ABSTRACT
The risk of meningococcal transmission is increased with crowding and prolonged close proximity between people. There have been numerous invasive meningococcal disease (IMD) outbreaks associated with mass gatherings and other overcrowded situations, including cramped accommodation, such as student and military housing, and refugee camps. In these conditions, IMD outbreaks predominantly affect adolescents and young adults. In this narrative review, we examine the situation in India, where the burden of IMD-related complications is significant but the reported background incidence of IMD is low. However, active surveillance for meningococcal disease is suboptimal and laboratory confirmation of meningococcal strain is near absent, especially in non-outbreak periods. IMD risk factors are prevalent, including frequent mass gatherings and overcrowding combined with a demographically young population. Since overcrowded situations are generally unavoidable, the way forward relies on preventive measures. More widespread meningococcal vaccination and strengthened disease surveillance are likely to be key to this approach.
Plain Language Summary
What is the context?
Invasive meningococcal disease (IMD) often presents as sepsis and meningitis. It may be fatal in up to 20% of cases, even when treated.
Mass gatherings may facilitate IMD outbreaks through close contact with infected individuals, constituting a public health concern.
What is new?
We studied the main settings where IMD outbreaks had occurred in association with mass gatherings as well as measures for outbreak control focusing on the situation in India. We found that:
The burden of IMD in India is estimated to be low, but there is a lack of comprehensive information on its epidemiology.
Many risk factors exist, including frequent mass gatherings (the largest having up to 150 million attendees) and overcrowding, combined with a demographically young population and high level of cross-border travel.
In the most recent outbreaks, adolescents and young adults were predominantly affected.
Vaccination is the key method of IMD prevention, but meningococcal vaccines are not included in India’s national immunization program.
What is the impact?
Without reliable information on its epidemiology, it is impossible to determine the true IMD burden in India. It is likely higher than estimated due to overcrowding and frequent mass gatherings.
Improved IMD detection and reporting systems are needed to have a clearer view of the situation.
Future healthcare strategies should favor preventive measures, including more widespread meningococcal vaccination.
Introduction
The World Health Organization (WHO) has described mass gatherings as events “characterized by the concentration of people at a specific location for a specific purpose over a set period of time and which has the potential to strain the planning and response resources of the country or community”.Citation1 While much of the literature describes gatherings exceeding 25,000 persons, a mass gathering can be as few as 1,000 people.Citation1 This could be an organized occasion, such as a social function, sports competition, or political, religious, or cultural gathering, or it may occur spontaneously, for example, in association with a funeral, social unrest, or social upheaval. The duration of the mass gathering may be long, as for some festivals or a displaced population in a refugee camp. It may be transitory or recurrent, in association with public transport, repeating sports events, or temple visits.
Mass gatherings are associated with public health challenges, including an increased risk of communicable disease transmission,Citation2 although they can also have positive health effects, ranging from feelings of well-being and belongingness that can last for a long time,Citation3–5 to beneficial economic effects. The benefits of each mass gathering must therefore be weighed against any increased risk of communicable disease and the availability of effective disease prevention measures.
The risk of communicable disease transmission increases in crowded conditions, where there is close contact with numerous individuals, and where there is a mixing of people from geographical areas with different disease endemicities.Citation1,Citation2 During mass gatherings, this risk is exacerbated by shared accommodation, compromised hygiene practices, and other behavioral factors, such as smoking, sharing food, and poor cough etiquette.Citation1,Citation6 The appetite for seeking healthcare is often low in this context, leading to continued infection exposure. Once an outbreak is recognized, there are healthcare burdens related not only to patient care but also to the impact on local resources, and potential exposure of the wider society to the pathogen. Additionally, for bacterial pathogens, increased antibiotic prescription and chemoprophylaxis of close contacts raise the risk of the emergence of drug-resistant strains.Citation7
The planning process for mass gatherings therefore needs to include a risk assessment and the introduction of measures to prevent and control communicable diseases.Citation1 For example, millions of people usually attend the annual Hajj in Mecca, Saudi Arabia. Free healthcare is provided to all attendees and public health teams assess arriving pilgrims, check their immunization status, and administer recommended prophylactic medicines.Citation8,Citation9 Different communicable diseases are monitored, including invasive meningococcal disease (IMD) in response to previous IMD outbreaks among pilgrims and close contacts.Citation10
IMD is primarily transmitted via direct contact or through the dispersion of respiratory droplets from an infected to a susceptible individual.Citation11,Citation12 Incidence estimates vary widely among countries because of diverse standards of IMD surveillance although it is recognized that its incidence is highest among children younger than 1 year and adolescents or young adults.Citation7 Carriage prevalence of Neisseria meningitidis increases throughout childhood, with estimates suggesting a peak of 24% in late adolescence.Citation13 Although a very small proportion of carriers develop IMD, meningococcal carriage represents the first step for disease transmission.Citation14 The onset of disease is often rapid, with case fatality rates of 4–20% despite appropriate treatment,Citation15 and serious long-term sequelae in up to 20% of survivors.Citation16,Citation17 Diagnosis of IMD therefore needs to be accompanied by prompt treatment.Citation18 Prevention strategies, most notably vaccination, are extremely effective in controlling meningococcal disease,Citation19 while mass chemoprophylaxis can provide temporary protection to recipients during outbreaks.Citation20
This article provides a narrative review of the main settings where IMD outbreaks have occurred in association with mass gatherings. We then focus on the situation in India, where mass gatherings and overcrowding are common for various socio-demographic reasons. A comprehensive review of the burden of meningococcal disease in this country has been published recently.Citation21 We highlight the difficulty in determining the current epidemiology of IMD in India and outline the measures used to contain outbreaks, presenting the case for adjusting the existing public health policy toward this vaccine-preventable disease ().
