ABSTRACT
The persistence of specific IgG after measles infection and after measles vaccination has not been sufficiently investigated. Current evidence suggests that immunity after the disease is life-long, whereas the response after two doses of measles-containing vaccine declines within 10–15 years. This study evaluated the proportion of individuals with detectable anti-measles IgG in two groups, those vaccinated with two doses of anti-MMR vaccine and those with a self-reported history of measles infection. Among the 611 students and residents who were tested, 94 (15%) had no detectable protective anti-measles IgG. This proportion was higher among vaccinated individuals (20%; GMT = 92.2) than among those with a self-reported history of measles (6%; GMT = 213.3; p < .0001). After one or two MMR vaccine booster doses, the overall seroconversion rate was 92%. An important proportion of people immunized for measles did not have a protective IgG titer in the years after vaccination, but among those who had a natural infection the rate was three-fold lower. This finding should be considered in the pre-elimination phase, given the resurgence of measles cases among individuals who after being vaccinated lost their circulating IgG after several years, especially if they failed to receive a natural booster.
Introduction
Measles is an acute viral respiratory illness caused by a single-stranded, enveloped RNA virus with a single serotype (genus Morbillivirus, family Paramyxoviridae). Humans are the only natural host of measles virus. Patients are considered to be contagious between 4 days before and 4 days after the rash appears.Citation1 Common complications of measles include otitis media, bronchopneumonia, laryngotracheobronchitis, and diarrhea. One out of every 1,000 measles patients will develop acute encephalitis and 1–3/1,000 children infected with measles will die from respiratory and neurologic complications. The most dreaded complication of measles is subacute sclerosing panencephalitis, that generally develop 7–10 years after measles infection.Citation1
Since the introduction of global mass vaccination, the safety,Citation2 cost-savingsCitation3 and efficacy of measles-containing vaccines have been repeatedly demonstrated. Vaccination has reduced the incidence of measles by 99.9%, with >20,000,000 lives saved throughout the world.Citation4 Nevertheless, in the post-vaccination era, the WHO estimated almost 90,000 measles-related deaths in 2016 and reported 353,236 cases of measles in 2018.Citation5
Measles virus replicates in the cytoplasm of infected human cells without the integration of the viral genome into that of the host cell. In addition, measles virus is considered sensitive to antibody-mediated clearance. Generally, measles infection and its effects on the immune system are limited to the period of viral replication, spread and clearance, during which time acute illness in the host develops. However, in natural infections of measles, the viral RNA can persist in lymphoid tissue and the immune system remains activated for many months.Citation6 This characteristic may explain the observed maturation of the immune response to the virus, which may be required to establish life-long protective immunity.Citation6 Immune activation and the proliferation of lymphocytes, particularly CD4 + T cells, is evident both during the acute phase and in the months after resolution of the rash. During this period, there is a shift in the production of cytokines to those promoting B cell maturation, thus allowing the continuous production of antibody-secreting cells.Citation6 The improvement over time in the quality of antibodies, as evidenced by their increasing avidity, suggests the continuous activity of follicular T-helper cells and the selection of B cells in the germinal centers of lymphoid tissue. The development of long-lived plasma cells is necessary to sustain life-long plasma antibody levels.Citation6
In Italy, a single-antigen measles vaccine was introduced in the 1970s.Citation7 Since 2003, the national vaccination schedule has recommended universal mass vaccination consisting of two doses of measles, mumps and rubella (MMR) vaccine administered in accordance with CDC recommendations (the first dose at 12–15 months and the second at 5–6 years of age).Citation8 According to pre-licensure data, one dose of MMR vaccine is 93% effective and two doses are 97% effective against measles.Citation8 The seroconversion rate is 95–98% after a single dose and 99% after two doses.Citation8 The live attenuated vaccine induces both antibody and cellular immune responses that mature over a period of months.
Although the immune responses induced by the vaccine are qualitatively similar to those induced by infection, antibody levels are lower after vaccination. Vaccination at a young age enhances the quality and quantity of the antibody response but has a minor effect on T cell responses. However, over time, virus-specific antibodies and vaccine-induced CD4 + T cells decrease, accounting for the secondary vaccine failure rate of 5% 10–15 years after immunization.Citation9
The aim of this study was to evaluate the proportion of seroprotected individuals in two populations: those vaccinated with two doses of anti-MMR vaccine and those with a history of measles infection. In addition, the GMTs were compared in the previously vaccinated and naturally infected.
