% and has been observed in both primary tumors and metastatic lesions.Citation8 Host immune system activation is the leading hypothesis for the mechanism. This view is supported by the observation that spontaneous regressions are often preceded by feverish infection.Citation9 Early studies therefore looked for evidence of spontaneous T-cell responses recognizing RCC.
The possibility of RCC eliciting cytotoxic immune responses was supported by the finding that 100% of RCC tumors expressed MHC class I, and 93% retained expression during tumor progression and metastasis.Citation10 In 1991, an RCC-specific cytotoxic T-lymphocyte (CTL) line was isolated from a primary tumor and displayed lytic specificity and IFN-γ production only in co-culture with autologous tumor but not lymphoblasts or allogeneic RCC tumor.Citation11 Subsequently, a CTL culture was isolated from a human leukocyte antigen (HLA)-A*02+ RCC tumor with limited representation of Vα genes and demonstrated cytotoxicity towards allogeneic HLA-A*02+ RCC tumors, suggesting T-cell clonal expansion in the tumor and the existence of shared RCC tumor antigens.Citation12 These studies were followed by the discovery of multiple RCC-associated antigens that were the targets for spontaneous T-cell responses in RCC patients.Citation13–20 These antigens were shown to arise through point mutations, the aberrant overexpression of genes and antisense transcripts, or alternative open reading frames likely generated from frameshift mutations ().
Table 1. RCC antigens recognized by spontaeously arising tumor-reactive CD8 CTL clones
Successful therapeutic manipulation of tumor-reactive T-cell responses was first demonstrated with systemic cytokines that came into clinical use for mRCC in the 1980s and 1990s. IL-2 activates post T-cell receptor (TCR) signaling in T-cells and promotes CD8+ T-cell proliferation, effector function and survival. In 1992, high-dose IL-2 was approved by the FDA as a monotherapy for mRCC. Objective responses (OR) were observed in 15% to 30% of patients receiving high-dose IL-2, with 5–8% of patients achieving durable and unmaintained complete responses (CR).Citation21–23 Type I interferons represent a component of an innate immune response to virus infection or neoplastic cells and serve a subsequent key role in priming the host adaptive immune response. Clinical trials of recombinant IFN-α treatment for mRCC have shown a response rate of 15%. However, CRs (< 5%) were incrementally less frequent or durable than for IL-2.Citation24–26 Collectively, the early evidence for spontaneous T-cell immunity to RCC antigens and clinical success in some patients receiving T cell-mediated systemic immunotherapy encouraged the search for more effective therapy strategies and focused attention on defining the mechanisms regulating tumor-reactive T-cell immunity as well as tumor escape from immune surveillance.
Phenotype of tumor-infiltrating lymphocytes (TIL) in RCC
More contemporary analysis of over 7,000 tumors from The Cancer Genome Atlas (TCGA) demonstrated clear cell RCC (ccRCC), the most common subtype of RCC, has the highest CD8+ T-cell infiltration among 23 solid tumor types.Citation27 Within the RCC tumor microenvironment, T-cells are the most prevalent immune subset (50%) followed by tumor-associated macrophages (25%), natural killer (NK) cells (9%), B cells (4%), and other immune cells including plasma cells, dendritic cells (DC’s), and neutrophils.Citation28 Moreover, ccRCC had the highest cytolytic activity index (geometric average of GZMA and PRF1 expression) compared to 17 other human cancers.Citation29 These observations suggest RCC can elicit tumor-reactive T-cell responses. Accumulating evidence emphasizes not just the quantity but also the functional quality of T-cells in the tumor microenvironment on favorable outcomes in mRCC. Features of TIL including low expression of immune checkpoint proteins,Citation30,Citation31 a T helper 1 (Th1)-type phenotype possibly mediated by chemokine recruitment of T-cells into the tumor,Citation32 above-median CD8+ T-cell/regulatory T-cell (Treg) and Th17/Th2 ratios,Citation33 in addition to the presence of mature dendritic cells and higher adaptive immune response gene expression in the tumor microenvironment are associated with favorable prognosis.Citation34
Another important aspect of antitumor efficacy is T-cell proliferation capacity in the tumor microenvironment that may reflect a response to tumor antigen. The ability of CD8+ T-cells to expand in tumors marked by Ki-67 expression is an independent favorable prognostic factor in RCC.Citation35 Recurrent TCR transcripts marked by uniquely rearranged complementarity-determining region 3 (CDR3) is another powerful marker indicating antigen-driven proliferation of individual T-cell clones in tumors. TCR transcripts with the same Vβ gene can represent up to 25–30% of the tumor-infiltrating TCR repertoire in RCC.Citation36,Citation37 Our research group’s ongoing single-cell analyses of RCC TIL identified clonally expanded T-cells unique to the tumor microenvironment not detected in normal adjacent renal cortex or peripheral blood that represented 8 to 24% of the total TIL repertoire. These expanded clones are enriched for an effector or effector memory CD8+ T-cell phenotype consistent with a tumor-reactive effector population. Moreover, IHC of the same tumor specimens revealed that Ki67+CD8+ T-cells were significantly more abundant in tumor compared to non-tumor regions, suggesting tumor antigen-driven expansion. However, a major challenge in the field is the technical difficulty in identifying the cognate antigens associated with clonally expanded TCRs.Citation38
Mechanisms that limit the antitumor activities of T-cells in RCC have been identified. CD8+ T-cells infiltrated into RCC tumors can be non-responsive to ex vivo stimulation, lack granule mobilization, cytolytic activity and cytokine production,Citation39 a phenotype associated with a low level of TCR-distal signaling.Citation40 Inhibitory co-receptors expressed on activated T-cells may contribute to T-cell anergy in the tumor microenvironment and RCC escape from immune surveillance. Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) regulates activated T-cells by competing with CD28 to bind to CD80 and/or CD86 ligands,Citation41 decreasing IL-2 secretion, T-cell proliferationCitation42 and promoting T-cell apoptosis.Citation43 PD-1 is expressed on activated effector T-cells, binding to PD-L1 and PD-L2, and limiting the T-cell effector responses.Citation44 PD-L1 was shown to be expressed by 23.9% of ccRCC tumors.Citation45 Patients with PD-L1+ tumors had significantly lower 5-year survival and higher metastatic cancer progression,Citation45 and patients with TIL expressing PD-1 were more likely to have aggressive PD-L1+ RCC tumors.Citation46 Similarly, infiltration of PD1+ T-cells was shown to predict distant metastases in ccRCC.Citation30,Citation47 Two other inhibitory receptors, lymphocyte activation gene 3 (LAG-3), and T-cell immunoglobulin and mucin domain-containing 3 (TIM-3) also have been found to play important roles in RCC TIL exhaustion. Analysis of TCGA data revealed that tumor LAG-3 expression was associated with poor overall survival (OS).Citation30 TIM-3 is induced by Th1 cytokines after T-cell activation in the tumor microenvironmentCitation48 and tumor-infiltrating PD-1+ CD8+ T-cells coexpressing TIM-3 were associated with a highly aggressive RCC phenotype.Citation49 In addition, TIM-3 expression has been detected on intratumoral Tregs,Citation49 contributing to the accumulation of dysfunctional CD8+ T-cells.Citation50
Immune checkpoint blockade therapy in RCC
Given the immunotherapy responsive phenotype for RCC as well as insight into mechanisms for tumor resistance, mRCC was one of the first cancers treated with immune checkpoint blocking antibodies. In 2015, the PD-1 blocking antibody nivolumab was approved by the FDA for the treatment of advanced RCC that had failed prior VEGF pathway targeted therapy. Approval was based on the phase III CheckMate 025 trial that showed superior OS and higher response rate (25 vs 5%) for nivolumab versus the mTOR inhibitor everolimus.Citation51 Nivolumab was subsequently combined with the CTLA-4 blocking antibody (ipilimumab) as a frontline treatment regimen and tested versus the TKI sunitinib in the CheckMate 214 trial.Citation4 In the primary analysis cohort of intermediate- and poor-risk RCC patients, the nivolumab plus ipilimumab treatment arm had superior OS, progression-free survival (PFS), and overall response rate (ORR) (42 vs 27%) versus sunitinib and the combination of nivolumab plus ipilimumab received FDA approval in 2018. Immune checkpoint blockade has also been combined with antiangiogenic TKI’s for frontline therapy of advanced RCC. In the phase III KEYNOTE-426 trial the PD-1 antibody pembrolizumab plus axitinib was compared to sunitinib monotherapyCitation5 and in the phase III JAVELIN Renal 101 trial, avelumab (anti-PD-L1) plus axitinib was also compared to sunitinib. Both studies showed positive clinical benefit for the immune checkpoint containing regimen leading to FDA approval for both doublets in 2019.Citation6 Taken together, systemic therapy with a two-drug regimen incorporating an immune checkpoint inhibitor is now the preferred treatment for most patients with advanced RCC.
RCC-antigen targets associated with immune checkpoint blockade
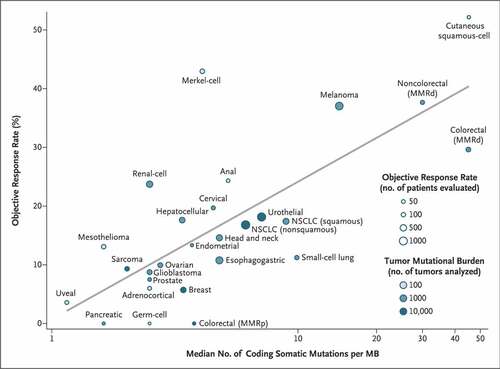
Tumor neoantigens created by tumor-specific mutations were first associated with response to immune checkpoint blockade for melanoma and NSCLC, tumors with a high single nucleotide variant mutation burden [10 to 400 mutations/megabase (Mb)].Citation52–54 Tumor mutation burden (TMB) has been evaluated as a more generalizable biomarker for response to immune checkpoint blockade showing a strong correlation for most tumor types () and high TBM [≥10 mutations/Mb)] is a validated tumor-agnostic biomarker for selecting treatment with pembrolizumab.Citation55,Citation56 However, despite a relatively low number of coding somatic mutations (1.8 mutations/Mb) RCC has a disproportionately high response rate to PD-1 inhibition (), a discrepancy further exacerbated by a higher response rate (38%) observed in more recent front-line data with PD-1 inhibition.Citation57 Of note, in a study of 19 cancer types, RCC was found to have the highest number and proportion of insertion and deletion (indel) mutations representing an alternate mechanism for neoantigen formation.Citation58 However, biomarker discovery efforts with tumor samples from RCC patients enrolled in studies of nivolumab monotherapy,Citation59 or front-line regimens with nivolumab plus ipilimumab,Citation60 avelumab plus axitinibCitation61 or atezolizumab plus bevacizumabCitation62 are consistent for failing to show an association of TMB or neoantigen density with clinical benefit for patients receiving immune checkpoint blockade. These data suggest other mechanisms separate from neoantigen expression may play an important role contributing to RCC immunogenicity.