Meningococcal disease outbreaks related to mass gatherings
lists the main settings, with select examples, where IMD outbreaks have occurred in association with crowded conditions. Religious pilgrimages are a well-known example, particularly outbreaks associated with the Hajj and Umrah mass gatherings in Saudi Arabia.Citation10 The first reported international meningococcal outbreak following the Hajj occurred in 1987, when a meningococcal serogroup A outbreak spread rapidly among pilgrims, Saudi residents, and was exported internationally via colonized pilgrims returning home and infecting close contacts.Citation22 The following year, immunization with the bivalent meningococcal AC polysaccharide vaccine became mandatory for all pilgrims entering Saudi Arabia. In the 2000/2001 season, two major Hajj-related meningococcal serogroup W outbreaks occurred. After the 2000 Hajj, over 400 serogroup W IMD cases were reported in Hajj pilgrims and their close contacts from 16 countries, followed by a smaller outbreak in 2001.Citation22 In 2001, chemoprophylaxis was introduced for all local pilgrims before returning to their families and in 2002, immunization with a quadrivalent meningococcal ACWY vaccine became mandatory. Combined with strict disease surveillance, this strategy of meningococcal vaccination has been exemplary in preventing further IMD outbreaks.Citation10
Table 1. Main settings and selected examples where invasive meningococcal disease outbreaks have occurred in association with a mass gathering or other over-crowded setting
IMD outbreaks have also been reported in association with ceremonial mass gatherings, such as funerals, as reported in Liberia, which is outside of the African meningitis belt (the region in sub-Saharan Africa with a very high incidence of meningococcal disease) and therefore not a country where meningococcal disease was suspected immediately.Citation23,Citation30 Across three counties, 31 cases of unexplained illness and death were reported among individuals who attended a two-day funeral in 2017. Subsequent laboratory testing, using confirmatory direct real-time polymerase chain reaction (PCR) assays, detected N. meningitidis in 14 patients, with serogroup C identified in 13 patients. Further, N. meningitidis was identified in all 11 fatal cases with specimens available.Citation30 This experience illustrated the importance of rapid laboratory confirmation where the etiology of the outbreak was unknown.
The second peak of IMD incidence, after the first in infancy, occurs in adolescents and young adults,Citation31 usually when individuals are living in close quarters, such as in conjunction with mass gatherings.Citation32 This occurred in association with the World Scout Jamboree held in 2015 in Japan, involving over 33,000 participants from 162 countries.Citation25 On their return home, five IMD cases were reported among Scottish and Swedish scouts, as well as an additional secondary case in a parent, all of which were caused by meningococcal serogroup W, which is rarely detected in Japan.Citation25,Citation33 This outbreak, therefore, highlighted the risk of IMD outbreaks in mass gatherings held in low-incidence settings. There have also been reports of outbreaks among adolescents and young adults that are likely to have been related to other crowded situations, such as school transportCitation26,Citation34 and college residence halls and social venues.Citation27,Citation35,Citation36 For example, 10 university-based outbreaks occurred in seven USA states during 2013–2018, involving between two and nine cases in each outbreak, and lasting up to 379 days.Citation27
Sporting events present another setting with an increased likelihood of crowding and close proximity among people for prolonged periods. IMD outbreaks have been reported following various sporting events in Europe.Citation24,Citation37–39 For example, following an international youth football tournament in Belgium in 1997 with over 1,300 participants,Citation24 11 cases of meningitis or sepsis were reported in four countries (Denmark, the Netherlands, Germany, and Belgium), all caused by meningococcal serogroup C. Eight of the cases were adolescent, and three cases were aged 29–39 years, one of whom died. The response to this outbreak involved international cooperation, although control measures varied according to national guidelines, emphasizing the need for greater consistency. These outbreaks also highlight the need to consider large sporting events as mass gatherings in national IMD prevention guidelines.
Other meningococcal outbreaks related to crowded conditions concern refugee populations, as reported in Italy, Turkey, Uganda, and the Democratic Republic of Congo.Citation28,Citation40–43 The military is also at higher risk of IMD because of communal and crowded living quarters combined with deployment or training in regions with different meningococcal serogroup epidemiology.Citation44 Various outbreaks have been reported,Citation29,Citation44,Citation45 pointing toward the need to offer vaccination to military recruits at commencement of training.Citation44
Mass gatherings in India
India is the world’s largest democracy, with more than 2,000 ethnic groupsCitation46 and an estimated population density in 2018 of 455 people per square kilometer of landCitation47 (). Because of India’s democratic constitution, ethnic diversity, and dense population, mass gatherings are regular occurrences. These include religious and spiritual ceremonies relevant to each ethnic group, and other cultural, political, entertainment, and sporting events.