The study was carried out in Apulia (southern Italy, ~4,000,000 inhabitants), where MMR vaccine coverage is ~91% (year 2017, birth cohort 2015)Citation10 and where in 2002/2003 a large outbreak of measles (around 20,000 cases) was documented,Citation11 followed by many outbreaks in subsequent yearsCitation12,Citation13 that included documented cases of nosocomial transmission.Citation12-15
Material and methods
This was a retrospective cohort study.
In accordance with the Italian Ministry of Health’s recommendations,Citation16 in April 2014 the Hygiene Department of the Bari Policlinico University Hospital implemented a biological risk prevention program for medical students and residents of the Medical School of the University of Bari. The protocol included obtaining a medical history and the determination of measles vaccination status and measles history. To increase the accuracy of the information, the parents of the medical students and residents were to be interviewed as well.
Thus, for each student or resident participant, a 5 mL serum sample was collected to assess the measles immunity/susceptibility status, determined in a chemiluminescence (CLIA) assay using LIAISON® Measles IgG, a semi-quantitative test performed using a standardized commercial method (Diasorin). The assay’s cut off value (>16.5 AU/mL) is equivalent to 175 mIU/mL (WHO Third International Standard for Anti-Measles, NIBSC code: 97/648).Citation16,Citation17 Individuals with equivocal tests were retested; if their results were still equivocal, their status was classified as negative.
Vaccinated individuals who had a non-protective IgG titer received a booster dose of MMR vaccine (M-M-RVAXPRO, administered subcutaneously in the deltoid). A second blood test was performed 20–25 days thereafter to remeasure the IgG titer. If the value exceeded the cutoff, the person was classified as having seroconverted; if the titer was still negative, another vaccine dose (28 days after the first booster) was administered and the IgG titer was again measured 20–25 days later. Individuals who were still seronegative were definitively classified as “non-responders” and an evaluation for measles infection as well as immunoglobulin administration in case of measles exposure were recommended.
Individuals with a self-reported natural history of vaccination who had a non-protective IgG titer received two booster doses of MMR vaccine (M-M-RVAXPRO, administered subcutaneously in the deltoid), 4 weeks apart. IgG titers were re-measured in a new blood test 20–25 days after the second booster dose. If the value of that test exceeded the cutoff, the person was classified as seroconverted; if the results were still negative, he was treated as described for vaccinated individuals.
This management protocol was consistent with the protocols applied in some US medical schools.Citation18 Study participants who received booster doses underwent a 1-month follow-up to assess the development of any adverse events following vaccination.
The population considered in the present study was composed of medical students and residents who attended the Hygiene Department from April 2014 to March 2019. Informed consent was routinely collected during clinical procedures. This study was carried out according to the principles of the Helsinki Declaration.
Our survey included only those medical students and residents who at the time of study enrollment had received two doses of MMR vaccine (vaccine basal routine) or who reported a history of measles infection. The vaccination status of enrolled participants was assessed using the Regional Immunization Database (GIAVA),Citation19 a computerized vaccination registry that allows every Apulian inhabitant to ascertain the vaccination history.
Participants without an available vaccination history, without a history of measles and never vaccinated, with a history of measles but who were also vaccinated, who were vaccinated with a single dose or ≥2 doses of MMR vaccine at baseline were excluded.
To calculate the sample size, individuals who had been vaccinated but lacked circulating anti-measles IgG were estimated to account for 15% of the study populationCitation20 and those who were naturally immunized but lacked circulating IgG for 4.5% (our hypothesis, since there are no studies on the topic). The groups were compared using a chi-square test, with a significance level (alpha) of 0.01 and a beta power of 95%. To improve the statistical analysis, a 1:2 allocation ratio of naturally immunized and vaccinated individuals was chosen. Thus, the preliminary sample comprised 537 participants: 358 in the vaccine group and 179 in the disease group. The two groups were matched for age, sex and chronic diseases. Since records with missing data were expected, data for 448 individuals from the vaccine group and 224 from the disease group (20% more than the minimum determined sample size) were extracted from the database. Among the extracted records, 38 from the vaccine group and 23 from the disease group were excluded due to missing data. The final sample therefore consisted of 611 individuals: 410 had been vaccinated (vaccine group) and 201 naturally immunized (disease group).