Figure 1. RCC has a high objective response rate to immune checkpoint blockade despite low TMB. Median number of coding somatic mutations per megabase (MB) of DNA for 27 tumor types is plotted in log scale versus objective response rates for patients who received PD-1 or PD-L1 inhibitors as described in published studies. RCC is within the top 25% of objective response rates while being within the bottom 33% of median number of coding somatic mutations per MB. MMRd denotes mismatch repair-deficient, MMRp mismatch repair proficient, and NSCLC non-small cell lung cancer. Reproduced from Yarchoan, Hopkins & JaffeeCitation55 with copyright permission
Accumulating evidence suggests that human endogenous retrovirus (hERV)-derived antigens contribute to immune checkpoint inhibitor-associated responses. These germline-encoded elements have integrated into the genome and are identified by unique sequences derived from 5′ and 3′ long terminal repeats (LTRs) and retroviral genes. These normally quiescent sequences can be translationally re-expressed due to epigenetic dysregulation preferentially in RCC versus other tumors.Citation63–67 Mechanistically, the up-regulation of the HIF2α transcription factor in ccRCC was found to target a response element in the proviral 5′LTR, turning on hERV-E expression in tumors.Citation68 HERV-encoded proteins can be immunogenic and T-cells specific for epitopes from the hERV-E 5′LTR and the hERV-E envelope gene have been identified.Citation69–71 HERVs may harbor immunogenic hotspots with multiple epitopes per retroelement, and the conserved retroviral epitopes are widely shared between patients.Citation71 In small studies, expression of select hERV elements has been associated with better clinical outcomes for ccRCC treated by immune checkpoint blockade including higher response rates and longer PFS further encouraging the study of hERV as biomarkers or as therapeutic targets.Citation71–73
Inducing tumor-specific T-cell immunity against RCC-associated antigens
The longstanding recognition of RCC as an immune responsive tumor has encouraged the development and clinical testing of numerous antigen-targeted vaccine and T-cell-engaging therapies. RCC tumor antigens defined by spontaneously arising T-cells were either patient-specific, expressed in normal tissues, or had low-frequency expression by tumors and were not suitable candidates for vaccine development. Thus, selection of RCC antigen targets has focused on inducing immunity against proteins that demonstrate tumor-specific expression and are broadly shared between tumors.
Single antigen vaccines and T-cell engaging therapies ()
Mutation or inactivation of the Von Hippel-Lindau (VHL) tumor suppressor gene occurs in most ccRCC tumors,Citation53 and results in constitutive activation of HIF transcription factors. Overexpressed proteins encoded by hypoxia-inducible genes represent widely shared target antigens for ccRCC tumors including carbonic anhydrase IX (CAIX), vascular endothelial growth factor receptor 1 (VEGFR1), and hypoxia-inducible protein 2 (HIG2). Mutant versions of the VHL protein itself have also been evaluated as a vaccine target.
Table 2. Clinical testing of single antigen-specific vaccination or T-cell engaging therapy for advanced RCC
Table 3. Clinical testing of multiple antigen vaccines for advanced RCC
CAIX
CAIX is one of the first characterized RCC-associated antigens Citation95 and high-level expression is observed in ccRCC tumors at the earliest stage of the disease.Citation96,Citation97 IHC staining revealed CAIX expression in over 85% of tumors but not normal kidney tissue.Citation95,Citation98 CAIX epitopes have been identified to induce cytolytic T-cell responses from bulk T-cell populations restricted by HLA-A*24, HLA-A*02, and HLA-DR. In a phase I study, synthetic peptides corresponding to three putative HLA-A*24-restricted CAIX T-cell epitopes were used to vaccinate 23 mRCC patients refractory to cytokine therapy.Citation74 After 6–9 intradermal doses of the vaccine, cytolytic T-cell reactivity specific for one or more peptides was observed in 76% of the patients. Three patients had a partial response with a regression of pulmonary metastases. In a separate phase I study, synthetic peptides corresponding to naturally processed CAIX epitopes restricted by HLA-A*02Citation99 and HLA-DRCitation100 were used to develop a DC-based vaccine. The two CAIX-derived peptides and keyhole limpet hemocyanin (KLH) adjuvant were loaded on autologous DC’s from five patients with cytokine-refractory mRCC. After five intradermal vaccinations, all patients developed humoral responses against KLH, however, none mounted detectable CAIX-peptide-specific cellular immunity, and no clinical responses were observed.Citation75
CAIX has also been targeted with a first-generation chimeric antigen receptor (CAR) vector retrovirally transduced into autologous peripheral blood T-cells from 12 RCC patients refractory to prior systemic therapy with cytokines or TKIs.Citation76 Four of the first eight patients experienced liver toxicity associated with CAIX expression on bile duct epithelium and CAR-T-cell infiltration. In four additional patients, a CAIX-blocking antibody administered before CAR-T-cell infusions attenuated the liver toxicity. However, no clinical responses were observed.Citation101
VEGR-R1
VEGFR1 is an important factor associated with RCC tumor angiogenesis. In a phase I vaccine trial, 18 patients were subcutaneously administered an HLA-A*0201- or HLA-A*2402-restricted VEGFR1-derived peptide weekly for 5 weeks, and then every 2 weeks.Citation77 Peptide-reactive CTL responses were observed in 15/18 patients and two patients showed a partial response.
HIG2
HIG2 is expressed in 86% of RCC tumors at an early stage of tumor development but not in normal kidney and functions as an autocrine factor enhancing tumor growth.Citation102 High HIG2 expression was found to correlate with disease stage and is a poor prognostic marker for RCC patient survival. A phase I dose-escalation study deployed an HLA-A*0201/0206-restricted HIG2–9–4 peptide to vaccinate nine patients with refractory mRCC after cytokine and/or TKI therapies.Citation78 The vaccine was administered subcutaneously weekly for 4 weeks in each cycle and vaccination cycles continued until disease progression. HIG2–9–4-specific CTL responses were detected in eight of nine patients; however, there were no objective responses.
VHL
A pilot study identified patient-specific VHL mutation-spanning peptides with computationally predicted high binding affinity to an autologous class I HLA molecule.Citation79 Among six metastatic ccRCC patients, two had frameshift mutations, creating completely new 12-mer or 13-mer sequences; others harbored centered point mutations with 8-mer peptides flanking each side. These patients were treated by subcutaneous vaccination with their personalized synthetic peptide. Peptide-specific CD8+ T-cell responses were detected in 4/5 evaluable patients; however, no responses were observed in patients with measurable disease at study enrollment.
5T4/Trophoblast glycoprotein
Cancer-testis antigens have highly restricted expression in normal adults limited to male germ cells in the testis, and to ovary and trophoblast T-cells in females, but can be aberrantly expressed by many tumor types. However, the most studied immunogenic cancer-testis antigens NY-ESO-1, MAGE-A4 and SAGE were shown to have limited expression in RCC.Citation103 5T4 is a glycosylated cancer-testis antigen that is a highly expressed in human trophoblast T-cells and is overexpressed in RCC and a wide range of other solid tumors including prostate, pancreatic, ovarian, breast, cervical, gastric, and non-small cell lung cancer but not normal adult tissue.Citation104–106 IHC staining has revealed over 95% of RCC tumors express at least focal 5T4 protein, and 75% of tumors have strong staining of cell surface 5T4. Expression is retained in metastatic tumors.Citation104 The observation of circulating preexisting CD8+ and CD4+ T-cell 5T4 responses in RCC patientsCitation107,Citation108 has encouraged the development of 5T4 targeted therapies. By IFN-γ ELISpot assays, to date 4 MHC class I-restricted 5T4 epitopes have been associated with common HLA alleles including HLA-A*0201, HLA-A*0101, and HLA-Cw7.Citation107,Citation109,Citation110
A Modified Vaccinia Ankara (MVA) virus was engineered to express the full-length 5T4 protein and shown to elicit 5T4-specific cellular or humoral responses in vaccinated RCC patients that correlated with clinical benefit in four phase I/II trials.Citation80–83,Citation111 In the phase III TRIST trial, patients were randomized to MVA-5T4 (n = 365) or placebo (n = 368) in combination with sunitinib, IL-2, or IFN-α as first-line mRCC therapy.Citation84 Although well-tolerated, MVA-5T4 failed to show enhanced OS of vaccinated patients versus placebo (HR 1.07; P = 0.55). In post hoc analysis, there was an association between enhanced patient survival and 5T4 antibody response (56% of treated patients) but not MVA antibody responses (96% of treated patients) linking induced 5T4-specific immunity with better clinical outcomes. However, during early phase clinical development of MVA-5T4, a cellular immune response against 5T4 measured by IFN-γ ELIspot was detectable in <50% of patients. It is therefore likely that in the TRIST trial, only a minority of patients vaccinated with MVA-5T4 mounted 5T4-specific T-cell responses.Citation112,Citation113
Naptumomab estafenatox (anti-5T4-SEA/E-120) is a fusion protein conjugating a bacterial superantigen variant to the Fab binding domain of a 5T4 monoclonal antibodyCitation114 in order to activate both CD4+ and CD8+ T-cells in proximity to 5T4-expressing tumor. In a phase II/III study, 512 patients with RCC were randomized to receive naptumomab estafenatox with IFN-α or IFN-α alone.Citation115,Citation116 However, this study failed to meet its primary endpoint of improved survival for naptumomab estafenatox treated patients (HR 1.08; P = 0.56).