Figure 2. Mass gathering and over-crowding settings in India.Citation46,Citation47
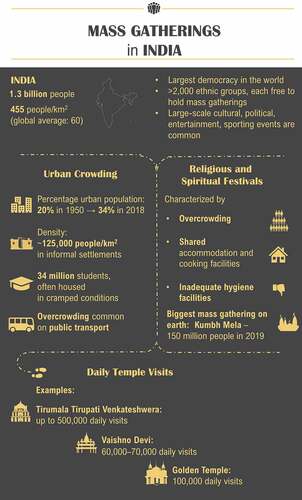
The biggest human mass gathering on earth is held in India: the Hindu festival, Kumbh Mela, a three-month-long event held every three years in one of four different cities (Allahabad, Nasik, Haridwar, and Ujjain) in the north of India.Citation48 An estimated 150 million people attended the 2019 Kumbh Mela.Citation49 Pilgrims travel to the Kumbh Mela by different means (by air, road, rail, and foot) from within India, making it difficult to monitor the health of visiting pilgrims.Citation48 The pilgrims generally stay in a make-shift city where there is overcrowding, sharing of accommodation and cooking facilities, and hygienic practices are often compromised. All these factors have implications for public health, increasing the risk of disease transmission.Citation48,Citation50
Temple visits in India can involve mass gatherings that occur on a daily basis. For example, the Tirumala Tirupati Venkateshwera temple in south India has daily visitor numbers of around 100,000, increasing to 500,000 during Hindu festivals. Vaishno Devi, a temple situated in a mountain cave in the northern state of Jammu, has approximately 60,000–70,000 visits daily. The Golden Temple in the state of Punjab, the holiest shrine for Sikhs, attracts an estimated 100,000 people per day, which can double during the festival of Gurupurub and Baisakhi. During visits, people sit together to eat, with up to 5,000 people served at each sitting from a ‘mega kitchen’.
As well as mass gatherings, overcrowded situations occur frequently in India, often in relation to living conditions and the movement of people in cities. Urban informal settlements are densely populated residential areas characterized by insufficient access to adequate water and sanitation services and households that frequently edge city drains, railway tracks, and low-lying flood-prone areas. The population density varies but is estimated to exceed 125,000 persons per square kilometer in the largest urban informal settlements in India, which are in Mumbai and Hyderabad.Citation51 Moreover, the proportion of residents in urban settlements increased from under 20% in 1950 to 34% in 2018, and projections indicate near doubling of the urban population in India between 2018 and 2050, with an additional 416 million urban dwellers.Citation52 This growth is largely attributed to rural-to-urban migration. Since migrants are often younger, on average, than the populations living in areas of origin or destination, migration tends to lower the average age in destination areas.Citation52
Another common setting in India of overcrowded living conditions is student accommodation. Globally, India has one of the youngest populations, with approximately 58% aged under 30 years.Citation53 More than 34 million students are enrolled in courses at universities across India and most live in small shared spaces in apartments and hostels.Citation54 A review of meningococcal carriage in high-risk settings found that university students had the highest rates of carriage (up to 71% but more usually around 10–30% carriage), although most data are from the USA or Europe.Citation12 Nevertheless, overcrowded student accommodations combined with social behaviors that encourage close physical contact suggest there is likely to be an elevated risk of meningococcal transmission among students in India. However, there has been only one study of meningococcal carriage among college students in India, which showed nasal carriage of 1.5% among 274 students from a single center in 2014.Citation55 Since this study was carried out in one north Indian setting, this finding was not generalizable to the whole country. Additionally, the effect of living in close proximity was not studied as the students were only surveyed at one timepoint; it was planned to re-test them after six weeks but this was not possible as the institutions had to close due to flash flooding.
The rapid growth of India’s urban population has put enormous strains on all public transport systems, with overcrowding common on trains and buses.Citation56 At peak times, passengers are packed together, and trains and buses routinely carry up to two times the actual capacity, forcing people to be in close proximity to each other in enclosed spaces. Moreover, the risk of exposure to meningococcal infection that originated outside of India is likely to have risen in recent years with increased international travel. In 2018, there were 10.6 million foreign tourist arrivals in India, an increase of 5.2% on the previous year, and 26.3 million Indian nationals made a cross-border journey, which had an annual growth rate of 9.8%.Citation57
Epidemiology of meningococcal disease in India
IMD is recognized as a notifiable disease in India and a recent literature review concluded there is a significant burden of meningococcal disease-related complications in this country.Citation21 The review also found that meningococcal disease is increasingly reported among adolescents and adults, with large outbreaks described in these populations, the most recent of which occurred in MeghalayaCitation58 and Tripura.Citation59 The Meghalaya outbreak occurred from January 2008 until June 2009 with 110 cases aged up to 18 years and seven deaths from one center as per the study by Hazarika et al.,Citation58 whereas the total number of cases in the state were 2100 with 260 deaths. The Tripura outbreak, which occurred from January to August 2009, included 285 suspected and confirmed cases and 62 deaths, and most cases were aged 20–30 years. In both outbreaks, IMD was caused by meningococcal serogroup A.58,Citation59 Meningococcal disease in India appears to be caused almost exclusively by serogroup A, although serogroups B, C, W, and Y have also been documented.Citation21 Further epidemiological information is also available from the military in India. In 2005–2010, the Indian Armed Forces had an average attack rate of 9 or 10 cases of meningococcal disease per year and the N. meningitidis carrier rate among recruits was estimated in the 1990s to be 12%.Citation60,Citation61 The largest outbreak occurred in 2006, in which 17 cases were reported among a group of soldiers, with disease caused by meningococcal serogroup A.44
The background incidence of IMD in India has been estimated to be low, with N. meningitidis responsible for 1.9% of the meningitis cases, although this estimation is based on data gathered from 1950 to 2007.Citation62 Moreover, as discussed in detail previously,Citation21 there is a lack of comprehensive information on the epidemiology of meningococcal disease, with few data from the last decade. Small outbreaks are likely to be unreported, particularly those in rural areas, and the true magnitude of larger-scale outbreaks is considered to be underestimated.Citation62,Citation63 This underestimation occurs because, although IMD is a notifiable disease, disease surveillance is not enforced and reporting is passive only.Citation21 The focus is therefore on disease management rather than the collection of surveillance data.Citation63 Most of the available epidemiological data were collected during outbreaksCitation64 and case reporting and disease monitoring activities were discontinued once the epidemic had resolved.Citation63 Moreover, as in other resource-limited countries,Citation65 diagnosis has been most commonly via bacterial culture rather than PCR. Culture-confirmed diagnosis of IMD is often hindered by early antibiotic treatment,Citation66 which is highly prevalent in India: between 2000 and 2015, antibiotic consumption doubled in India, to 6.5 billion defined daily doses.Citation67 Combined with resourcing constraints on government reference laboratories for processing clinical samples, these factors have led to a lack of reliable data on IMD incidence and the prevalence of meningococcal carriage in India.