For every enrollee, a specific form was generated in which information on patient id, sex, age at enrollment, age at measles infection, dates of routine MMR vaccine, measles IgG titer, date of first booster dose, IgG titer after first booster (vaccine group), date of second booster dose and IgG titer after second booster were recorded. Data from the compiled forms were entered into a database generated using Excel and analyzed using STATA MP16 software.
Continuous variables are reported as the mean±standard deviation and range, categorical variables as proportions, with the 95% confidence interval (95%CI), when appropriate. Skewness and kurtosis test was conducted to evaluate the normality of the continuous variables; in case of a non-normal distribution, a normalization model was established. Student’s t-test for independent data (parametric) and the Wilcoxon rank sum test (non-parametric) tests were used to compare continuous variables between groups; chi-square and Fisher’s exact tests were used to compare proportions.
To assess the determinants of seroprotection at the time of study enrollment, a multivariate logistic regression model was used in which seroprotection was the outcome and sex (male vs. female), age (years) at study enrollment, group assignment (vaccine vs. disease) and the presence of chronic disease (yes/no) were the determinants. The adjusted Odds Ratio (aOR) was calculated with the 95%CI. The Hosmer-Lemeshow test was used to evaluate the goodness-of-fit of the multivariate logistic regression model.
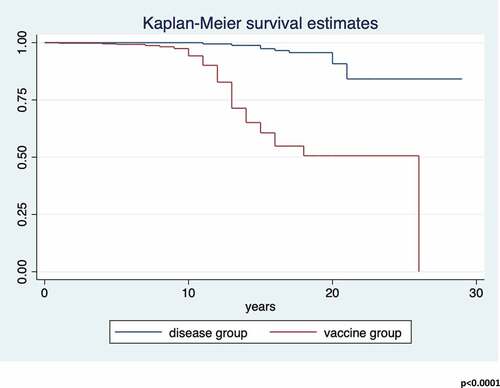
Protective antibody survival (PAS), defined as the time elapsed from the second dose of routine MMR vaccine to the evaluation of antibody titer (years) or the time elapsed between natural measles infection to the evaluation of antibody titer (years), was determined.
PAS was assessed using Kaplan-Meier curves, and the differences between groups using the log-rank test. The median PAS time as well as the incidence rate per 100 person-years of loss of seroprotection were estimated, both with their 95%CIs. The Incidence Rate Ratio (IRR), in which the number of naturally immunized individuals was the denominator and the number of vaccinated individuals the numerator, was calculated with the 95%CI.
The determinants of PAS were identified by applying a multivariate Cox semiparametric regression, in which the risk predictors were sex (male vs. female), age (years) at study enrollment, group assignment (vaccine vs. disease) and the presence of chronic disease (yes/no). The adjusted Hazard Ratio (aHR) was calculated with the 95%CI. The Schoenfeld and scaled Schoenfeld residuals test was used to evaluate the proportionality assumption of the multivariate Cox semiparametric regression model, and the Gronnesby and Borgan test to evaluate the goodness-of-fit of the model.
For all tests, a two-sided p-value<0.05 was considered to indicate statistical significance.
Results
The study sample comprised 611 medical students and residents: 201 (32.9%) in the disease group and 410 (67.1%) in the vaccine group. The characteristics of the participants at enrollment are described in .
Table 1. Characteristics of the two study groups at baseline
On average, members of the disease group had contracted measles at age 5.6 ± 3.3 (range: 0–18) years. Members in the vaccine group had been given the first dose of MMR vaccine at age 17.0 ± 3.0 (range: 6–23) months and the second dose at age 10.9 ± 3.6 (range: 1–29) years.
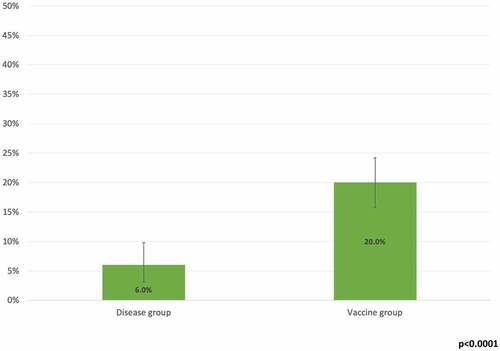
The proportion of all participants without circulating antibodies at enrollment was 15.4% (n = 94/611; 95%CI = 12.6-18.5%). The difference between the two groups was statistically significant (p < .0001; ).
