ABSTRACT
The worldwide implementation of pneumococcal conjugate vaccines (PCVs) in children has reduced the overall pneumococcal disease burden. Two PCVs are widely available for infant vaccination: the pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) and the 13-valent PCV (PCV13). While these PCVs differ in serotype composition (PCV13 includes polysaccharides of serotypes 3, 6A and 19A; PHiD-CV does not), their impact on the overall pneumococcal disease burden in children is comparable. This commentary summarizes the evidence of comparability between PHiD-CV and PCV13 and explores why differences in serotype composition may not necessarily translate into a differential clinical impact. Both vaccines confer similarly high protection against disease caused by vaccine serotypes and lead to a partial replacement by non-vaccine serotypes. PHiD-CV does not protect against serotype 3 disease (not included in the vaccine) and PCV13’s effect on this serotype has been inconsistent. PHiD-CV provides some cross-protection against disease caused by vaccine-related serotype 19A but neither vaccine has fully controlled 19A disease. While protection against 19A is higher for PCV13 than PHiD-CV, replacement by non-PCV13 serotypes in settings with a PCV13 program appears to compensate for this difference. This results in a similar residual overall disease burden with both vaccines.
PLAIN LANGUAGE SUMMARY
What is the context?
The pneumococcus bacterium can cause infections of the meninges, blood, lung, middle ear and sinuses.
Two vaccines, Synflorix (GSK) and Prevnar 13 (Pfizer Inc.), are widely used to protect young children against these infections.
The vaccines’ compositions differ: Synflorix includes antigens from 10 pneumococcus strains (or “serotypes”) and Prevnar 13 from 13 serotypes.
However, both have a similar effect on the total pneumococcal disease burden in children.
What does this commentary highlight?
This commentary summarizes the evidence behind the two vaccines’ comparable impact on pneumococcal disase.
It also looks at why the vaccines have a similar effect on the total pneumococcal disease burden despite their different compositions.
What is the impact on current thinking?
Given that Synflorix and Prevnar 13 have a comparable impact on pneumococcal disease, a country’s choice between the two vaccines will depend on vaccine supply, cost, logistical factors (e.g., transport, storage, training requirements of health workers) and the local pneumococcal epidemiology.
© 2021 GlaxoSmithKline Biologicals SA. Published with license by Taylor & Francis Group, LLC
Introduction
Before the widespread use of effective pediatric pneumococcal vaccines, Streptococcus pneumoniae killed >800,000 children aged 1–59 months worldwide each year (based on estimates for 2000).Citation1 These deaths resulted from an estimated 14.5 million cases of invasive pneumococcal disease (IPD; including bacteremic pneumonia, meningitis and sepsis) and non-bacteremic pneumonia.Citation1 While these numbers declined substantially after the worldwide implementation of pneumococcal conjugate vaccines (PCVs) in children, the latest estimates (from 2015) still show a significant burden: 9.2 million cases and >300,000 deaths per year, nearly 10% of all deaths in children aged 1–59 months.Citation2 S. pneumoniae is also one of the main bacterial pathogens responsible for upper respiratory tract infections, including sinusitis and otitis media (OM).Citation3
One hundred different pneumococcal serotypes have been identified, characterized by serologically distinct polysaccharide (PS) capsules.Citation4 While most serotypes can cause disease, and significant variations in their distribution are seen by geographical region, time, age and disease manifestation, a small proportion of serotypes are responsible for most IPD in young children.Citation3,Citation5–8 Serotypes causing non-bacteremic pneumonia and OM are less well characterized as these diseases are primarily diagnosed through clinical assessment. The first PCV to enter the market – the seven-valent PCV (PCV7, Prevnar/Prevenar, Pfizer Inc.), in 2000 – contained PS of the seven serotypes that were most prevalent in pediatric IPD isolates in the United States: 