Mucin 1 (MUC1)
MUC1 is transmembrane glycoprotein restricted to the luminal surface of epithelial cells.Citation117 In ccRCC, MUC1 is overexpressed, aberrantly glycosylated and diffused from the luminal surface, promoting cancer cell differentiation and metastasis.Citation118 High MUC1 expression is an independent prognostic factor for advanced disease and metastasis in ccRCC.Citation117,Citation119,Citation120 MUC1 has a variable number tandem repeat (VNTR) of 20-amino-acids each with five potential sites of O-glycosylation.Citation121 The hypoglycosylation of VNTR sequences in malignant T-cells can produce tumor-specific glycopeptide antigens. HLA-A*02-restricted T-cell epitopes from the VNTR core were discovered,Citation122,Citation123 and MUC1-specific CTL clones from HLA-A*02+ healthy donors were isolated that recognized a variety of cancer cell lines including renal tumor lines.Citation122,Citation124,Citation125 In addition, a non-MHC-restricted recognition of tumoral MUC1 epitopes by CD8+ T-cells was documented in different tumor types.Citation126
In a phase I trial, 20 HLA-A2+ metastatic RCC patients with MUC1 expressing tumors were vaccinated with autologous DCs pulsed with HLA-A2 binding MUC1 peptides plus the pan-DR helper peptide PADRE.Citation85 Twelve of 18 evaluable patients developed detectable MUC1 peptide CTL responses and 10/18 had PADRE-specific responses. Three objective responses were observed including one complete response.
TG-4010 is an MVA-based vaccine that is designed to express both IL-2 and MUC1 protein. The safety profile was confirmed in several phase I studies. MUC1-specific T-cell and antibody responses were seen in some pancreatic cancer patients,Citation127 while in another phase I study, only T-cell responses to MUC1 were observed with various-advanced cancers.Citation128 The best clinical responses were observed in non-small cell lung cancer.Citation129 A phase II study enrolled 37 mRCC patients and reported 5/28 evaluable patients developed a MUC1-specific CD4+ T-cell response during therapy and 6/23 had MUC1-specific CD8+ T-cells detected before or during therapy. However, there were no objective responses.Citation86
Wilms’ tumor 1 (WT-1)
WT1 is expressed in normal gonad, uterus, kidney, mesothelium, and hematopoietic progenitor cells and is frequently overexpressed in RCC.Citation130 In a 2007 phase I study, two patients with RCC were treated with an HLA-A*2402-binding WT1 peptide with a modified anchor residue.Citation87 Peptide-specific T-cells were detected from PBMCs in both patients; however, neither experienced an objective response. In another phase I study, five patients with metastatic or relapsed RCC expressing WT1 were treated with HLA-matched WT1 peptide-loaded DCs and OK-432 adjuvant,Citation88 a toll-like receptor 4 ligand shown to facilitate the maturation of DCs and stimulate Th1-type cytokine secretion.Citation131 Vaccinations were given in combination with a TKI or mTOR inhibitor. Peptide-reactive CTL responses were detected in all five patients by tetramer staining, ELISPOT, or cytoplasmic IFN-γ assays. However, no objective responses were observed.
Multi-antigen vaccines ()
In contrast to vaccines targeting single antigens, multi-antigen vaccine platforms are anticipated to increase the likelihood for antigen priming and vaccine-induced T-cell responses. A multi-valent immune response may also reduce the potential for tumor escape from immune surveillance.
Personalized peptide vaccines
In 2007, an RCC vaccination protocol enrolled 10 patients with refractory metastatic disease in which four HLA class I restricted synthetic peptides per patient were selected from a pool of 25 HLA-A*24-restricted and 23 HLA-A*02 peptides, based on the presence of preexisting peptide-specific CTLs in PBMC and IgG in the plasma of RCC patients.Citation89 Peptide-reactive CTL responses were detected in only 2/10 patients post-treatment, peptide-specific IFN-γ production in post-vaccination PBMC was only minimally increased, and no objective responses were observed.
Survivin and telomerase
Survivin and telomerase are overexpressed in the majority of RCC tumors. Survivin is an oncofetal protein that inhibits apoptosis and regulates cell division, while telomerase regulates telomere shortening during DNA replication and is activated in a majority of human cancers. Histological analysis of 634 ccRCC tumors demonstrated that survivin was expressed in all tumors, with 31.2% tumors having high expression (> 15 cells/mmCitation2).Citation132 A meta-analysis of 12 independent studies showed that increased survivin expression predicted poor prognosis in RCC patients.Citation133 In a phase I/II multi-peptide vaccination trial, eight telomerase- and 11 survivin-derived HLA-A*02-restricted peptides (or tumor lysate for the non-HLA-A*02 cohort) were loaded on autologous DCs to vaccinate 13 HLA-A*02+ patients.Citation90 In 14 non-HLA-A*02+ patients, autologous DCs were pulsed with tumor lysate prior to administration, in hope to present tumor antigen to CTLs. Cytotoxic T-cell responses against one or more survivin/telomerase peptides were found in 6/6 evaluated HLA-A*02+ patients. No objective responses were observed.
IMA-901
IMA-901 is a vaccine developed from multiple tumor-associated peptides that are naturally presented in human RCC tissue.Citation134 The discovery workflow included mass spectrometry sequencing of HLA-associated peptides eluted from primary RCC tumors, mRNA expression profiling of the genes encoding HLA-bound peptides to select those preferentially expressed in tumor versus healthy tissues, and assessment of peptide immunogenicity by in vitro priming of peptide-specific T-cells in PBMC of healthy donors. Nine HLA-A*02-presented peptides and one HLA-DR-restricted peptide from highly overexpressed genes in tumor (PLIN2, APOL1, CCND1, GUCY1A3, PRUNE2, MET, MUC1, RGS5, MMP7) were included in the vaccine, and granulocyte macrophage-colony stimulating factor (GM-CSF) was used as a local immune adjuvant.Citation91 In a phase I trial, 20/27 patients developed a T-cell response to at least one peptide antigen, and 8/27 patients responded to more than one peptide.Citation135 A phase II trial in 68 patients demonstrated similar immune response efficacy where 64% of patients treated with IMA901 plus GM-CSF with or without a single infusion of cyclophosphamide-developed T-cell responses, and 26% responded to more than one peptide.Citation91 Better OS was associated with T-cell responses against multiple peptides. In the subsequent phase III IMPRINT study of HLA-A*02+ patients randomized to IMA-901 (n = 204) plus sunitinib versus sunitinib monotherapy (n = 135),Citation92 no improvement in OS was observed for the vaccinated patients (HR 1.34, P = 0.087). Notably, the magnitude of T-cell responses was threefold lower compared with the preceding phase II study, and there was no clear association between T-cell responses and clinical outcomes.
AGS-003
In a phase I study, renal tumor RNA-transfected DCs were administered to 10 patients. The vaccine successfully induced T-cell responses against a broad set of tumor antigens including hTERT, CAIX, and OFA, but not against antigens expressed by autologous normal renal tissue.Citation136 AGS-003 (Rocapuldencel-T) is a DC-based vaccine in which autologous mature DCs are electroporated with tumor lysate-derived mRNA and synthetic CD40L RNA in order to present patient-specific tumor antigens (mutated and non-mutated; class I and class II), and to activate co-stimulatory signals in T-cells.Citation137 Following a promising result in a phase II study of 21 patients (median OS of 30.1 months),Citation93 the phase III ADAPT study was performed in which 462 mRCC patients undergoing cytoreductive nephrectomy were randomly assigned to AGS-003 plus sunitinib versus sunitinib monotherapy.Citation94 By intent to treat analysis, there was no significant OS benefit for combination therapy (HR 1.10). Of interest, for the 70% of the vaccine-treated patients with a positive T-cell activation biomarker assay, the magnitude of the T-cell response positively correlated with OS.
Discussion and future directions
Extensive clinical development of vaccine and other antigen targeting strategies for RCC has culminated with four completed phase III clinical trials. However, a successful primary endpoint for better survival in patients who received the investigational therapies has not yet been demonstrated, and there are currently no FDA-approved vaccine or other tumor antigen-specific compounds for advanced RCC.
Multiple early peptide vaccine studies showed encouraging rates for T-cell priming against the target antigen. However, conventional immune monitoring post-peptide-based vaccination may overestimate the frequency of true tumor-reactive T-cells in tumor by detecting a T-cell pool with a wide range of antigen affinity/avidity in periphery. T-cells with low avidity respond to peptide-loaded targets but may not recognize lower antigen density on tumor.Citation85 Multi-peptide vaccines therefore were developed to increase the frequency of T-cell responses and also decrease the potential for tumor escape from immune surveillance. Despite deploying a panel of ten synthetic tumor antigen peptides, the phase III IMPRINT study failed to show improved survival in the vaccine cohort associated with immune response data significantly inferior to prior phase I and II data with the same compound.Citation92
Compared to synthetic peptides, recombinant vaccinia vaccines that require T-cell recognition of naturally processed antigen may stimulate T-cells with higher avidity and more robust anti-tumor activity. Such constructs also are anticipated to prime both antigen-specific CD8+ and CD4+ T-cells and therefore may elicit better helper function than synthetic peptide vaccines stimulating only CD8+ T-cell responses. Nevertheless, MVA-based vaccines incorporating 5T4 or MUC1 achieved detectable antigen-specific T-cell priming in < 50% of vaccinated patients. Rapid development of serologic immunity to the MVA vector likely limited the ability of repeat vaccination to drive sustained T-cell responses Citation84 and contributed to the failure of MVA-5T4 to show a survival benefit in the phase III TRIST trial. A prime-boost protocol with sequential heterologous recombinant 5T4 expression vectors (ChAdOx1-MVA-5T4) has yielded considerably higher specific T-cells responsesCitation138 in recent phase I testing in prostate cancer.Citation139
The naptumomab estafenatox fusion protein in principle could overcome the non-responder phenotype observed in vaccine trials by delivering antigen-directed T-cell engagement to all treated patients. However, the bacterial derived superantigen component is immunogenic, and like the MVA reagents, serologic immunity to the bacterial protein also appeared to diminish the activity.Citation115,Citation116 While the primary analysis of the phase II/III trial with naptumomab estafenatox was negative for improved survival versus the IFN-γ control arm, the patient subgroup having below median of baseline anti-SEA/E-120 showed a trend toward improved OS and PFS.