Measures to prevent and contain meningococcal disease outbreaks in India
The measures used to contain IMD outbreaks in India are summarized in .Citation64 These are implemented by a rapid response team, consisting of epidemiologists, microbiologists, and medical professionals, which is deployed for immediate action, although more remote areas of the country often face a lag time in the response. If diagnostic facilities are not available locally, as is typical in remote areas, patient samples are sent to the National Center for Disease Control (NCDC) for diagnostic testing.
Table 2. Actions recommended to be undertaken immediately after confirmation of a meningococcal disease outbreak in India.Citation64 These actions are implemented by a rapid response team, typically composed of an epidemiologist, microbiologist, and medical professionals
Chemoprophylaxis of close contacts is recommended to decrease the risk of transmission,Citation18 usually with ciprofloxacin or, alternatively, rifampin or ceftriaxone. However, antimicrobial resistance is a major threat to public health, particularly given the high prevalence of antibiotic treatment and misuse in India.Citation51,Citation67 Addressing antimicrobial resistance is recognized as one of the top 10 priorities for collaborative work between the Indian Ministry of Health and the WHO.Citation68 A national action plan on antimicrobial resistance has been implemented since 2017, including the aim of reducing infection by effective prevention or control measures.
The WHO recommends large-scale meningococcal vaccination programs in countries with high or moderate endemic rates of IMD and in countries with frequent outbreaks.Citation69 A growing number of countries have included vaccination against meningococcal disease in their national immunization programs.Citation21 In India, routine vaccines are administered via the state-sponsored Expanded Program on Immunization (EPI) and meningococcal vaccination is not included in the EPI list. Consequently, meningococcal vaccination is mainly limited to outbreak control. Since the immunological response to vaccination is not immediately protective,Citation70 the effectiveness of this reactive approach is limited if the outbreak is abrupt and short-lasting, or if there is a delay in reporting cases to the NCDC, which may occur in remote parts of the country.Citation63
There are however circumstances where meningococcal vaccination is administered outside of outbreak control. The Indian Academy of Pediatrics (IAP) recommends vaccination of children with high-risk conditions, such as terminal complement component deficiencies or functional/anatomic asplenia, and persons with HIV infection.Citation64 Beyond this, a quadrivalent ACWY vaccine is given to all travelers to Mecca, in accordance with the Saudi Arabian national policy, within 3 years of travelCitation22 and meningococcal vaccination is recommended for those studying abroad and travelers to countries in the African meningitis belt.Citation64 In 2012, an immunization program with a quadrivalent polysaccharide vaccine was introduced in India for all military cadets and recruits.Citation63 Currently, vaccination of military personnel is recommended but is not mandatory.
The IAP recommends conjugate vaccines rather than polysaccharide-only meningococcal vaccines because polysaccharide-only vaccines are T cell-independent and so are only effective for short-term protection in children older than 2 years and adults.Citation69,Citation71 Conjugate vaccines elicit longer-term protection from birth and induce immune memoryCitation72,Citation73 and may prevent the acquisition of meningococcal carriage.Citation73 A polysaccharide bivalent (serogroups AC) vaccine and polysaccharide or conjugate quadrivalent (ACWY) vaccines are licensed against IMD in India. Polysaccharide vaccines are used most widely via the EPI or where vaccination is mandatory, while conjugate vaccines are more commonly used in the private sector. The most recent example of mass vaccination by using the polysaccharide AC meningococcal vaccine was during the Meghalaya and Tripura outbreak in 2008–2009. Any vaccine not included in the EPI schedule is purchased privatelyCitation74 and there is little focus in India on immunization beyond early childhood, with no published national immunization guidelines for adults.Citation75,Citation76
Meningococcal disease prevention and control policies for mass gatherings
The WHO has outlined key considerations for preventing and controlling infections associated with mass gatherings, as summarized in .Citation1 These practical suggestions are based on experience from various events, highlighting the multitude of logistical issues that are relevant for improving the public health response to both planned and unplanned mass gatherings. Briefly, before each event, there needs to be an exchange of information on applied measures and their effects, experience and consultations with previous event organizers, and an understanding of the risk of infection from the disease endemicity both locally and in participating nations. During the event, procedures should be in place for early detection and response to infection, and, post-event, there should be continued awareness of any communicable disease related to the gathering, which may require international cooperation.
Table 3. Practical suggestions provided by the World Health Organization to prevent and control infection during mass gatherings.Citation1.