4, 6B, 9V, 14, 18C, 19F and 23F.Citation5,Citation7 Higher-valent PCVs were subsequently developed, with serotype compositions that provided better global coverage and included serotypes that emerged after PCV7 introduction.Citation5,Citation8 Currently, two PCVs are widely available for vaccination in infants and young children: pneumococcal non-typeable Haemophilus influenzae (NTHi) protein D conjugate vaccine (PHiD-CV, Synflorix, GSK) and 13-valent PCV (PCV13, Prevnar 13/Prevenar 13, Pfizer Inc.). PHiD-CV contains PS from serotypes 1, 5 and 7F in addition to the seven PCV7 serotypes; PCV13 contains PS from the ten PHiD-CV serotypes and 3, 6A and 19A.Citation3,Citation9 PHiD-CV and PCV13 also differ in the amount of PS they contain for each serotype (more than double in PCV13 for seven of the ten serotypes shared with PHiD-CV, exceptions being 4, 18C and 19F), the carrier proteins conjugated to the PS (NTHi protein D for eight serotypes, tetanus and diphtheria toxoids for the remaining two in PHiD-CV; diphtheria toxoid variant CRM197 in PCV13) and the conjugation method.Citation9,Citation10 Despite these differences, the available evidence does not indicate that the two vaccines differ in their impact on the overall pneumococcal disease burden in children (i.e., the combined burden of disease caused by vaccine serotypes [VT] and non-VT).Citation3
In this commentary, we summarize the evidence behind the comparable impact of PHiD-CV and PCV13 on pneumococcal disease, with an emphasis on the overall rather than VT-specific disease burden, as this is most relevant for public health. We explore why differences in serotype composition may not necessarily translate into a differential clinical impact. We focus on the vaccines’ effect on IPD (as this is the most frequently measured and etiologically characterized pneumococcal disease outcome) but also include a section describing their impact on pneumonia and OM.
Comparable impact of PHiD-CV and PCV13 on overall IPD
The impact of PHiD-CV and PCV13 on IPD was evaluated in comprehensive systematic reviews. A systematic review of PCV effectiveness and impact studies in Latin America showed that both PHiD-CV and PCV13 have a significant impact on reducing IPD-related hospitalizations in <5-y-olds, with no evidence of one vaccine being superior over the other.Citation11 Another systematic review (“PCV review of impact evidence [PRIME]”) evaluated both vaccines’ immunogenicity, and their effectiveness/impact on nasopharyngeal carriage, pneumonia, IPD and mortality.Citation9,Citation12 The review served as a basis for the latest update of the World Health Organization (WHO) opinion paper on PCVs in <5-y-olds, which states that, while differences were found in the impact on the three additional PCV13 serotypes and 6C, there is at present insufficient evidence of a difference in the net impact of the two products on the overall pneumococcal disease burden (from IPD and pneumonia).Citation3
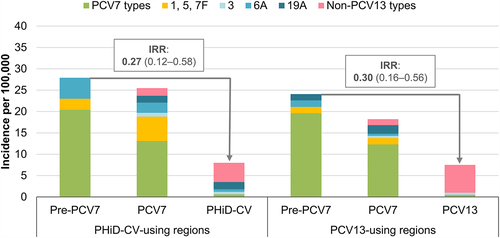
Both reviews based their conclusions on indirect comparisons between the two vaccines, since no prospective, randomized, head-to-head studies assessing the effects of PHiD-CV and PCV13 on pneumococcal diseases have been performed. However, a single study in Sweden has been published since, that compared the vaccines when given to equivalent populations in parallel time periods and similar settings, which is the closest available to a head-to-head study.Citation13 In Sweden, PCV7 was introduced in some regions from 2007 and throughout the country in 2009. It was replaced by PHiD-CV in some and PCV13 in other regions from 2009 onwards.Citation13 Declines in overall IPD (IPD caused by any serotype) in 0–2-y-old children were not statistically significantly different between PHiD-CV- and PCV13-using regions (thereby confirming the overall conclusions of the systematic reviews): incidence rate ratios (IRRs) compared to the pre-PCV7 period were 0.27 (95% confidence interval [CI]: 0.12–0.58) and 0.30 (0.16–0.56), respectively ().Citation13
Figure 1. Invasive pneumococcal disease incidence rates in children 0–2 y of age in PHiD-CV- or PCV13-using Swedish regions before PCV7 and before and after PHiD-CV or PCV13 implementation.