Figure 1. Proportion (%) of study participants in the vaccine and disease groups without circulating anti-measles IgG at study enrollment
The average GMT of the enrollees was 92.2 (95%CI = 82.6–103.0), with a statistically significant difference between the disease group (GMT = 213.3; 95%CI = 185.4–245.5) and the vaccine group (GMT = 60.5; 95%CI = 53.0–69.1; p < .0001).
Following vaccination of 7 of the 12 (58.3%) non-seroprotected members of the disease group according to the vaccination protocol (two doses of MMR vaccine 4 weeks apart), the titer evaluation revealed seroconversion in all 7 (100%; 95%CI = 59.0–100.0%), with a post-administration GMT of 239.8 (95%CI = 179.5–320.5).
In the vaccine group, 54 of the 82 (65.9%) seronegative individuals received a third booster dose of MMR vaccine, which resulted in the seroconversion of 42 of 54 (77.8%; 95%CI = 64.4–88.0%); 10 of the 12 (83.3%) still seronegatives individuals received a fourth booster dose of vaccine, of whom 3 of 10 (30.0%; 95%CI = 6.7–65.2%) seroconverted (overall seroconversion rate in the vaccine group: 90.0%; 95%CI = 78.2–96.7%). The GMT of those individuals after the booster(s) was 52.9 (95%CI = 38.4–73.0).
The multivariate logistic analysis showed a statistically significant association between evidence of circulating antibodies at enrollment and the group assignment (vaccine vs. disease; aOR = 0.25; 95%CI = 0.13–0.47). There were no further associations between the outcome and the determinants in the analysis (p > .05; ).
Table 2. Multivariate logistic regression analysis of the determinants of seropositivity at enrollment
The average PAS time was 13.2 ± 4.4 years (range = 0–29). For seronegatives, the incidence rate ×100 person-years was 1.2 (95%CI = 1.0–1.4).
The PAS between the groups differed significantly (log-rank p < .00001; ). The incidence rate ×100 person-years for the loss of circulating IgG was 0.4 (95%CI = 0.2–0.7) in the disease group and 1.7 (95%CI = 1.4–2.1) in the vaccine group, with an IRR of 4.6 (95%CI = 2.5–9.3; p < .0001).
The multivariate analysis identified belonging to the vaccine group (aHR = 11.8; 95%CI = 6.1–22.9) and age (aHR = 0.88; 95%CI = 0.80–0.95) as determinants of the loss of circulating antibodies. There were no associations between the PAS and the other determinants in the analysis (p > .05; ).
Table 3. Multivariate cox semiparametric regression analysis of the risk predictors of PAS
Discussion
Our study showed that 15% of the screened participants lacked detectable circulating anti-measles IgG and one or more booster doses was needed for seroconversion; this value is higher than the one reported in a 2020 meta-analysisCitation21 on Italian HCWs (equal to 9%), probably due to the young age of our sample. The difference between the two groups (20% vs. 6%) is consistent with literature reports and provides further evidence that natural immunity is more long-lasting than vaccine immunity. Additional support for this conclusion comes from the significantly higher baseline GMT in the naturally immunized group (213 vs. 61; p < .0001); these results are consistent with the ones highlighted by a 2020 Italian study,Citation22 which concluded that among subjects who received two doses of measles vaccine, the neutralizing antibody titer tended to decline over time, on contrary of natural immunized subjects.
The seroconversion rate after two doses of MMR vaccine in the disease group was 100% (95%CI = 59–100%), while in the vaccinated group it was 86% (95%CI = 73–94%). The difference in the response to the booster dose(s) may have reflected the greater persistence of immunological memory in naturally immunized individuals. Also in this case, the GMT measured after the booster(s) was significantly higher in the naturally immunized than in the vaccinated participants (240 vs. 53). The overall seroconversion after a booster(s) in subjects found seronegative after the first blood sample was 92.2% (95%CI = 80.7–97.1%).