The tumor RNA-transfected DC platform for the AGS-003 vaccine addressed several limitations inherent in the other vaccine and naptumomab estafenatox therapies. Antigen targets expressed in AGS-003 are naturally processed proteins that are expected to prime high avidity T-cell responses. Antigens are personalized based on autologous tumor RNA and can include both mutated and nonmutated antigens without requiring the laborious effort needed to identify patient-specific antigen targets. AGS-003 is also fully autologous without foreign elements and should be suitable for serial administration and T-cell boosting without eliciting vector-specific immunity. Despite these advantages, the phase III ADAPT trial failed to show a survival benefit for patients receiving AGS-003. One limitation of individualized antigen priming is the inability to conduct quantitative antigen-specific immune monitoring, leaving substantial uncertainty about the magnitude and durability of T-cell priming against tumor antigens with this approach.
It is noteworthy that all of the phase III vaccine studies were conducted during the cytokine or targeted therapy era of RCC, and it has been suggested that the combining partner for the tested vaccines may have contributed to the lack of efficacy. For example, reduced monocyte counts in the IMPRINT study has been associated with sunitinib and suggested to contribute to the poor immune response outcomes noted.Citation92 Therapeutic combinations of tumor vaccine and immune checkpoint blockade may produce better synergy to activate and maintain effective anti-tumor T-cell immunity.Citation138 Currently active studies include the personalized neoantigen peptide vaccine NeoMax in combination with ipilimumab at the vaccine injection site to direct anti-CTLA4 activity to the vaccine-draining lymph nodes (NCT02950766), the WT1 multi-peptide vaccine DSP-7888 administered in combination with anti-PD-1 antibodies nivolumab or pembrolizumab (NCT03311334), and an mRNA-based personalized neoantigen multi-epitope vaccine in combination with the anti-PD-L1 antibody atezolizumab (NCT03289962).
The impressive success of engineered T-cells expressing chimeric antigen receptors (CARs) to achieve complete remissions of refractory acute lymphocytic leukemia and non-Hodgkin lymphomaCitation140,Citation141 has created intense interest to extend engineered, re-directed T-cells as a therapeutic modality to treat other cancers including solid tumors. Promising early results with T-cell receptor (TCR) engineered T-cell therapy targeting NY-ESO-1, MAGE-A4 and human papillomavirus proteins for melanomas, sarcomas, and cervical cancers encourage further development of tumor antigen-specific TCR in addition to CAR vectors.Citation142–144 Adoptive T-cell therapy appears capable of generating far higher numbers of anti-tumor effector T-cells in treated patients than can be generated with available vaccine technologies and provides an anti-tumor product to all treated patients in contrast to vaccines that suffer from high frequencies of non-responders. However, the initial experience in RCC patients with CAR-T-cells targeting CAIX associated with off-tumor toxicity without tumor response reinforces the need for both careful target selection and also strategies to augment the anti-tumor potency.Citation76 Cellular therapies lend themselves to further optimizations including the targeting receptor gene vector (TCR affinity and expression enhancement,Citation145 CAR signaling domain optimizationCitation146), selecting the phenotype and effector potential of transduced T-cells (CD4+, CD8+,Citation147 central memory T-cellCitation148), and further genetic manipulations of the transduced cell, (CRISPR/cas9 targeted disruption of the native TCR locus,Citation149 or inhibitory co-receptorsCitation150). Safe and effective cellular products may subsequently be combined with synergistic non-specific immunotherapies including checkpoint blocking antibodies or T-cell agonist cytokines. Emerging phase I and II trials enrolling RCC patients are actively testing engineered T-cells with CAR vectors targeting CD70, or c-MET in addition to TCR’s specific for personalized neoantigens or an HLA-A*11 presented hERV-E epitope ().
Table 4. Active trials with engineered T-cell therapy enrolling metastatic RCC patients
5T4 is the most extensively studied RCC antigen and has been targeted in phase III trials by vaccination with MVA-5T4 and the antibody-superantigen conjugate naptumomab estafenatox without detection of on-target, off-tumor toxicities. Our group has sequenced the TCRs from seven high avidity CTL clones recognizing a defined HLA-A2 presented 5T4 epitope.Citation151 CD8+ T-cells from healthy donors transduced with these 5T4-specific TCRs recognized 5T4-expressing tumor lines and primary RCC tumors, but not normal renal tubular epithelial cells. Clinical testing of TCR engineered T-cells redirected to target 5T4 would closely parallel the emerging success with engineered T-cells targeting single cancer-testis class antigens NY-ESO-1 and MAGE-A4 in other solid tumors.
Lastly, discovery of novel tumor antigen targets in RCC is a research priority, with highest interest in the antigen targets associated with sustained and deep tumor regression seen in some patients receiving checkpoint blocking immunotherapies. However, a major technical challenge is the identification of the cognate antigen for defined TCR sequences. T-cell antigen discovery may be accelerated by the recent developments in high-throughput methods for identifying TCR-epitope pairs.Citation152–154 Our group and others are applying whole-genome CRISPR-Cas9 screening to facilitate the discovery of tumor antigens targeted by RCC TIL.Citation155,Citation156 Emerging computational approaches that consider information such as TCR sequence similarity, structural information, V gene usage bias, CDR3 length, and HLA types are also in development to predict peptide antigen sequences for select TCRs. Success in these efforts may serve to prioritize among known RCC tumor antigens those with the highest clinical value for therapeutic targeting.
Disclosure of potential conflicts of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551.
- Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530–40. doi:10.1200/JCO.1999.17.8.2530.
- Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000;163(2):408–17. doi:10.1016/S0022-5347(05)67889-5.
- Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthelemy P, Porta C, George S, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90. doi:10.1056/NEJMoa1712126.
- Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulieres D, Melichar B, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–27. doi:10.1056/NEJMoa1816714.
- Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–15. doi:10.1056/NEJMoa1816047.
- Bumpus HC. The apparent disappearance of pulmonary metastasis in a case of hypernephroma following nephrectomy. J Urol. 1928;20(2):185–92. doi:10.1016/S0022-5347(17)73147-3.
- Janiszewska AD, Poletajew S, Wasiutynski A. Spontaneous regression of renal cell carcinoma. Contemp Oncol (Pozn). 2013;17(2):123–27. doi:10.5114/wo.2013.34613.
- Hobohm U. Fever therapy revisited. Br J Cancer. 2005;92(3):421–25. doi:10.1038/sj.bjc.6602386.
- Gastl G, Ebert T, Finstad CL, Sheinfeld J, Gomahr A, Aulitzky W, Bander NH. Major histocompatibility complex class i and class ii expression in renal cell carcinoma and modulation by interferon gamma. J Urol. 1996;155(1):361–67. doi:10.1016/S0022-5347(01)66661-8.
- Finke JH, Rayman P, Edinger M, Tubbs RR, Stanley J, Klein E, Bukowski R. Characterization of a human renal cell carcinoma specific cytotoxic cd8+ t cell line. J Immunother. 1991–1992;11(1):1–11. doi:10.1097/00002371-199201000-00001.
- Schendel DJ, Gansbacher B, Oberneder R, Kriegmair M, Hofstetter A, Riethmuller G, Segurado OG. Tumor-specific lysis of human renal cell carcinomas by tumor-infiltrating lymphocytes. I. Hla-a2-restricted recognition of autologous and allogeneic tumor lines. J Immunol. 1993;151:4209–20.
- Brandle D, Brasseur F, Weynants P, Boon T, Van den Eynde B. A mutated hla-a2 molecule recognized by autologous cytotoxic t lymphocytes on a human renal cell carcinoma. J Exp Med. 1996;183(6):2501–08. doi:10.1084/jem.183.6.2501.
- Gaudin C, Kremer F, Angevin E, Scott V, Triebel F. A hsp70–2 mutation recognized by ctl on a human renal cell carcinoma. J Immunol. 1999;162:1730–38.
- Gaugler B, Brouwenstijn N, Vantomme V, Szikora JP, Van der Spek CW, Patard JJ, Boon T, Schrier P, Van den Eynde BJ. A new gene coding for an antigen recognized by autologous cytolytic t lymphocytes on a human renal carcinoma. Immunogenetics. 1996;44(5):323–30. doi:10.1007/BF02602776.
- Morel S, Levy F, Burlet-Schiltz O, Brasseur F, Probst-Kepper M, Peitrequin AL, Monsarrat B, Van Velthoven R, Cerottini JC, Boon T, et al. Processing of some antigens by the standard proteasome but not by the immunoproteasome results in poor presentation by dendritic cells. Immunity. 2000;12(1):107–17. doi:10.1016/S1074-7613(00)80163-6.
- Probst-Kepper M, Stroobant V, Kridel R, Gaugler B, Landry C, Brasseur F, Cosyns JP, Weynand B, Boon T, Van den Eynde BJ. An alternative open reading frame of the human macrophage colony-stimulating factor gene is independently translated and codes for an antigenic peptide of 14 amino acids recognized by tumor-infiltrating cd8 t lymphocytes. J Exp Med. 2001;193(10):1189–98. doi:10.1084/jem.193.10.1189.
- Ronsin C, Chung-Scott V, Poullion I, Aknouche N, Gaudin C, Triebel F. A non-aug-defined alternative open reading frame of the intestinal carboxyl esterase mrna generates an epitope recognized by renal cell carcinoma-reactive tumor-infiltrating lymphocytes in situ. J Immunol. 1999;163:483–90.
- Van Den Eynde BJ, Gaugler B, Probst-Kepper M, Michaux L, Devuyst O, Lorge F, Weynants P, Boon T. A new antigen recognized by cytolytic t lymphocytes on a human kidney tumor results from reverse strand transcription. J Exp Med. 1999;190(12):1793–800. doi:10.1084/jem.190.12.1793.
- Hanada K, Yewdell JW, Yang JC. Immune recognition of a human renal cancer antigen through post-translational protein splicing. Nature. 2004;427(6971):252–56. doi:10.1038/nature02240.
- Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–96. doi:10.1200/JCO.1995.13.3.688.
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–16. doi:10.1200/JCO.1999.17.7.2105.
- Belldegrun AS, Klatte T, Shuch B, LaRochelle JC, Miller DC, Said JW, Riggs SB, Zomorodian N, Kabbinavar FF, Dekernion JB, et al. Cancer-specific survival outcomes among patients treated during the cytokine era of kidney cancer (1989–2005): a benchmark for emerging targeted cancer therapies. Cancer. 2008;113(9):2457–63. doi:10.1002/cncr.23851.
- Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20(1):289–96. doi:10.1200/JCO.2002.20.1.289.
- Palmer PA, Vinke J, Philip T, Negrier S, Atzpodien J, Kirchner H, Oskam R, Franks CR, Von der Maase H, Thatcher N. Prognostic factors for survival in patients with advanced renal cell carcinoma treated with recombinant interleukin-2. Ann Oncol. 1992;3(6):475–80. doi:10.1093/oxfordjournals.annonc.a058239.
- Fossa SD, Kramar A, Droz JP. Prognostic factors and survival in patients with metastatic renal cell carcinoma treated with chemotherapy or interferon-alpha. Eur J Cancer. 1994;30A(9):1310–14. doi:10.1016/0959-8049(94)90179-1.
- Varn FS, Wang Y, Mullins DW, Fiering S, Cheng C. Systematic pan-cancer analysis reveals immune cell interactions in the tumor microenvironment. Cancer Res. 2017;77(6):1271–82. doi:10.1158/0008-5472.CAN-16-2490.
- Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, Ries CH, Ailles L, Jewett MAS, Moch H, et al. An immune atlas of clear cell renal cell carcinoma. Cell. 2017;169(4):736–749 e718. doi:10.1016/j.cell.2017.04.016.
- Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1–2):48–61. doi:10.1016/j.cell.2014.12.033.
- Giraldo NA, Becht E, Pages F, Skliris G, Verkarre V, Vano Y, Mejean A, Saint-Aubert N, Lacroix L, Natario I, et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin Cancer Res. 2015;21(13):3031–40. doi:10.1158/1078-0432.CCR-14-2926.
- Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean MC, Riquet M, Crozet L, Ouakrim H, Goc J, Cazes A, et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res. 2013;19(15):4079–91. doi:10.1158/1078-0432.CCR-12-3847.
- Kondo T, Nakazawa H, Ito F, Hashimoto Y, Osaka Y, Futatsuyama K, Toma H, Tanabe K. Favorable prognosis of renal cell carcinoma with increased expression of chemokines associated with a th1-type immune response. Cancer Sci. 2006;97(8):780–86. doi:10.1111/j.1349-7006.2006.00231.x.
- Senbabaoglu Y, Gejman RS, Winer AG, Liu M, Van Allen EM, de Velasco G, Miao D, Ostrovnaya I, Drill E, Luna A, et al. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger rna signatures. Genome Biol. 2016;17(1):231. doi:10.1186/s13059-016-1092-z.
- Ghatalia P, Gordetsky J, Kuo F, Dulaimi E, Cai KQ, Devarajan K, Bae S, Naik G, Chan TA, Uzzo R, et al. Prognostic impact of immune gene expression signature and tumor infiltrating immune cells in localized clear cell renal cell carcinoma. J Immunother Cancer. 2019;7(1):139. doi:10.1186/s40425-019-0621-1.
- Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral cd8(+) t-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–36.
- Gaudin C, Dietrich PY, Robache S, Guillard M, Escudier B, Lacombe MJ, Kumar A, Triebel F, Caignard A. In vivo local expansion of clonal t cell subpopulations in renal cell carcinoma. Cancer Res. 1995;55:685–90.
- Weidmann E, Logan TF, Yasumura S, Kirkwood JM, Trucco M, Whiteside TL. Evidence for oligoclonal t-cell response in a metastasis of renal cell carcinoma responding to vaccination with autologous tumor cells and transfer of in vitro-sensitized vaccine-draining lymph node lymphocytes. Cancer Res. 1993;53:4745–49.
- Massa C, Robins H, Desmarais C, Riemann D, Fahldieck C, Fornara P, Seliger B. Identification of patient-specific and tumor-shared t cell receptor sequences in renal cell carcinoma patients. Oncotarget. 2017;8(13):21212–28. doi:10.18632/oncotarget.15064.
- Prinz PU, Mendler AN, Masouris I, Durner L, Oberneder R, Noessner E. High dgk-alpha and disabled mapk pathways cause dysfunction of human tumor-infiltrating cd8+ t cells that is reversible by pharmacologic intervention. J Immunol. 2012;188(12):5990–6000. doi:10.4049/jimmunol.1103028.
- Noessner E, Brech D, Mendler AN, Masouris I, Schlenker R, Prinz PU. Intratumoral alterations of dendritic-cell differentiation and cd8(+) t-cell anergy are immune escape mechanisms of clear cell renal cell carcinoma. Oncoimmunology. 2012;1(8):1451–53. doi:10.4161/onci.21356.
- Krummel MF, Allison JP. Cd28 and ctla-4 have opposing effects on the response of t cells to stimulation. J Exp Med. 1995;182(2):459–65. doi:10.1084/jem.182.2.459.
- Krummel MF, Allison JP. Ctla-4 engagement inhibits il-2 accumulation and cell cycle progression upon activation of resting t cells. J Exp Med. 1996;183(6):2533–40. doi:10.1084/jem.183.6.2533.
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated b7-h1 promotes t-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi:10.1038/nm730.
- Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between pd-1 and pd-l1 promote tolerance by blocking the tcr-induced stop signal. Nat Immunol. 2009;10(11):1185–92. doi:10.1038/ni.1790.
- Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, et al. Tumor b7-h1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–85.
- Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. Pd-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13(6):1757–61. doi:10.1158/1078-0432.CCR-06-2599.
- Kang MJ, Kim KM, Bae JS, Park HS, Lee H, Chung MJ, Moon WS, Lee DG, Jang KY. Tumor-infiltrating pd1-positive lymphocytes and foxp3-positive regulatory t cells predict distant metastatic relapse and survival of clear cell renal cell carcinoma. Transl Oncol. 2013;6(3):282–89. doi:10.1593/tlo.13256.
- Anderson AC, Joller N, Kuchroo VK. Lag-3, tim-3, and tigit: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989–1004. doi:10.1016/j.immuni.2016.05.001.
- Granier C, Dariane C, Combe P, Verkarre V, Urien S, Badoual C, Roussel H, Mandavit M, Ravel P, Sibony M, et al. Tim-3 expression on tumor-infiltrating pd-1(+)cd8(+) t cells correlates with poor clinical outcome in renal cell carcinoma. Cancer Res. 2017;77(5):1075–82. doi:10.1158/0008-5472.CAN-16-0274.
- Sakuishi K, Ngiow SF, Sullivan JM, Teng MW, Kuchroo VK, Smyth MJ, Anderson AC. Tim3(+)foxp3(+) regulatory t cells are tissue-specific promoters of t-cell dysfunction in cancer. Oncoimmunology. 2013;2(4):e23849. doi:10.4161/onci.23849.
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. doi:10.1056/NEJMoa1510665.
- Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–39. doi:10.1038/nature12634.
- Cancer Genome Atlas Research N. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499(7456):43–49. doi:10.1038/nature12222.
- de Velasco G, Wankowicz SA, Madison R, Ali SM, Norton C, Duquette A, Ross JS, Bosse D, Lalani AA, Miller VA, et al. Targeted genomic landscape of metastases compared to primary tumours in clear cell metastatic renal cell carcinoma. Br J Cancer. 2018;118(9):1238–42. doi:10.1038/s41416-018-0064-3.
- Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to pd-1 inhibition. N Engl J Med. 2017;377(25):2500–01. doi:10.1056/NEJMc1713444.
- Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase ii keynote-158 study. J Clin Oncol. 2020;38(1):1–10. doi:10.1200/JCO.19.02105.
- McDermott DF, Lee J-L, Bjarnason GA, Larkin JMG, Gafanov R, Kochenderfer MD, Jensen NV, Donskov F, Malik J, Poprach A, et al. First-line pembrolizumab (pembro) monotherapy in advanced clear cell renal cell carcinoma (ccrcc): updated follow-up for keynote-427 cohort a. J Clin Oncol. 2020;38(15_suppl):5069–5069.
- Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL, Wong YNS, Rowan A, Kanu N, Al Bakir M, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 2017;18(8):1009–21. doi:10.1016/S1470-2045(17)30516-8.
- Braun DA, Hou Y, Bakouny Z, Ficial M, Sant’ Angelo M, Forman J, Ross-Macdonald P, Berger AC, Jegede OA, Elagina L, et al. Interplay of somatic alterations and immune infiltration modulates response to pd-1 blockade in advanced clear cell renal cell carcinoma. Nat Med. 2020;26(6):909–18. doi:10.1038/s41591-020-0839-y.
- Motzer RJ, Choueiri TK, McDermott DF, Powles T, Yao J, Ammar R, Papillon-Cavanagh S, Saggi SS, McHenry BM, Ross-Macdonald P, et al. Biomarker analyses from the phase iii checkmate 214 trial of nivolumab plus ipilimumab (n+i) or sunitinib (s) in advanced renal cell carcinoma (arcc). J Clin Oncol. 2020;38(15_suppl):5009–5009. doi:10.1200/JCO.2020.38.15_suppl.5009.
- Motzer RJ, Robbins PB, Powles T, Albiges L, Haanen JB, Larkin J, Mu XJ, Ching KA, Uemura M, Pal SK, et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 javelin renal 101 trial. Nat Med. 2020;26:1733–41. doi:10.1038/s41591-020-1044-8.
- McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, Fong L, Joseph RW, Pal SK, Reeves JA, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749–57. doi:10.1038/s41591-018-0053-3.
- Schmitt K, Reichrath J, Roesch A, Meese E, Mayer J. Transcriptional profiling of human endogenous retrovirus group herv-k(hml-2) loci in melanoma. Genome Biol Evol. 2013;5(2):307–28. doi:10.1093/gbe/evt010.