In relation to India, its current IMD prevention and control policies need to be viewed in the context of various socio-demographic factors that are likely to increase the risk of IMD further, as described earlier. Half of the population is projected to be urban by 2050, up from one-third in 2018,Citation52 and this is accompanied by unplanned and crowded settlements, poor sanitation, and a high frequency of travel from other regions and countries. A variety of travel means are used to reach mass gatherings, which makes it difficult to monitor the immunization status and health of attendees, a key part of the WHO recommendations.Citation1 Monitoring is easier for mass gatherings where the majority of attendees arrive by air, such as the Hajj.Citation48 The relatively young age of the general population in India is another factor to be taken into account, given the peak in IMD incidence in the adolescent/young adult age group.Citation7,Citation62
Since IMD is a vaccine-preventable disease,Citation73 these risk factors could be addressed with routine meningococcal vaccination but currently however, only vaccination during an outbreak, and vaccination of certain high-risk groups, students studying abroad, and travelers to the Hajj and sub-Saharan Africa, are sponsored by the Indian government.Citation63,Citation64,Citation71 The decision to not include meningococcal vaccination in the EPI is based on a perceived low burden of IMDCitation64 but, as already discussed, there is a lack of accurate data on its epidemiology. There is therefore a need for better IMD surveillance in India and, as concluded by attendees of the Global Meningococcal Initiative India, this surveillance needs to integrate disease reporting from both the public and private sectors.Citation63
Conclusions
In conclusion, without reliable surveillance data and laboratory confirmation with strain characterization, it is impossible to determine the true IMD burden in India but it is probably safe to presume that it is higher than estimated. IMD risk factors abound in India, with frequent mass gatherings and overcrowding, combined with a demographically young population and high level of travel within India and internationally. Avoidance of mass gatherings and overcrowding is extremely difficult, as is the logistics of monitoring the health of those attending large events. Moreover, with the inclusion in the national immunization program of the Haemophilus influenzae B vaccine and pneumococcal conjugate vaccine, the etiology of meningitis in India may shift toward N. meningitidis.Citation77 Future healthcare strategies are likely to favor strengthening meningococcal disease surveillance and introducing preventive measures that simplify outbreak control and avoid increasing the threat of antimicrobial resistance to public health. Given the abundance of meningococcal disease risk factors and the potential life-threatening nature of IMD, there is a rationale for offering vaccination against meningococcal disease to the wider community in India.
Abbreviations
EPI, Expanded Program on Immunization; IAP, Indian Academy of Pediatrics; IMD, invasive meningococcal disease; NCDC, National Center for Disease Control; WHO, World Health Organization.
Disclosure of potential conflicts of interest
VA, SK, and SA are employed by the GSK group of companies and VA and SK hold shares in the GSK group of companies. APD and RDH have nothing to disclose. Authors do not disclose any non-financial conflicts of interest.
Contributorship
Someya Agrawal contributed to the study design and performed the literature search. All authors participated in the development of this manuscript. All authors gave final approval before submission.
Acknowledgments
The authors thank the Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Amandine Radziejwoski coordinated the manuscript development and editorial support. Joanne Knowles (independent medical writer for Business & Decision Life Sciences) provided medical writing support.
Additional information
Funding
References
- World Health Organization. Public health for mass gatherings: key considerations. Geneva (Switzerland): 2015. [accessed 2020 Aug 19]. https://apps.who.int/iris/bitstream/handle/10665/162109/WHO_HSE_GCR_2015.5_eng.pdf;jsessionid=6034907890651AF5EBA19A260A799013?sequence=1.
- Memish ZA, Steffen R, White P, Dar O, Azhar EI, Sharma A, Zumla A. Mass gatherings medicine: public health issues arising from mass gathering religious and sporting events. Lancet. 2019;393(10185):2073–84. doi:10.1016/s0140-6736(19)30501-x.
- Tewari S, Khan S, Hopkins N, Srinivasan N, Reicher S, Holme P. Participation in mass gatherings can benefit well-being: longitudinal and control data from a North Indian Hindu pilgrimage event. PLoS One. 2012;7(10):e47291. doi:10.1371/journal.pone.0047291.
- Hopkins N, Reicher S. The psychology of health and well-being in mass gatherings: A review and a research agenda. J Epidemiol Glob Health. 2016;6(2):49–57. doi:10.1016/j.jegh.2015.06.001.
- Wood C. Time to recognize the positive impact and health benefits of mass gatherings. Travel Med Infect Dis. 2018;24:12. doi:10.1016/j.tmaid.2018.06.013.
- Koul PA, Mir H, Saha S, Chadha MS, Potdar V, Widdowson MA, Lal RB, Krishnan A. Respiratory viruses in returning Hajj & Umrah pilgrims with acute respiratory illness in 2014-2015. Indian J Med Res. 2018;148(3):329–33. doi:10.4103/ijmr.IJMR_890_17.
- Acevedo R, Bai X, Borrow R, Caugant DA, Carlos J, Ceyhan M, Christensen H, Climent Y, De Wals P, Dinleyici EC, et al. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines. 2019;18(1):15–30. doi:10.1080/14760584.2019.1557520.
- Al-Tawfiq JA, Memish ZA. The Hajj: updated health hazards and current recommendations for 2012. Euro Surveill. 2012;17:20295.
- Al-Tawfiq JA, Memish ZA, Hajj T. 2019 vaccine requirements and possible new challenges. J Epidemiol Glob Health. 2019;9(3):147–52. doi:10.2991/jegh.k.190705.001.
- Yezli S. The threat of meningococcal disease during the Hajj and Umrah mass gatherings: A comprehensive review. Travel Med Infect Dis. 2018;24:51–58. doi:10.1016/j.tmaid.2018.05.003.
- Caugant DA, Maiden MC. Meningococcal carriage and disease–population biology and evolution. Vaccine. 2009;27 Suppl 2(4):B64–70. doi:10.1016/j.vaccine.2009.04.061.