An analysis of the determinants of seroprotection showed that the detection of circulating IgG at baseline was associated with natural immunization (aOR = 0.25; 95%CI = 0.13–0.47). The survival analysis also indicated a greater persistence of circulating antibodies in the naturally immunized. Although a stronger antibody response (titer) is induced by natural disease than by vaccination, a 1994 studyCitation23 found that for MMR immunity, serological memory after vaccination is similar to that after natural infection. However, the second dose of the MMR vaccine is essential, as the antibody titer undergoes since a slow decline during the first 10 years after the first vaccination of the basal routine.Citation23 The levels of neutralizing antibodies 10 years after the second dose of vaccine remain above the level considered protective and confer long-lasting immunity, although they fall in the years thereafter.Citation24 A 2019 Italian study estimated that circulating anti-measles IgG antibodies decrease 10–15 years after the second dose of MMR vaccine administered according to the basal routine.Citation20
A strength of our study was it large sample size. Its main limitation arose from the source of the information on the natural history of measles in the enrollees, as it relied on the historical memory of the interviewed participants (and their parents), whose recall may not have been accurate. In addition, individuals naturally immunized as children may still have been vaccinated by their pediatrician; however, if they had no memory of the event and there was no record of it, because in the past vaccination was not consistently reported, then bias may have inadvertently been introduced into the study. This problem has been discussed in the literature, although self-reported information is still considered to be a good investigative tool.Citation25-27 In particular, an Italian 2007 study showed that a self-reported measles history had a positive predictive value of 94.7%Citation28 and a 2006 survey among HCWs showed a predictive value of 92%.Citation29 Moreover, our results may have been partly influenced by the epidemiological change that has occurred in recent years, which has made exposure to natural boosters less frequent. Finally, the level of functional antibodies, i.e. neutralizing antibodies, measured through virus neutralization assays and cellular immunity have not been measured and so next studies must focus on these elements to achieve more robust conclusions.
A key to the interpretation of our data is to define the role of circulating antibodies and memory B cells in protecting against wild virus. Protection correlates better with the quality and quantity of the induced neutralizing antibodies, but the development of immunity against the disease is probably largely determined by T cells.Citation9 Studies on macaques have shown that neutralizing antibodies provide protection from the disease (rash) but not necessarily from infection and that T cells alone do not protect against either infection or disease but instead facilitate the clearance of viral RNA.Citation9 Indeed, the role of cell-mediated immunity in the long-term response to the vaccine/disease (and consequent protection against measles) is discussed controversially in the scientific literature. Amanna et al., in a 2017 study,Citation30 conducted a prospective observational analysis of antibody titer changes in 45 individuals over a period of more than 26 years. Antigen-specific memory B cells were measured and their levels compared with those of the corresponding antibodies. The authors determined an association between the levels of memory B-cell and the concentration of antibodies against measles, based on the assumption that serum antibodies and memory B cell levels are equally stable but independently maintained. However, a direct cause-and-effect relationship could not be established.Citation30 A 1975 study highlighted the role of cellular immunity and postulated that the cell-associated immune system is the main host defense against measles. The findings were based on the observed responses to measles in agammaglobulinemic children and the death of these children but not those with a thymus deficiency who also contracted measles.Citation24 However, a 2016 study found that the contribution of T cells to protection is generally minor compared to that of neutralizing antibodies.Citation6 Nonetheless, field experience has shown that during measles outbreaks vaccinated individuals have been among the infected.Citation31,Citation32
While further research is needed, our study clearly showed that natural immunity is both more robust and longer-lasting than vaccine immunity. However, this finding should not lead to a questioning of the role of measles vaccination. It is well-established that the complications of measles are more frequent and more serious than any vaccine-related adverse reaction.Citation1,Citation33 For example, in a recent study published in Science,Citation34 Mina et al. described the long-term damage to immune memory caused by measles infection. They found that measles infection can greatly diminish previously acquired immune memory, potentially leaving individuals at risk of infection by other pathogens. The same authors showed that the MMR vaccine does not impair the immune repertoire and that the loss of antibodies that occurs in measles virus infection does not appear to accompany MMR vaccination.Citation34 In light of this evidence, the MMR vaccine remains the most effective, safe and cost-effective tool for preventing measles.
The elimination of measles is a 20-year objective of national and international Public Health institutions.Citation35 The results of this study highlight the risk of a loss of antibodies over time. Thus, from now until the next 10–20 years, the vaccinated population can be expected to lose circulating antibodies such that their susceptibility to measles may increase. Moreover, since it is highly unlikely that measles will be eliminated in the immediate future, a part of individuals vaccinated several years ago will soon lose their circulating antibodies, such that outbreaks of the disease in the coming years can be expected. Recently, Kurata et al. described a cluster of measles cases, seven of which (including the index case) involved fully vaccinated individuals.Citation36 The confirmation of our results may lead to a revision of the mathematical algorithms used in disease elimination strategies. Current mathematical modelsCitation37 applied to reach the elimination goal consider the vaccinated population to be 100% immunized, ignoring the possibility of vaccination failure or the waning of circulating antibodies in those previously vaccinated (20% in our sample) or with a history of measles (6%).