While this study is – to our knowledge – the only one comparing the effects of the two vaccines on IPD when used in the same time period and the same country, other countries/regions used both vaccines consecutively in their infant immunization programs, providing an opportunity to evaluate the vaccines in similar settings and populations (but different time periods). We found a single study in such settings (in Quebec [Canada]) that calculated the effectiveness against IPD for both vaccines (see Supplementary material for details on the literature search).Citation14 In Quebec, PCV7 was introduced for routine infant immunization in 2004 and replaced by PHiD-CV in 2009. PHiD-CV was replaced by PCV13 in 2011Citation14 and was reinstated in 2018.Citation15 A case-control study assessing laboratory-confirmed IPD cases from 2–59-month-old children, notified during 2005–2013, showed comparable effectiveness of PHiD-CV and PCV13 against overall IPD: 72% (95% CI: 46–85) and 66% (29–83) for ≥1 dose of the vaccines, respectively.Citation14
Why can PHiD-CV and PCV13 have a comparable impact on overall IPD?
As highlighted in the introduction, PCVs only cover a small fraction of all pneumococcal serotypes.Citation9 Undeniably, the direct effectiveness against VT disease and the relative contribution of VT to the overall disease burden in the population targeted for vaccination are the major components determining the maximal vaccine effect. The net impact can be increased if the vaccine confers “cross-protection” against non-VT that serologically resemble one of the VT (i.e., vaccine-related types).Citation16 By contrast, the net impact on disease can be diminished as a result of “serotype replacement”, a consequence of prolonged PCV use, where VT are replaced by non-VT in nasopharyngeal carriage and disease.Citation17
In the following sections, we discuss how the combined effect on VT, vaccine-related serotypes and non-VT can result in a similar impact of PHiD-CV and PCV13 on overall IPD in children. We also briefly discuss herd protection.
Protection against VT IPD
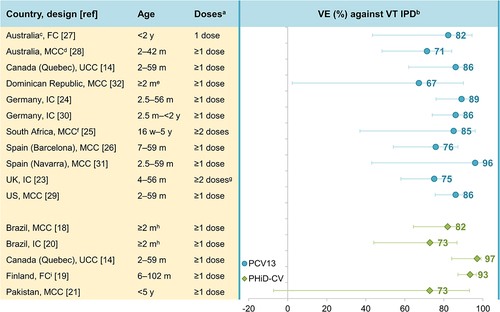
We evaluated data from effectiveness studies to assess whether PHiD-CV and PCV13 protect equally well against VT IPD and if protection is equally high for each serotype (see Supplementary material for details on the literature search and Supplementary table 1 for study details). Effectiveness studies using case-control, indirect or full cohort designs have shown statistically significant effectiveness against VT IPD: 73–97% for PHiD-CV (against PHiD-CV types)Citation14,Citation18–22 and 67%–96% for PCV13 (against PCV13 types) ().Citation14,Citation22–32 This is in line with results from two double-blind, randomized, controlled PHiD-CV trials showing efficacy/effectiveness estimates of 92–100% against VT IPD.Citation33,Citation34 No randomized efficacy trials have been performed with PCV13 in children.
Figure 2. Effectiveness of PCV13 and PHiD-CV against VT IPD in children.
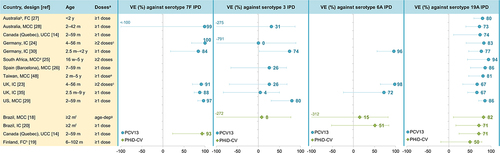
The question of whether PCVs protect equally well against each VT is harder to address because the number of IPD cases included in individual studies is often too low to calculate serotype-specific effectiveness. Nevertheless, effectiveness estimates are available from several studies for serotypes 1, 3 and 7F, as well as for 6A and 19A (covered in the next section). Effectiveness against 7F IPD was high across studies for both PCVs: 93% for PHiD-CVCitation14 and 84–100% for PCV13 ().Citation23,Citation24,Citation28–30,Citation35 Effectiveness estimates against serotype 1 IPD were only available for PCV13 and were also high across most studies (83–89%),Citation23,Citation25,Citation26,Citation30 although a lower estimate was found in a UK study (66%).Citation35 By contrast, no consistent effect of PCV13 on IPD due to serotype 3 (included in PCV13, not in PHiD-CV) has been demonstrated, in line with the immunologic hyporesponsiveness observed for this serotype.Citation36,Citation37 Most studies showed no statistically significant effectiveness against serotype 3 IPD, and estimates varied widely across studies (0–80%) ().Citation23,Citation24,Citation26,Citation28–30,Citation35 As expected, given that PHiD-CV does not contain serotype 3, PHiD-CV has no effect on serotype 3 IPD.Citation12,Citation18 Consistent with these findings, serotype 3 is still present as a cause of pediatric IPD in PHiD-CV- and PCV13-using countries,Citation12,Citation13,Citation38–41 and is frequently associated with PCV13 vaccine failure.Citation13,Citation42,Citation43 Serotype-specific effectiveness estimates for PHiD-CV and PCV13 against IPD caused by the seven VT contained in PCV7 are available from a limited number of studiesCitation18,Citation20,Citation26,Citation28 because in most settings, PCV7 had been used for several years before higher-valent PCV introduction, resulting in too low numbers of PCV7-type IPD cases to calculate serotype-specific effectiveness.