- Wang-Johanning F, Liu J, Rycaj K, Huang M, Tsai K, Rosen DG, Chen DT, Lu DW, Barnhart KF, Johanning GL. Expression of multiple human endogenous retrovirus surface envelope proteins in ovarian cancer. Int J Cancer. 2007;120(1):81–90. doi:10.1002/ijc.22256.
- Contreras-Galindo R, Kaplan MH, Leissner P, Verjat T, Ferlenghi I, Bagnoli F, Giusti F, Dosik MH, Hayes DF, Gitlin SD, et al. Human endogenous retrovirus k (hml-2) elements in the plasma of people with lymphoma and breast cancer. J Virol. 2008;82(19):9329–36. doi:10.1128/JVI.00646-08.
- Florl AR, Lower R, Schmitz-Drager BJ, Schulz WA. DNA methylation and expression of line-1 and herv-k provirus sequences in urothelial and renal cell carcinomas. Br J Cancer. 1999;80(9):1312–21. doi:10.1038/sj.bjc.6690524.
- Siebenthall KT, Miller CP, Vierstra JD, Mathieu J, Tretiakova M, Reynolds A, Sandstrom R, Rynes E, Haugen E, Johnson A, et al. Integrated epigenomic profiling reveals endogenous retrovirus reactivation in renal cell carcinoma. EBioMedicine. 2019;41:427–42. doi:10.1016/j.ebiom.2019.01.063.
- Cherkasova E, Malinzak E, Rao S, Takahashi Y, Senchenko VN, Kudryavtseva AV, Nickerson ML, Merino M, Hong JA, Schrump DS, et al. Inactivation of the von hippel-lindau tumor suppressor leads to selective expression of a human endogenous retrovirus in kidney cancer. Oncogene. 2011;30(47):4697–706. doi:10.1038/onc.2011.179.
- Takahashi Y, Harashima N, Kajigaya S, Yokoyama H, Cherkasova E, McCoy JP, Hanada K, Mena O, Kurlander R, Tawab A, et al. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an herv-e antigen by t cells. J Clin Invest. 2008;118(3):1099–109. doi:10.1172/JCI34409C1.
- Cherkasova E, Scrivani C, Doh S, Weisman Q, Takahashi Y, Harashima N, Yokoyama H, Srinivasan R, Linehan WM, Lerman MI, et al. Detection of an immunogenic herv-e envelope with selective expression in clear cell kidney cancer. Cancer Res. 2016;76(8):2177–85. doi:10.1158/0008-5472.CAN-15-3139.
- Smith CC, Selitsky SR, Chai S, Armistead PM, Vincent BG, Serody JS. Alternative tumour-specific antigens. Nat Rev Cancer. 2019;19(8):465–78. doi:10.1038/s41568-019-0162-4.
- Smith CC, Beckermann KE, Bortone DS, De Cubas AA, Bixby LM, Lee SJ, Panda A, Ganesan S, Bhanot G, Wallen EM, et al. Endogenous retroviral signatures predict immunotherapy response in clear cell renal cell carcinoma. J Clin Invest. 2018;128(11):4804–20. doi:10.1172/JCI121476.
- Pignon J-C, Jegede O, Shukla SA, Braun DA, Horak C, Wind-Rotolo M, Ishii Y, Catalano PJ, Freeman GJ, Jennings RB, et al. Association of human endogenous retrovirus (herv) expression with clinical efficacy of pd-1 blockade in metastatic clear cell renal cell carcinoma (mccrcc). J Clin Oncol. 2019;37(15_suppl):4568–4568. doi:10.1200/JCO.2019.37.15_suppl.4568.
- Uemura H, Fujimoto K, Tanaka M, Yoshikawa M, Hirao Y, Uejima S, Yoshikawa K, Itoh K. A phase i trial of vaccination of ca9-derived peptides for hla-a24-positive patients with cytokine-refractory metastatic renal cell carcinoma. Clin Cancer Res. 2006;12(6):1768–75. doi:10.1158/1078-0432.CCR-05-2253.
- Bleumer I, Tiemessen DM, Oosterwijk-Wakka JC, Voller MC, De Weijer K, Mulders PF, Oosterwijk E. Preliminary analysis of patients with progressive renal cell carcinoma vaccinated with ca9-peptide-pulsed mature dendritic cells. J Immunother. 2007;30(1):116–22. doi:10.1097/01.cji.0000211318.22902.ec.
- Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, Vulto A, den Bakker M, Oosterwijk E, Debets R, et al. Treatment of metastatic renal cell carcinoma with caix car-engineered t cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21(4):904–12. doi:10.1038/mt.2013.17.
- Yoshimura K, Minami T, Nozawa M, Uemura H. Phase i clinical trial of human vascular endothelial growth factor receptor 1 peptide vaccines for patients with metastatic renal cell carcinoma. Br J Cancer. 2013;108(6):1260–66. doi:10.1038/bjc.2013.90.
- Obara W, Karashima T, Takeda K, Kato R, Kato Y, Kanehira M, Takata R, Inoue K, Katagiri T, Shuin T, et al. Effective induction of cytotoxic t cells recognizing an epitope peptide derived from hypoxia-inducible protein 2 (hig2) in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2017;66(1):17–24. doi:10.1007/s00262-016-1915-5.
- Rahma OE, Ashtar E, Ibrahim R, Toubaji A, Gause B, Herrin VE, Linehan WM, Steinberg SM, Grollman F, Grimes G, et al. A pilot clinical trial testing mutant von hippel-lindau peptide as a novel immune therapy in metastatic renal cell carcinoma. J Transl Med. 2010;8(1):8. doi:10.1186/1479-5876-8-8.
- Amato RJ, Shingler W, Naylor S, Jac J, Willis J, Saxena S, Hernandez-McClain J, Harrop R. Vaccination of renal cell cancer patients with modified vaccinia ankara delivering tumor antigen 5t4 (trovax) administered with interleukin 2: A phase ii trial. Clin Cancer Res. 2008;14(22):7504–10. doi:10.1158/1078-0432.CCR-08-0668.
- Kaufman HL, Taback B, Sherman W, Kim DW, Shingler WH, Moroziewicz D, DeRaffele G, Mitcham J, Carroll MW, Harrop R, et al. Phase ii trial of modified vaccinia ankara (mva) virus expressing 5t4 and high dose interleukin-2 (il-2) in patients with metastatic renal cell carcinoma. J Transl Med. 2009;7(2). doi:10.1186/1479-5876-7-2.
- Hawkins RE, Macdermott C, Shablak A, Hamer C, Thistlethwaite F, Drury NL, Chikoti P, Shingler W, Naylor S, Harrop R. Vaccination of patients with metastatic renal cancer with modified vaccinia ankara encoding the tumor antigen 5t4 (trovax) given alongside interferon-alpha. J Immunother. 2009;32(4):424–29. doi:10.1097/CJI.0b013e31819d297e.
- Amato RJ, Shingler W, Goonewardena M, de Belin J, Naylor S, Jac J, Willis J, Saxena S, Hernandez-McClain J, Harrop R. Vaccination of renal cell cancer patients with modified vaccinia ankara delivering the tumor antigen 5t4 (trovax) alone or administered in combination with interferon-alpha (ifn-alpha): A phase 2 trial. J Immunother. 2009;32(7):765–72. doi:10.1097/CJI.0b013e3181ace876.
- Amato RJ, Hawkins RE, Kaufman HL, Thompson JA, Tomczak P, Szczylik C, McDonald M, Eastty S, Shingler WH, de Belin J, et al. Vaccination of metastatic renal cancer patients with mva-5t4: A randomized, double-blind, placebo-controlled phase iii study. Clin Cancer Res. 2010;16(22):5539–47. doi:10.1158/1078-0432.CCR-10-2082.
- Wierecky J, Muller MR, Wirths S, Halder-Oehler E, Dorfel D, Schmidt SM, Hantschel M, Brugger W, Schroder S, Horger MS, et al. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res. 2006;66(11):5910–18. doi:10.1158/0008-5472.CAN-05-3905.
- Oudard S, Rixe O, Beuselinck B, Linassier C, Banu E, Machiels JP, Baudard M, Ringeisen F, Velu T, Lefrere-Belda MA, et al. A phase ii study of the cancer vaccine tg4010 alone and in combination with cytokines in patients with metastatic renal clear-cell carcinoma: clinical and immunological findings. Cancer Immunol Immunother. 2011;60(2):261–71. doi:10.1007/s00262-010-0935-9.
- Iiyama T, Udaka K, Takeda S, Takeuchi T, Adachi YC, Ohtsuki Y, Tsuboi A, Nakatsuka S, Elisseeva OA, Oji Y, et al. Wt1 (wilms’ tumor 1) peptide immunotherapy for renal cell carcinoma. Microbiol Immunol. 2007;51(5):519–30. doi:10.1111/j.1348-0421.2007.tb03940.x.
- Ogasawara M, Miyashita M, Ota S. Vaccination of urological cancer patients with wt1 peptide-pulsed dendritic cells in combination with molecular targeted therapy or conventional chemotherapy induces immunological and clinical responses. Ther Apher Dial. 2018;22(3):266–77. doi:10.1111/1744-9987.12694.
- Suekane S, Nishitani M, Noguchi M, Komohara Y, Kokubu T, Naitoh M, Honma S, Yamada A, Itoh K, Matsuoka K, et al. Phase i trial of personalized peptide vaccination for cytokine-refractory metastatic renal cell carcinoma patients. Cancer Sci. 2007;98(12):1965–68. doi:10.1111/j.1349-7006.2007.00631.x.
- Berntsen A, Trepiakas R, Wenandy L, Geertsen PF, Thor Straten P, Andersen MH, Pedersen AE, Claesson MH, Lorentzen T, Johansen JS, et al. Therapeutic dendritic cell vaccination of patients with metastatic renal cell carcinoma: a clinical phase 1/2 trial. J Immunother. 2008;31(8):771–80. doi:10.1097/CJI.0b013e3181833818.
- Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, et al. Multipeptide immune response to cancer vaccine ima901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254–61. doi:10.1038/nm.2883.