In the absence of a revised strategy, our combined screening and vaccination approach allows safe access to healthcare environments by ensuring that HCWs are immune to circulating pathogens responsible for preventable diseases. The introduction of a third MMR dose for serosusceptible HCWs, both vaccinated and naturally immunized, showed high levels of efficacy and safety; furthermore, the above described strategy showed good compliance by health personnel, a critical determinant in the immunization of HCWs, as evidenced by many studies in literatureCitation38,Citation39 The benefits of our approach also include economic ones, as it will lead to a lowering of the risk of measles outbreaks and therefore their associated costs;Citation40 indeed, the cost of serological screening eventually followed by third MMR dose has less impact on public funds compared to the measures required in the context of epidemic outbreaks. Our screening model is applicable and implementable in a short time for HCWs in epidemiological contexts similar to that described by us; over time the results of further experiences will confirm the effectiveness of this strategy and the effects on the field could be measured with a zeroing of nosocomial clusters of measles in the structures where it is applied. Finally, a 2019 meta-analysisCitation21 showed a prevalence of Italian HCWs susceptible to measles equal to 12% and so firm measures of control and prevention are needed to reduce the risk of measles in nosocomial environment and its complication especially in high risk patients.
Abbreviations
| CDC | = | Center for Disease Control and Prevention |
| MMR | = | Measles, mumps, rubella |
| HCWs | = | Healthcare workers |
| GIAVA | = | Regional Immunization Database |
| CLIA | = | Chemiluminescent immunoassay |
| PAS | = | Protective antibody survival |
| IRR: | = | Incidence rate ratio |
| GMT | = | Geometric mean titer |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed. The manuscript has not previously been presented in any meeting.
Additional information
Funding
References
- CDC. Measles (Rubeola). [accessed 2019 November 11]. https://www.cdc.gov/measles/hcp/index.html.
- CDC. Measles, mumps, and rubella (MMR) vaccine safety. [accessed 2018 Sep 15]. https://www.cdc.gov/vaccinesafety/vaccines/mmr-vaccine.html.
- Zhou F, Reef S, Massoudi M, Papania MJ, Yusuf HR, Bardenheier B, Zimmerman L, McCauley MM. An economic analysis of the current universal 2-dose Measles-mumps-rubella vaccination program in the United States. J Infect Dis. 2004;189(Suppl 1):S131–45. doi:10.1086/378987.
- Dabbagh A, Patel MK, Dumolard L, Gacic-Dobo M, Mulders MN, Okwo-Bele JM, Kretsinger K, Papania MJ, Rota PA, Goodson JL. Progress towards regional Measles elimination – worldwide, 2000–2016. Wkly Epidemiol Rec. 2017;92:649–59.
- WHO. Mumps reported cases. 2019 July 15 (data received as of 1-July-19) [accessed 2019 November 10]. http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencemumps.html.
- Griffin DE. The immune response in measles: virus control, clearance and protective immunity. Viruses. 2016 Oct 12;8(10):282. doi:10.3390/v8100282.
- Bechini A, Boccalini S, Tiscione E, Pesavento G, Mannelli F, Peruzzi M, Rapi S, Mercurio S, Bonanni P. Progress towards measles and rubella elimination in Tuscany, Italy: the role of population seroepidemiological profile. Eur J Public Health. 2012 Feb;22(1):133–39. doi:10.1093/eurpub/ckq134.
- CDC. The pink book. Epidemiology and Prevention of Vaccine-Preventable Diseases. Measles; accessed 2019 October 31]. https://www.cdc.gov/vaccines/pubs/pinkbook/meas.html.
- Griffin DE. Measles vaccine. Virun Immunol. 2018;31(2):86–95. doi:10.1089/vim.2017.0143.
- D’Ancona F, D’Amario C, Maraglino F, Rezza G, Ricciardi W, Iannazzo S. Introduction of new and reinforcement of existing compulsory vaccinations in Italy: first evaluation of the impact on vaccination coverage in 2017. Euro Surveill. 2018;23(22). doi:10.2807/1560-7917.ES.2018.23.22.1800238.