Figure 3. Effectiveness of PCV13 and PHiD-CV against serotype-specific IPD in children.
In summary, while both vaccines are highly effective against VT IPD, protection is not equally high for all serotypes; in particular, PCV13 does not seem effective against serotype 3 IPD in most settings where the vaccine was evaluated.Citation23,Citation24,Citation26,Citation28,Citation35
(Cross-)protection against IPD caused by serotypes 6A, 6C and 19A
Data on the cross-protection of PHiD-CV against 6A IPD (mediated by cross-reactive 6B antibodiesCitation44) are scarce given the low baseline levels of 6A, particularly in countries with a previous PCV7 program (due to PCV7 cross-protection against 6A disease).Citation12,Citation16 Case-control and indirect cohort studies in Brazil (which introduced PHiD-CV as first PCV in its infant immunization program) did not show significant effectiveness of PHiD-CV against 6A IPD ().Citation18,Citation20 However, population-based cohort studies in Finland (which also introduced PHiD-CV as first PCV) noted a significant and sustained reduction in 6A IPD in vaccine-eligible children over a 3- and 6-y follow-up, suggesting long-lasting direct cross-protection against 6A IPD.Citation45,Citation46 In Sweden, 6A IPD declined (non-significantly) in PHiD-CV-using regions (IRR versus PCV7 period in 0–2-y-old children: 0.23, 95% CI: 0.02–2.24) ().Citation13 For PCV13, effectiveness estimates against 6A IPD were 72–98% across the assessed studies but not all estimates were statistically significant because of the low baseline 6A IPD rates ().Citation23,Citation30,Citation35
Evidence of PCV13 cross-protection against 6C IPD (mediated by cross-reactive 6A antibodiesCitation47) is sparse although positive effectiveness estimates were found in an indirect cohort study in the UK (70%, 95% CI: 2–92)Citation35 and a case-control study in Australia (80%, <−100–98).Citation28 PHiD-CV effectiveness against 6C IPD was not assessed in the evaluated studies.Citation14,Citation18–20
Cross-protection has been observed for PHiD-CV against 19A IPD (mediated by cross-reactive 19F antibodies).Citation44 Significant effectiveness against 19A IPD was shown in two studies in Brazil using either a case-control or indirect cohort design (82% and 71%) and in a case-control study in Quebec (71%), with the latter showing similar effectiveness for PCV13 (74%) ().Citation14,Citation18,Citation20 Positive but non-significant effectiveness was also observed for PHiD-CV in a study in Finland (50%, full cohort design).Citation19 Population-based studies in Finland showed a significant reduction (62%, 95% CI: 20–85) in 19A IPD in a vaccine-eligible cohort after a 3-y follow-up post-PHiD-CV implementation,Citation45 but this reduction was no longer significant after a 6-y follow-up (26%, −13–51).Citation46 Possible explanations suggested by the authors were waning of the initial cross-protection afforded by PHiD-CV or an increasing infection pressure from 19A in Finland during the later follow-up years.Citation46 In Sweden, no reduction in the incidence of 19A IPD was seen in 0–2-y-olds in PHiD-CV-using regions (versus the PCV7 period), while, over the same time period, the 19A IPD incidence decreased significantly in PCV13-using regions (no cases identified in the PCV13 period) ().Citation13 Interestingly, while effectiveness estimates for PCV13 against 19A IPD (67–94%)Citation14,Citation23–30,Citation35,Citation48 tend to be higher than those for PHiD-CV, they were lower than those against 7F and 6A measured in the same studies (),Citation23,Citation24,Citation28–30,Citation35,Citation49 suggesting that PCV13 may be less effective against 19A than against other VT. The seemingly lower effectiveness of both vaccines against 19A IPD is consistent with the observation that 19A is still circulating in various settings several years after these vaccines were introduced,Citation38–41,Citation50,Citation51 and with the observation that 19A is among the most common VT responsible for PCV13 vaccine failure.Citation42,Citation43
In summary, PHiD-CV provides some cross-protection against 19A, but neither PHiD-CV nor PCV13 has fully controlled 19A IPD. For 6A and 6C, data are too limited to draw firm conclusions, but some of the available evidence is suggestive of cross-protection by PHiD-CV against 6A and by PCV13 against 6C.