- Peterson ME, Mile R, Li Y, Nair H, Kyaw MH. Meningococcal carriage in high-risk settings: A systematic review. Int J Infect Dis. 2018;73:109–17. doi:10.1016/j.ijid.2018.05.022.
- Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–61. doi:10.1016/s1473-3099(10)70251-6.
- Gianchecchi E, Piccini G, Torelli A, Rappuoli R, Montomoli E. An unwanted guest: neisseria meningitidis - carriage, risk for invasive disease and the impact of vaccination with insight on Italy incidence. Expert Rev Anti Infect Ther. 2017;15(7):689–701. doi:10.1080/14787210.2017.1333422.
- Wang B, Santoreneos R, Giles L, Haji Ali Afzali H, Marshall H. Case fatality rates of invasive meningococcal disease by serogroup and age: A systematic review and meta-analysis. Vaccine. 2019;37(21):2768–82. doi:10.1016/j.vaccine.2019.04.020.
- Viner RM, Booy R, Johnson H, Edmunds WJ, Hudson L, Bedford H, Kaczmarski E, Rajput K, Ramsay M, Christie D. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. Lancet Neurol. 2012;11(9):774–83. doi:10.1016/s1474-4422(12)70180-1.
- Olbrich KJ, Müller D, Schumacher S, Beck E, Meszaros K, Koerber F. Systematic review of invasive meningococcal disease: sequelae and quality of life impact on patients and their caregivers. Infect Dis Ther. 2018;7(4):421–38. doi:10.1007/s40121-018-0213-2.
- World Health Organization. Meningococcal meningitis. 2018. [accessed 2020 Aug 19]. https://www.who.int/en/news-room/fact-sheets/detail/meningococcal-meningitis#.
- Borrow R, Alarcón P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echániz-Aviles G, Findlow J, Head C, et al. The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16(4):313–28. doi:10.1080/14760584.2017.1258308.
- McNamara LA, MacNeil JR, Cohn AC, Stephens DS. Mass chemoprophylaxis for control of outbreaks of meningococcal disease. Lancet Infect Dis. 2018;18(9):e272–e281. doi:10.1016/s1473-3099(18)30124-5.
- Dutta AK, Swaminathan S, Abitbol V, Kolhapure S, Sathyanarayanan S. A comprehensive review of meningococcal disease burden in India. Infect Dis Ther. 2020:1–23. Forthcoming. doi:10.1007/s40121-020-00323-4.
- Yezli S, Bin Saeed AA, Assiri AM, Alhakeem RF, Yunus MA, Turkistani AM, Booy R, Alotaibi BM. Prevention of meningococcal disease during the Hajj and Umrah mass gatherings: past and current measures and future prospects. Int J Infect Dis. 2016;47:71–78. doi:10.1016/j.ijid.2015.12.010.
- Doedeh J, Frimpong JA, Yealue KDM 2nd, Wilson HW, Konway Y, Wiah SQ, Doedeh V, Bao U, Seneh G, Gorwor L, et al. Rapid field response to a cluster of illnesses and deaths - Sinoe County, Liberia, April-May, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(42):1140–43. doi:10.15585/mmwr.mm6642a4.
- Reintjes R, Kistemann T, MacLehose L, McKee M, Gill N, Weinberg J, Schaefer O, Camaroni I, Fulop N, Brand H. Detection and response to a meningococcal disease outbreak following a youth football tournament with teams from four European countries. Int J Hyg Environ Health. 2002;205(4):291–96. doi:10.1078/1438-4639-00156.
- Smith-Palmer A, Oates K, Webster D, Taylor S, Scott KJ, Smith G, Parcell B, Lindstrand A, Wallensten A, Fredlund H, et al. Outbreak of Neisseria meningitidis capsular group W among scouts returning from the World Scout Jamboree, Japan, 2015. Euro Surveill. 2016;21(45):45. doi:10.2807/1560-7917.es.2016.21.45.30392.
- Beard FH, McAnulty JM, Tapsall JW, Zaia AM. Probable transmission of meningococcal disease on a school bus. Med J Aust. 2006;184(2):90. doi:10.5694/j.1326-5377.2006.tb00128.x.
- Soeters HM, McNamara LA, Blain AE, Whaley M, MacNeil JR, Hariri S, Mbaeyi SA. University-based outbreaks of meningococcal disease caused by serogroup B, United States, 2013-2018. Emerg Infect Dis. 2019;25(3):434–40. doi:10.3201/eid2503.181574.
- Stefanelli P, Neri A, Vacca P, Picicco D, Daprai L, Mainardi G, Rossolini GM, Bartoloni A, Anselmo A, Ciammaruconi A, et al. Meningococci of serogroup X clonal complex 181 in refugee camps, Italy. Emerg Infect Dis. 2017;23(5):870–72. doi:10.3201/eid2305.161713.
- Kushwaha AS, Aggarwal SK, Arora MM. Outbreak of meningococcal infection amongst soldiers deployed in operations. Med J Armed Forces India. 2010;66(1):4–8. doi:10.1016/s0377-1237(10)80082-6.
- Patel JC, George J, Vuong J, Potts CC, Bozio C, Clark TA, Thomas J, Schier J, Chang A, Waller JL, et al. Rapid laboratory identification of Neisseria meningitidis serogroup C as the cause of an outbreak - Liberia, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(42):1144–47. doi:10.15585/mmwr.mm6642a5.
- Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. 2016;59(2 Suppl):S3–S11. doi:10.1016/j.jadohealth.2016.04.012.