- Lopalco PL, Prato R, Pastore R, Martinelli D, Caputi G, Germinario C. Epidemiological analysis of measles in the Apulian region based on the use of current data sources. J Prev Med Hyg. 2005;46:132.
- Prato R, Chironna M, Caputi G, Sallustio A, Martinelli D, Falco A, Germinario CA. An outbreak of measles in Apulia, Italy, November 2006 – January 2007. Euro Surveill. 2007 Apr 5;12(14):E070405.1.
- Caputi G, Tafuri S, Chironna M, Martinelli D, Sallustio A, Falco A, Germinario CA, Prato R, Quarto M. An outbreak of measles including nosocomial transmission in Apulia, south-east Italy, January-March 2008 - a preliminary report. Euro Surveill. 2008 Apr 17;13(16):18839.
- Tafuri S, Germinario C, Rollo M, Prato R. Occupational risk from measles in healthcare personnel: a case report. J Occup Health. 2009;51(1):97–99. doi:10.1539/joh.N8006. Epub 2008 Dec 19.
- Tafuri S, Gallone MS, Gallone MF, Pappagallo MT, Larocca AMV, Germinario C. Monitoring the process of measles elimination by serosurveillance data: the Apulian 2012 study. Vaccine. 2016;34(18):2092–95. doi:10.1016/j.vaccine.2016.03.011.
- Gallone MS, Germinario C, Larocca AMV, Tafuri S. Long time immunogenicity of measles vaccine in the vaccination era: an open question. Hum Vaccin Immunother. 2017;13(1):117–19. doi:10.1080/21645515.2016.1227519.
- DiaSorin. The diagnostic specialist. LIAISON® Measles IgG and IgM. The fully automated solution for antibody detection. [accessed 2019 Nov 12]. https://www.diasorin.com/sites/default/files/allegati_prodotti/ese_brochure_liaison_Measles_0413_low.pdf
- Cabrillo College. Clinical compliance basics. Health screening. Measles, mumps, rubella (MMR). [Last accessed 2019 November 04]. https://www.cabrillo.edu/services/health/clinical-compliance/clinical-mumps.html.
- Bianchi FP, Gallone MS, Gallone MF, Larocca AMV, Vimercati L, Quarto M, Tafuri S. HBV seroprevalence after 25 years of universal mass vaccination and management of non-responders to the anti-Hepatitis B vaccine: an Italian study among medical students. J Viral Hepat. 2018;26:136–44. doi:10.1111/jvh.13001.
- Bianchi FP, Stefanizzi P, De Nitto S, Larocca AMV, Germinario C, Tafuri S. Long-term immunogenicity of measles vaccine: an Italian retrospective cohort study. J Infect Dis. 2020;221(5):721–28.
- Bianchi FP, Mascipinto S, Stefanizzi P, de Nitto S, Germinario CA, Lopalco P, Tafuri S. Prevalence and management of measles susceptibility in healthcare workers in Italy: a systematic review and meta-analysis. Expert Rev Vaccines. 2020 Jul;19(7):611–20. doi:10.1080/14760584.2020.1791091.
- Anichini G, Gandolfo C, Fabrizi S, Miceli GB, Terrosi C, Gori Savellini G, Prathyumnan S, Orsi D, Battista G, Cusi MG. Seroprevalence to measles virus after vaccination or natural infection in an adult population, in Italy. Vaccines (Basel). 2020 Feb 3;8(1):66. doi:10.3390/vaccines8010066.
- Christenson B, Böttiger M. Measles antibody: comparison of long-term vaccination titres, early vaccination titres and naturally acquired immunity to and booster effects on the measles virus. Vaccine. 1994;12(2):129–33. doi:10.1016/0264-410X(94)90049-3.
- Ruckdeschel JC, Graziano KD, Mardiney MR Jr. Additional evidence that the cell-associated immune system is the primary host defense against measles (rubeola). Cell Immunol. 1975;17(1):11–18. doi:10.1016/S0008-8749(75)80002-5.
- Kawada T, Suzuki S. Validation study on self-reported height, weight, and blood pressure. Percept Mot Skills. 2005 Aug;101( no. 1):187–91. doi:10.2466/pms.101.1.187-191.
- Gilbert GH, Rose JS, Shelton BJ. A prospective study of the validity of data on self‐reported dental visits. Community Dent Oral Epidemiol. 2002;30(5):352–62. doi:10.1034/j.1600-0528.2002.00062.x.