Replacement by non-VT
Previous experience with PCV7 showed a complete replacement of VT by non-VT in the nasopharynx after vaccine introduction, but an incomplete replacement in disease.Citation17,Citation38,Citation52 Consequently, PCV7 had no net impact on pneumococcal carriage but its net impact on pneumococcal disease was substantial in many settings. This seeming discrepancy can be explained by a lower virulence of the replacing non-VT compared to VT.Citation17,Citation38 Similar observations have been made for PHiD-CV and PCV13, with both vaccines leading to serotype replacement in carriageCitation53–57 and disease.Citation38–40
We found two published, randomized, controlled trials that assessed the impact of both vaccines on carriage (see Supplementary material for search string).Citation58,Citation59 No major differences were found in either study between the carriage prevalence of VT, non-VT or any pneumococcal serotype in PHiD-CV- compared to PCV13-vaccinated children.Citation58,Citation59
In terms of IPD, as mentioned previously, no randomized, head-to-head studies are available but the study in Sweden (the closest available to a head-to-head comparison) showed that the incidence of IPD due to non-PCV13 serotypes in 0–2-y-olds increased non-significantly in PHiD-CV-using regions (IRR compared to the PCV7 period: 2.48, 95% CI: 0.51–12.0) and significantly in PCV13-using regions (IRR compared to the PCV7 period: 4.78, 1.22–18.7) ().Citation13 The residual IPD burden in 0–2-y-olds in Sweden was very similar in PHiD-CV- and PCV13-using regions but the share taken up by 6A and 19A in regions using PHiD-CV was taken up by non-PCV13 serotypes in those using PCV13 (). Therefore, considering the redistribution of serotypes, there were no differences in the impact of these PCVs on overall IPD in this age group.Citation13 Previous reviews of surveillance studies and reports showed replacement of VT by non-VT in disease in both PHiD-CV- and PCV13-using countries but did not allow direct comparisons between the two vaccines.Citation38–40
In summary, both PHiD-CV and PCV13 cause serotype replacement. At present, there is insufficient evidence to conclude whether replacement is more pronounced with one vaccine or the other.
Herd protection
The total benefit of PCV programs does not only depend on direct effects on the vaccinated population but also on indirect (herd) effects on unvaccinated populations. VT IPD and nasopharyngeal carriage have declined in various age groups of unvaccinated children and adults after implementation of both vaccines.Citation9,Citation12 The study in Sweden, comparing PHiD-CV- and PCV13-using regions, showed similar indirect effects on overall IPD with both vaccines.Citation13
Comparable impact of PHiD-CV and PCV13 on pneumonia and OM
As for IPD, no prospective, head-to-head studies directly comparing the effect of PHiD-CV and PCV13 on pneumonia or OM have been performed. The systematic review on effectiveness and impact studies in Latin America and the PRIME systematic review found evidence of both PCVs’ impact on clinical and chest X-ray confirmed pneumonia in young children and concluded that there was no systematic evidence that one product has a greater impact than the other on pneumonia.Citation9,Citation11,Citation12 Time-series analyses of national-level mortality data from Latin American and Caribbean countries showed declines in mortality due to all-cause pneumonia in countries using either vaccine.Citation60
Two studies in Sweden compared OM-related endpoints in PHiD-CV- and PCV13-using regions.Citation61,Citation62 A first study retrospectively analyzed national registry data and showed that the incidence of outpatient acute OM (AOM), AOM hospital admissions, myringotomies and ventilation tube insertions declined in <5-y-olds both after PHiD-CV and PCV13 introduction.Citation61 The decrease in outpatient AOM and ventilation tube insertions appeared more pronounced in PHiD-CV-using regions; however, cautious interpretation is warranted because geographical differences in incidence were large before vaccine introduction.Citation61 The second study, a retrospective cohort study using linked regional and national databases, assessed the OM incidence in one PHiD-CV- and one PCV13-using region. Descriptive time-series analyses showed declines in the OM incidence in ≤2-y-olds after both PHiD-CV and PCV13 implementation. Effectiveness estimates obtained by modeling adjusted for age, period and cohort effects were only statistically significant for PHiD-CV in ≤5-y-olds.Citation62
In summary, both vaccines seem to have a comparable impact on pneumonia and although some studies suggest that PHiD-CV may offer better protection against OM-related endpoints, further evidence is necessary to confirm these findings.