- Badahdah AM, Rashid H, Khatami A, Booy R. Meningococcal disease burden and transmission in crowded settings and mass gatherings other than Hajj/Umrah: A systematic review. Vaccine. 2018;36(31):4593–602. doi:10.1016/j.vaccine.2018.06.027.
- Kanai M, Kamiya H, Smith-Palmer A, Takahashi H, Hachisu Y, Fukusumi M, Saitoh T, Ohnishi M, Sunagawa T, Matsui T, et al. Meningococcal disease outbreak related to the World Scout Jamboree in Japan, 2015. Western Pac Surveill Response J. 2017;8(2):25–30. doi:10.5365/wpsar.2016.7.3.007.
- Harrison LH, Armstrong CW, Jenkins SR, Harmon MW, Ajello GW, Miller GB Jr., Broome CV. A cluster of meningococcal disease on a school bus following epidemic influenza. Arch Intern Med. 1991;151(5):1005–09. doi:10.1001/archinte.1991.00400050141028.
- Mbaeyi SA, Joseph SJ, Blain A, Wang X, Hariri S, MacNeil JR. Meningococcal disease among college-aged young adults: 2014-2016. Pediatrics. 2019;143(1):e20182130. doi:10.1542/peds.2018-2130.
- Marshall GS, Dempsey AF, Srivastava A, Isturiz RE. US college students are at increased risk for serogroup B meningococcal disease. J Pediatric Infect Dis Soc. 2020;9(2):244–47. doi:10.1093/jpids/piz024.
- Cummiskey J, Borrione P, Bachil N, Ergen E, Pigozzi F. Report of a serious reportable communicable disease at a major sporting event. J Sports Med Phys Fitness. 2008;48:125–28.
- Orr H, Kaczmarski E, Sarangi J, Pankhania B, Stuart J. Cluster of meningococcal disease in rugby match spectators. Commun Dis Public Health. 2001;4:316–18.
- Gonçalves G, Castro L, Correia AM, Queirós L. Infectious diseases surveillance activities in the north of Portugal, during the EURO 2004 football tournament. Euro Surveill. 2005;10(4):86–89. doi:10.2807/esm.10.04.00532-en.
- Tezer H, Ozkaya-Parlakay A, Kanik-Yuksek S, Gülhan B, Güldemir DA. Syrian patient diagnosed with meningococcal meningitis serogroup B. Hum Vaccin Immunother. 2014;10(8):2482. doi:10.4161/hv.28951.
- Santaniello-Newton A, Hunter PR. Management of an outbreak of meningococcal meningitis in a Sudanese refugee camp in Northern Uganda. Epidemiol Infect. 2000;124(1):75–81. doi:10.1017/s0950268899003398.
- Heyman SN, Ginosar Y, Niel L, Amir J, Marx N, Shapiro M, Maayan S. Meningococcal meningitis among Rwandan refugees: diagnosis, management, and outcome in a field hospital. Int J Infect Dis. 1998;2(3):137–42. doi:10.1016/s1201-9712(98)90115-1.
- Haelterman E, Boelaert M, Suetens C, Blok L, Henkens M, Toole MJ. Impact of a mass vaccination campaign against a meningitis epidemic in a refugee camp. Trop Med Int Health. 1996;1(3):385–92. doi:10.1046/j.1365-3156.1996.d01-49.x.
- Millar BC, Moore PJA, Moore JE. Meningococcal disease: has the battle been won? J R Army Med Corps. 2017;163(4):235–41. doi:10.1136/jramc-2016-000695.
- Grecki M, Bienias M. Outbreak of invasive meningococcal disease among soldiers in Skwierzyna, Poland, March 2006. Euro Surveill. 2006;11(7):E060706 060704. doi:10.2807/esw.11.27.02996-en.
- India State-Level Disease Burden Initiative Collaborators. Nations within a nation: variations in epidemiological transition across the states of India, 1990-2016 in the Global Burden of Disease Study. Lancet. 2017;390(10111):2437–60. doi:10.1016/s0140-6736(17)32804-0.
- The World Bank. Population density (people per sq. km of land area). 2020. [accessed 2020 Aug 19]. https://data.worldbank.org/indicator/EN.POP.DNST.
- David S, Roy N. Public health perspectives from the biggest human mass gathering on earth: Kumbh Mela, India. Int J Infect Dis. 2016;47:42–45. doi:10.1016/j.ijid.2016.01.010.
- Raines K, Centers for Disease Control and Prevention. CDC India helps protect 150 million people at the world’s largest gathering. 2019. [accessed 2020 Aug 19]. https://www.cdc.gov/globalhealth/stories/kumbh-mela.html.
- Sridhar S, Gautret P, Brouqui P. A comprehensive review of the Kumbh Mela: identifying risks for spread of infectious diseases. Clin Microbiol Infect. 2015;21(2):128–33. doi:10.1016/j.cmi.2014.11.021.
- Nadimpalli ML, Marks SJ, Montealegre MC, Gilman RH, Pajuelo MJ, Saito M, Tsukayama P, Njenga SM, Kiiru J, Swarthout J, et al. Urban informal settlements as hotspots of antimicrobial resistance and the need to curb environmental transmission. Nat Microbiol. 2020;5(6):787–95. doi:10.1038/s41564-020-0722-0.
- United Nations. World urbanization prospects: the 2018 revision (ST/ESA/SER.A/420). New York (USA): 2019. https://population.un.org/wup/Publications/Files/WUP2018-Report.pdf.
- Office of the Registrar General & Census Commissioner (India). 2011 census data. Population enumeration data (final population). 2020. [accessed 2020 Aug 19]. https://censusindia.gov.in/2011census/population_enumeration.html.