- Rini BI, Stenzl A, Zdrojowy R, Kogan M, Shkolnik M, Oudard S, Weikert S, Bracarda S, Crabb SJ, Bedke J, et al. Ima901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (imprint): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(11):1599–611. doi:10.1016/S1470-2045(16)30408-9.
- Amin A, Dudek AZ, Logan TF, Lance RS, Holzbeierlein JM, Knox JJ, Master VA, Pal SK, Miller WH Jr., Karsh LI, et al. Survival with ags-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (rcc): phase 2 study results. J Immunother Cancer. 2015;3(14). doi:10.1186/s40425-015-0055-3.
- Figlin RA, Tannir NM, Uzzo RG, Tykodi SS, Chen DYT, Master V, Kapoor A, Vaena D, Lowrance WT, Bratslavsky G, et al. Results of the adapt phase 3 study of rocapuldencel-t in combination with sunitinib as first-line therapy in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2020;26:2327–36. doi:10.1158/1078-0432.CCR-19-2427.
- Oosterwijk E, Ruiter DJ, Hoedemaeker PJ, Pauwels EK, Jonas U, Zwartendijk J, Warnaar SO. Monoclonal antibody g 250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidney. Int J Cancer. 1986;38(4):489–94. doi:10.1002/ijc.2910380406.
- Pastorek J, Pastorekova S. Hypoxia-induced carbonic anhydrase ix as a target for cancer therapy: from biology to clinical use. Semin Cancer Biol. 2015;31:52–64. doi:10.1016/j.semcancer.2014.08.002.
- Mandriota SJ, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM, Wykoff CC, Maher ER, Harris AL, Ratcliffe PJ, et al. Hif activation identifies early lesions in vhl kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell. 2002;1(5):459–68. doi:10.1016/S1535-6108(02)00071-5.
- Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158(3):905–19.
- Vissers JL, De Vries IJ, Schreurs MW, Engelen LP, Oosterwijk E, Figdor CG, Adema GJ. The renal cell carcinoma-associated antigen g250 encodes a human leukocyte antigen (hla)-a2.1-restricted epitope recognized by cytotoxic t lymphocytes. Cancer Res. 1999;59:5554–59.
- Vissers JL, De Vries IJ, Engelen LP, Scharenborg NM, Molkenboer J, Figdor CG, Oosterwijk E, Adema GJ. Renal cell carcinoma-associated antigen g250 encodes a naturally processed epitope presented by human leukocyte antigen-dr molecules to cd4(+) t lymphocytes. Int J Cancer. 2002;100(4):441–44. doi:10.1002/ijc.10518.
- Li H, Ding J, Lu M, Liu H, Miao Y, Li L, Wang G, Zheng J, Pei D, Zhang Q. Caix-specific car-t cells and sunitinib show synergistic effects against metastatic renal cancer models. J Immunother. 2020;43(1):16–28. doi:10.1097/CJI.0000000000000301.
- Togashi A, Katagiri T, Ashida S, Fujioka T, Maruyama O, Wakumoto Y, Sakamoto Y, Fujime M, Kawachi Y, Shuin T, et al. Hypoxia-inducible protein 2 (hig2), a novel diagnostic marker for renal cell carcinoma and potential target for molecular therapy. Cancer Res. 2005;65(11):4817–26. doi:10.1158/0008-5472.CAN-05-0120.
- Soga N, Hori Y, Yamakado K, Ikeda H, Imai N, Kageyama S, Nakase K, Yuta A, Hayashi N, Shiku H, et al. Limited expression of cancer-testis antigens in renal cell carcinoma patients. Mol Clin Oncol. 2013;1(2):326–30. doi:10.3892/mco.2012.40.
- Griffiths RW, Gilham DE, Dangoor A, Ramani V, Clarke NW, Stern PL, Hawkins RE. Expression of the 5t4 oncofoetal antigen in renal cell carcinoma: a potential target for t-cell-based immunotherapy. Br J Cancer. 2005;93(6):670–77. doi:10.1038/sj.bjc.6602776.
- Stern PL, Harrop R. 5t4 oncofoetal antigen: an attractive target for immune intervention in cancer. Cancer Immunol Immunother. 2017;66(4):415–26. doi:10.1007/s00262-016-1917-3.
- Southall PJ, Boxer GM, Bagshawe KD, Hole N, Bromley M, Stern PL. Immunohistological distribution of 5t4 antigen in normal and malignant tissues. Br J Cancer. 1990;61(1):89–95. doi:10.1038/bjc.1990.20.
- Smyth LJ, Elkord E, Taher TE, Jiang HR, Burt DJ, Clayton A, van Veelen PA, de Ru A, Ossendorp F, Melief CJ, et al. Cd8 t-cell recognition of human 5t4 oncofetal antigen. Int J Cancer. 2006;119(7):1638–47. doi:10.1002/ijc.22018.
- Elkord E, Burt DJ, Drijfhout JW, Hawkins RE, Stern PL. Cd4+ t-cell recognition of human 5t4 oncofoetal antigen: implications for initial depletion of cd25+ t cells. Cancer Immunol Immunother. 2008;57(6):833–47. doi:10.1007/s00262-007-0419-8.
- Shingler WH, Chikoti P, Kingsman SM, Harrop R. Identification and functional validation of mhc class i epitopes in the tumor-associated antigen 5t4. Int Immunol. 2008;20(8):1057–66. doi:10.1093/intimm/dxn063.
- Redchenko I, Harrop R, Ryan MG, Hawkins RE, Carroll MW. Identification of a major histocompatibility complex class i-restricted t-cell epitope in the tumour-associated antigen, 5t4. Immunology. 2006;118(1):50–57. doi:10.1111/j.1365-2567.2006.02338.x.
- Said R, Amato RJ. Identification of pre- and post-treatment markers, clinical, and laboratory parameters associated with outcome in renal cancer patients treated with mva-5t4. Front Oncol. 2013;3(185). doi:10.3389/fonc.2013.00185.
- Elkord E, Dangoor A, Drury NL, Harrop R, Burt DJ, Drijfhout JW, Hamer C, Andrews D, Naylor S, Sherlock D, et al. An mva-based vaccine targeting the oncofetal antigen 5t4 in patients undergoing surgical resection of colorectal cancer liver metastases. J Immunother. 2008;31(9):820–29. doi:10.1097/CJI.0b013e3181876ab3.
- Harrop R, Connolly N, Redchenko I, Valle J, Saunders M, Ryan MG, Myers KA, Drury N, Kingsman SM, Hawkins RE, et al. Vaccination of colorectal cancer patients with modified vaccinia ankara delivering the tumor antigen 5t4 (trovax) induces immune responses which correlate with disease control: a phase i/ii trial. Clin Cancer Res. 2006;12(11 Pt 1):3416–24. doi:10.1158/1078-0432.CCR-05-2732.
- Forsberg G, Skartved NJ, Wallen-Ohman M, Nyhlen HC, Behm K, Hedlund G, Nederman T. Naptumomab estafenatox, an engineered antibody-superantigen fusion protein with low toxicity and reduced antigenicity. J Immunother. 2010;33(5):492–99. doi:10.1097/CJI.0b013e3181d75820.
- Hawkins RE, Gore M, Shparyk Y, Bondar V, Gladkov O, Ganev T, Harza M, Polenkov S, Bondarenko I, Karlov P, et al. A randomized phase ii/iii study of naptumomab estafenatox + ifnalpha versus ifnalpha in renal cell carcinoma: final analysis with baseline biomarker subgroup and trend analysis. Clin Cancer Res. 2016;22(13):3172–81. doi:10.1158/1078-0432.CCR-15-0580.
- Elkord E, Burt DJ, Sundstedt A, Nordle O, Hedlund G, Hawkins RE. Immunological response and overall survival in a subset of advanced renal cell carcinoma patients from a randomized phase 2/3 study of naptumomab estafenatox plus ifn-alpha versus ifn-alpha. Oncotarget. 2015;6(6):4428–39. doi:10.18632/oncotarget.2922.
- Leroy X, Zerimech F, Zini L, Copin MC, Buisine MP, Gosselin B, Aubert JP, Porchet N. Muc1 expression is correlated with nuclear grade and tumor progression in pt1 renal clear cell carcinoma. Am J Clin Pathol. 2002;118(1):47–51. doi:10.1309/1F99-BPDY-7DHH-9G97.
- Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–55.
- Kraus S, Abel PD, Nachtmann C, Linsenmann HJ, Weidner W, Stamp GW, Chaudhary KS, Mitchell SE, Franke FE, Lalani El N. Muc1 mucin and trefoil factor 1 protein expression in renal cell carcinoma: correlation with prognosis. Hum Pathol. 2002;33(1):60–67. doi:10.1053/hupa.2002.29682.
- Fujita K, Denda K, Yamamoto M, Matsumoto T, Fujime M, Irimura T. Expression of muc1 mucins inversely correlated with post-surgical survival of renal cell carcinoma patients. Br J Cancer. 1999;80(1–2):301–08. doi:10.1038/sj.bjc.6690355.
- Roulois D, Gregoire M, Fonteneau JF. Muc1-specific cytotoxic t lymphocytes in cancer therapy: induction and challenge. Biomed Res Int. 2013;(2013):871936. doi:10.1155/2013/871936.
- Brossart P, Heinrich KS, Stuhler G, Behnke L, Reichardt VL, Stevanovic S, Muhm A, Rammensee HG, Kanz L, Brugger W. Identification of hla-a2-restricted t-cell epitopes derived from the muc1 tumor antigen for broadly applicable vaccine therapies. Blood. 1999;93(12):4309–17. doi:10.1182/blood.V93.12.4309.
- Ninkovic T, Kinarsky L, Engelmann K, Pisarev V, Sherman S, Finn OJ, Hanisch FG. Identification of o-glycosylated decapeptides within the muc1 repeat domain as potential mhc class i (a2) binding epitopes. Mol Immunol. 2009;47(1):131–40. doi:10.1016/j.molimm.2008.09.032.
- Brossart P, Schneider A, Dill P, Schammann T, Grunebach F, Wirths S, Kanz L, Buhring HJ, Brugger W. The epithelial tumor antigen muc1 is expressed in hematological malignancies and is recognized by muc1-specific cytotoxic t-lymphocytes. Cancer Res. 2001;61:6846–50.