- Short ME, Goetzel RZ, Pei X, Tabrizi MJ, Ozminkowski RJ, Gibson TB, Dejoy DM, Wilson MG. How accurate are self-reports? Analysis of self-reported health care utilization and absence when compared with administrative data. J Occup Environ Med. 2009;51(7):786–96. doi:10.1097/JOM.0b013e3181a86671.
- Trevisan A, Frasson C, Morandin M, Beggio M, Bruno A, Davanzo E, Di Marco L, Simioni L, Amato G. Immunity against infectious diseases: predictive value of self-reported history of vaccination and disease. Infect Control Hosp Epidemiol. 2007;28(5):564–69. doi:10.1086/516657.
- Celikbas A, Ergonul O, Aksaray S, Tuygun N, Esener H, Tanir G, Eren S, Baykam N, Guvener E, Dokuzoguz B. Measles, rubella, mumps, and varicella seroprevalence among health care workers in Turkey: is prevaccination screening cost-effective? Am J Infect Control. 2006;34(9):583–87. doi:10.1016/j.ajic.2006.04.213.
- Amanna IJ, Carlson NE, Slifka MK. Duration of umoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–15. doi:10.1056/NEJMoa066092.
- Hahné SJ, Nic Lochlainn LM, van Burgel ND, Kerkhof J, Sane J, Yap KB, van Binnendijk RS. Measles outbreak among previously immunized healthcare workers, the Netherlands, 2014. J Infect Dis. 2016;214(12):1980–86. doi:10.1093/infdis/jiw480. Epub 2016 Oct 7.
- Rota JS, Hickman CJ, Sowers SB, Rota PA, Mercader S, Bellini WJ. Two case studies of modified measles in vaccinated physicians exposed to primary measles cases: high risk of infection but low risk of transmission. J Infect Dis. 2011;204 Suppl 1:S559–63. doi:10.1093/infdis/jir098.
- Stefanizzi P, Stella P, Ancona D, Malcangi KN, Bianchi FP, De Nitto S, Ferorelli D, Germinario CA, Tafuri S. Adverse events following measles-mumps-rubella-varicella vaccination and the case of seizures: a post marketing active surveillance in Puglia Italian region, 2017-2018. Vaccines (Basel). 2019;7(4). doi:10.3390/vaccines7040140.
- Mina MJ, Kula T, Leng Y, Li M, de Vries RD, Knip M, Siljander H, Rewers M, Choy DF, Wilson MS, et al. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science. 2019;366(6465):599–606. doi:10.1126/science.aay6485.
- WHO. Measles elimination field guide. [accessed 2019 November 12]. https://apps.who.int/iris/rest/bitstreams/920747/retrieve.
- Kurata T, Kanbayashi D, Egawa K, Kinoshita M, Yoshida H, Miyazono M, Motomura K. A measles outbreak from an index case with immunologically confirmed secondary vaccine failure. Vaccine. 2020 Feb 5;38(6):1467–75. doi:10.1016/j.vaccine.2019.11.075.
- Stephen E, Kitengeso RE, Kiria GT, Felician N, Mwema GG, Mafarasa AP. A mathematical model for control and elimination of the transmission dynamics of measles. Appl Comput Maths. 2015;4(6):396–408. doi:10.11648/j.acm.20150406.12.
- Bianchi FP, Vimercati L, Mansi F, De Nitto S, Stefanizzi P, Rizzo LA, Fragnelli GR, Cannone ESS, De Maria L, Larocca AMV, et al. Compliance with immunization and a biological risk assessment of health care workers as part of an occupational health surveillance program: the experience of a university hospital in southern Italy. Am J Infect Control. 2020 Apr;48(4):368–74. doi:10.1016/j.ajic.2019.09.024.
- Karafillakis E, Dinca I, Apfel F, Cecconi S, Wűrz A, Takacs J, Suk J, Celentano LP, Kramarz P, Larson HJ. Vaccine hesitancy among healthcare workers in Europe: A qualitative study. Vaccine. 2016 Sep 22;34(41):5013–20. doi:10.1016/j.vaccine.2016.08.029.
- Giri P, Basu S, Farrow D, Adisesh A. Cost-effectiveness analysis of MMR immunization in health care workers. Occup Med (Lond). 2013;63:422–24.