- Frank K. Global student property 2019. 2020. [accessed 2020 Aug 19]. https://content.knightfrank.com/research/1775/documents/en/global-student-property-report-2019-may-2019-6426.pdf.
- Bali NK, Mir H, Tantray VG, Ali S, Kakru DK, Koul PA. Meningococcal carriage among college freshmen in Kashmir, North India - A single centre study. J Clin Diagn Res. 2017;11:13–17.
- Pucher J, Korattyswaroopam N, Ittyerah N. The crisis of public transport in India: overwhelming needs but limited resources. J Public Trans. 2004;7(4):1–20. doi:10.5038/2375-0901.7.4.1.
- Ministry of Tourism, Government of India. Tourism statistics at a glance - 2019. 2019. [accessed 2020 Aug 19]. http://tourism.gov.in/sites/default/files/2020-04/India%20Tourism%20Statistics%20at%20a%20Glance%202019.pdf.
- Dass Hazarika R, Deka NM, Khyriem AB, Lyngdoh WV, Barman H, Duwarah SG, Jain P, Borthakur D. Invasive meningococcal infection: analysis of 110 cases from a tertiary care centre in North East India. Indian J Pediatr. 2013;80(5):359–64. doi:10.1007/s12098-012-0855-0.
- Majumdar T, Bhattacharya S, Barman D, Begum R. Laboratory confirmed outbreak of meningococcal infections in Tripura. Indian J Med Microbiol. 2011;29(1):74–76. doi:10.4103/0255-0857.76533.
- Gorthi SP, Nagpal AK. Meningococcal vaccine: which, when and for whom? Med J Armed Forces India. 2010;66(1):2–3. doi:10.1016/s0377-1237(10)80081-4.
- Jha D, Ghosh MK. Epidemiology of meningococcal carrier state amongst recruits of a military training centre. J Commun Dis. 1995;27:250–55.
- Sinclair D, Preziosi MP, Jacob John T, Greenwood B. The epidemiology of meningococcal disease in India. Trop Med Int Health. 2010;15(12):1421–35. doi:10.1111/j.1365-3156.2010.02660.x.
- John TJ, Gupta S, Chitkara AJ, Dutta AK, Borrow R. An overview of meningococcal disease in India: knowledge gaps and potential solutions. Vaccine. 2013;31(25):2731–37. doi:10.1016/j.vaccine.2013.04.003.
- Indian Academy of Pediatrics. IAP guidebook on immunization 2018-2019. New Delhi, India; 2020.
- Aye AMM, Bai X, Borrow R, Bory S, Carlos J, Caugant DA, Chiou CS, Dai VTT, Dinleyici EC, Ghimire P, et al. Meningococcal disease surveillance in the Asia-Pacific region (2020): the global meningococcal initiative. J Infect. 2020;81(5):698–711. In press. doi:10.1016/j.jinf.2020.07.025.
- Fox AJ, Taha MK, Vogel U. Standardized nonculture techniques recommended for European reference laboratories. FEMS Microbiol Rev. 2007;31(1):84–88. doi:10.1111/j.1574-6976.2006.00048.x.
- Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, Goossens H, Laxminarayan R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115(15):E3463–E3470. doi:10.1073/pnas.1717295115.
- Nilgiriwala KS. Basis of science policies for infectious disease challenges in India. Indian J Public Health. 2018;62(3):193–96. doi:10.4103/ijph.IJPH_306_17.
- World Health Organization. Meningococcal vaccines: WHO position paper, November 2011. Wkly Epidemiol Rec. 2011;86(47):521–39.
- World Health Organization. International travel and health. Meningococcal disease. 2020. [accessed 2020 Aug 19]. https://www.who.int/ith/vaccines/meningococcal/en/#:~:text=A%20protective%20antibody%20response%20occurs,decline%20rapidly%20after%202%E2%80%933%20years.
- Vashishtha VM, Choudhury P, Kalra A, Bose A, Thacker N, Yewale VN, Bansal CP, Mehta PJ. Indian Academy of Pediatrics (IAP) recommended immunization schedule for children aged 0 through 18 years–India, 2014 and updates on immunization. Indian Pediatr. 2014;51(10):785–800. doi:10.1007/s13312-014-0504-y.
- Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meer HC, Baker CJ, Messonnier NE. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62:1–28.
- Parikh S, Campbell H, Bettinger JA, Harrison LH, Marshall HS, Martinon-Torres F, Safadi MA, Shao Z, Zhu B, von Gottberg A, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81(4):483–98. In press. doi:10.1016/j.jinf.2020.05.079.
- John TJ, Dandona L, Sharma VP, Kakkar M. Continuing challenge of infectious diseases in India. Lancet. 2011;377(9761):252–69. doi:10.1016/s0140-6736(10)61265-2.
- Verma R, Khanna P, Chawla S. Adult immunization in India: importance and recommendations. Hum Vaccin Immunother. 2015;11(9):2180–82. doi:10.4161/hv.29342.
- Dash R, Agrawal A, Nagvekar V, Lele J, Di Pasquale A, Kolhapure S, Parikh R. Towards adult vaccination in India: a narrative literature review. Hum Vaccin Immunother. 2020;16(4):991–1001. doi:10.1080/21645515.2019.1682842.
- Oordt-Speets AM, Bolijn R, van Hoorn RC, Bhavsar A, Kyaw MH. Global etiology of bacterial meningitis: A systematic review and meta-analysis. PLoS One. 2018;13(6):e0198772. doi:10.1371/journal.pone.0198772.