- Scheikl-Gatard T, Tosch C, Lemonnier F, Rooke R. Identification of new muc1 epitopes using hla-transgenic animals: implication for immunomonitoring. J Transl Med. 2017;15(1):154. doi:10.1186/s12967-017-1254-0.
- Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic t cells. Proc Natl Acad Sci U S A. 1989;86(18):7159–63. doi:10.1073/pnas.86.18.7159.
- Ramanathan RK, Lee KM, McKolanis J, Hitbold E, Schraut W, Moser AJ, Warnick E, Whiteside T, Osborne J, Kim H, et al. Phase i study of a muc1 vaccine composed of different doses of muc1 peptide with sb-as2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54(3):254–64. doi:10.1007/s00262-004-0581-1.
- Rochlitz C, Figlin R, Squiban P, Salzberg M, Pless M, Herrmann R, Tartour E, Zhao Y, Bizouarne N, Baudin M, et al. Phase i immunotherapy with a modified vaccinia virus (mva) expressing human muc1 as antigen-specific immunotherapy in patients with muc1-positive advanced cancer. J Gene Med. 2003;5(8):690–99. doi:10.1002/jgm.397.
- Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, Koralewski P, Breton JL, Stoelben E, Braun D, et al. Therapeutic vaccination with tg4010 and first-line chemotherapy in advanced non-small-cell lung cancer: A controlled phase 2b trial. Lancet Oncol. 2011;12(12):1125–33. doi:10.1016/S1470-2045(11)70259-5.
- Nakatsuka S, Oji Y, Horiuchi T, Kanda T, Kitagawa M, Takeuchi T, Kawano K, Kuwae Y, Yamauchi A, Okumura M, et al. Immunohistochemical detection of wt1 protein in a variety of cancer cells. Mod Pathol. 2006;19(6):804–14. doi:10.1038/modpathol.3800588.
- Okamoto M, Furuichi S, Nishioka Y, Oshikawa T, Tano T, Ahmed SU, Takeda K, Akira S, Ryoma Y, Moriya Y, et al. Expression of toll-like receptor 4 on dendritic cells is significant for anticancer effect of dendritic cell-based immunotherapy in combination with an active component of ok-432, a streptococcal preparation. Cancer Res. 2004;64(15):5461–70. doi:10.1158/0008-5472.CAN-03-4005.
- Parker AS, Leibovich BC, Lohse CM, Sheinin Y, Kuntz SM, Eckel-Passow JE, Blute ML, Kwon ED, Parker AS, Leibovich BC, et al. Development and evaluation of bioscore: A biomarker panel to enhance prognostic algorithms for clear cell renal cell carcinoma. Cancer. 2009;115(10):2092–103. doi:10.1002/cncr.24263.
- Xie Y, Ma X, Gu L, Li H, Chen L, Li X, Gao Y, Fan Y, Zhang Y, Yao Y, et al. Prognostic and clinicopathological significance of survivin expression in renal cell carcinoma: A systematic review and meta-analysis. Sci Rep. 2016;6:29794. doi:10.1038/srep29794.
- Weinschenk T, Gouttefangeas C, Schirle M, Obermayr F, Walter S, Schoor O, Kurek R, Loeser W, Bichler KH, Wernet D, et al. Integrated functional genomics approach for the design of patient-individual antitumor vaccines. Cancer Res. 2002;62(20):5818–27.
- Kirner A, Mayer-Mokler A, Reinhardt C. Ima901: A multi-peptide cancer vaccine for treatment of renal cell cancer. Hum Vaccin Immunother. 2014;10(11):3179–89. doi:10.4161/21645515.2014.983857.
- Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J, Coleman D, Niedzwiecki D, Gilboa E, Vieweg J. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor rna-transfected dendritic cells. Cancer Res. 2003;63:2127–33.
- Calderhead DM, DeBenedette MA, Ketteringham H, Gamble AH, Horvatinovich JM, Tcherepanova IY, Nicolette CA, Healey DG. Cytokine maturation followed by cd40l mrna electroporation results in a clinically relevant dendritic cell product capable of inducing a potent proinflammatory ctl response. J Immunother. 2008;31(8):731–41. doi:10.1097/CJI.0b013e318183db02.
- Cappuccini F, Pollock E, Stribbling S, Hill AVS, Redchenko I. 5t4 oncofoetal glycoprotein: an old target for a novel prostate cancer immunotherapy. Oncotarget. 2017;8(29):47474–89. doi:10.18632/oncotarget.17666.
- Cappuccini F, Bryant R, Pollock E, Carter L, Verrill C, Hollidge J, Poulton I, Baker M, Mitton C, Baines A, et al. Safety and immunogenicity of novel 5t4 viral vectored vaccination regimens in early stage prostate cancer: A phase i clinical trial. J Immunother Cancer. 2020;8(1):e000928. doi:10.1136/jitc-2020-000928.
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. Chimeric antigen receptor-modified t cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. doi:10.1056/NEJMoa1215134.
- Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, Hawkins R, Chaney C, Cherian S, Chen X, et al. Immunotherapy of non-hodgkin’s lymphoma with a defined ratio of cd8+ and cd4+ cd19-specific chimeric antigen receptor-modified t cells. Sci Transl Med. 2016;8(355):355ra116. doi:10.1126/scitranslmed.aaf8621.
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with ny-eso-1. J Clin Oncol. 2011;29(7):917–24. doi:10.1200/JCO.2010.32.2537.
- Doran SL, Stevanovic S, Adhikary S, Gartner JJ, Jia L, Kwong MLM, Faquin WC, Hewitt SM, Sherry RM, Yang JC, et al. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: a first-in-human, phase i/ii study. J Clin Oncol. 2019;37(30):2759–68. doi:10.1200/JCO.18.02424.
- Hong DS, Van Tine BA, Olszanski AJ, Johnson ML, Liebner DA, Trivedi T, Lin Q, Elefant E, Dryer-Minnerly R, Navenot J-M, et al. Phase I dose escalation and expansion trial to assess the safety and efficacy of ADP-A2M4 SPEAR T cells in advanced solid tumors. J. Clin. Oncol. 2020;38(15_suppl):102. doi:10.1200/JCO.2020.38.15_suppl.102.
- Thomas S, Mohammed F, Reijmers RM, Woolston A, Stauss T, Kennedy A, Stirling D, Holler A, Green L, Jones D, et al. Framework engineering to produce dominant t cell receptors with enhanced antigen-specific function. Nat Commun. 2019;10(1):4451. doi:10.1038/s41467-019-12441-w.
- Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunol. 2019;8(5):e1049. doi:10.1002/cti2.1049.
- Yang Y, Kohler ME, Chien CD, Sauter CT, Jacoby E, Yan C, Hu Y, Wanhainen K, Qin H, Fry TJ. Tcr engagement negatively affects cd8 but not cd4 car t cell expansion and leukemic clearance. Sci Transl Med. 2017;9(417):eaag1209. doi:10.1126/scitranslmed.aag1209.
- Wang X, Popplewell LL, Wagner JR, Naranjo A, Blanchard MS, Mott MR, Norris AP, Wong CW, Urak RZ, Chang WC, et al. Phase 1 studies of central memory-derived cd19 car t-cell therapy following autologous hsct in patients with b-cell nhl. Blood. 2016;127(24):2980–90. doi:10.1182/blood-2015-12-686725.
- Morton LT, Reijmers RM, Wouters AK, Kweekel C, Remst DFG, Pothast CR, Falkenburg JHF, Heemskerk MHM. Simultaneous deletion of endogenous tcralphabeta for tcr gene therapy creates an improved and safe cellular therapeutic. Mol Ther. 2020;28(1):64–74. doi:10.1016/j.ymthe.2019.10.001.
- Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, Mangan PA, Kulikovskaya I, Gupta M, Chen F, et al. Crispr-engineered t cells in patients with refractory cancer. Science. 2020;367(6481):eaba7365. doi:10.1126/science.aba7365.
- Xu Y, Morales AJ, Cargill MJ, Towlerton AMH, Coffey DG, Warren EH, Tykodi SS. Preclinical development of t-cell receptor-engineered t-cell therapy targeting the 5t4 tumor antigen on renal cell carcinoma. Cancer Immunol Immunother. 2019;68(12):1979–93. doi:10.1007/s00262-019-02419-4.
- Yossef R, Tran E, Deniger DC, Gros A, Pasetto A, Parkhurst MR, Gartner JJ, Prickett TD, Cafri G, Robbins PF, et al. Enhanced detection of neoantigen-reactive t cells targeting unique and shared oncogenes for personalized cancer immunotherapy. JCI Insight. 2018;3(19). doi:10.1172/jci.insight.122467.
- Kula T, Dezfulian MH, Wang CI, Abdelfattah NS, Hartman ZC, Wucherpfennig KW, Lyerly HK, Elledge SJ. T-scan: a genome-wide method for the systematic discovery of t cell epitopes. Cell. 2019;178(4):1016–1028 e1013. doi:10.1016/j.cell.2019.07.009.
- Gee MH, Han A, Lofgren SM, Beausang JF, Mendoza JL, Birnbaum ME, Bethune MT, Fischer S, Yang X, Gomez-Eerland R, et al. Antigen identification for orphan t cell receptors expressed on tumor-infiltrating lymphocytes. Cell. 2018;172(3):549–563 e516. doi:10.1016/j.cell.2017.11.043.
- Crowther MD, Dolton G, Legut M, Caillaud ME, Lloyd A, Attaf M, Galloway SAE, Rius C, Farrell CP, Szomolay B, et al. Genome-wide crispr-cas9 screening reveals ubiquitous t cell cancer targeting via the monomorphic mhc class i-related protein mr1. Nat Immunol. 2020;21(2):178–85. doi:10.1038/s41590-019-0578-8.
- Patel SJ, Sanjana NE, Kishton RJ, Eidizadeh A, Vodnala SK, Cam M, Gartner JJ, Jia L, Steinberg SM, Yamamoto TN, et al. Identification of essential genes for cancer immunotherapy. Nature. 2017;548(7669):537–42. doi:10.1038/nature23477.