Limitations
Our commentary was not intended as an exhaustive review of the impact of both PCVs but rather as an overview of their comparability. It was therefore based on evidence generated in previous systematic reviews providing indirect comparisons, complemented by studies where both vaccines were assessed in the same settings. We did not perform a comprehensive systematic search to retrieve these studies and may therefore have missed some of the available evidence. Claims of comparability of the two vaccines in terms of effectiveness against pneumococcal diseases are limited by the lack of prospective, randomized, head-to-head studies. Indirect comparisons between studies are hindered by differences in surveillance methods, clinical practice, local epidemiology, PCV vaccination schedule, previous PCV use and duration of the PCV programs. Our analysis of serotype-specific effectiveness is hampered by the fact that data for some of the serotypes are sparse or absent, particularly for PHiD-CV.
Conclusions
Studies with different designs assessing various pneumococcal-related endpoints in different settings have shown that PHiD-CV and PCV13 have a comparable impact on the overall pneumococcal disease burden. For IPD, this comparability can be explained by the similarly high effectiveness of both vaccines against VT disease, the limited effect of PCV13 on serotype 3 disease and PHiD-CV’s observed cross-protection against 19A (and possibly 6A) disease. While the effect of PHiD-CV on 19A is lower than that of PCV13, neither vaccine has fully controlled 19A IPD. Moreover, the comparative study in Sweden has shown that, in PCV13-using settings, 19A (and 6A) are replaced by disease-causing non-PCV13 serotypes, hence resulting in a comparable residual IPD burden. Both PCVs have also shown similar clinical outcomes for OM and pneumonia.
Considering the comparable impact of both vaccines on the overall pneumococcal disease burden (despite their different compositions), product choice will ultimately depend on programmatic characteristics, vaccine supply, price and local pneumococcal epidemiology.Citation3
Disclosure of potential conflicts of interest
P Izurieta and J Nieto Guevara are employees of the GSK group of companies and declare financial (including shares) and non-financial relationships and activities.
Author contributions
P Izurieta and J Nieto Guevara contributed to the conceptualization (structure and scope) of the paper, P Izurieta contributed to writing the original draft and both authors contributed to the review and editing of the manuscript.
Trademark statement
Synflorix is a trademark of the GSK group of companies. Prevnar/Prevenar and Prevnar 13/Prevenar 13 are trademarks of Pfizer Inc.
Supplemental Material
Download MS Word (85.1 KB)Acknowledgments
The authors would like to thank Modis for editorial assistance and manuscript coordination, on behalf of GSK: Natalie Denef provided writing assistance and Stéphanie Deroo provided publication coordination support.
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1872341.
Additional information
Funding
References
- O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–8. doi:10.1016/s0140-6736(09)61204-6.
- Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, Luksic I, Nair H, McAllister DA, Campbell H, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health. 2018;6(7):e744–e757. doi:10.1016/s2214-109x(18)30247-x.
- World Health Organization. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper – February 2019. Wkly Epidemiol Rec. 2019;94(8):85–104.
- Ganaie F, Saad JS, McGee L, van Tonder AJ, Bentley SD, Lo SW, Gladstone RA, Turner P, Keenan JD, Breiman RF, et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral streptococcus. mBio. 2020;11(3):e00937–00920. doi:10.1128/mBio.00937-20.
- Cherian T. WHO expert consultation on serotype composition of pneumococcal conjugate vaccines for use in resource-poor developing countries, 26-27 October 2006, Geneva. Vaccine. 2007;25(36):6557–64. doi:10.1016/j.vaccine.2007.06.044.
- Hausdorff WP, Bryant J, Kloek C, Paradiso PR, Siber GR. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin Infect Dis. 2000;30(1):122–40. doi:10.1086/313609.
- Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis. 2000;30(1):100–21. doi:10.1086/313608.
- Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, Muenz LR, O’Brien KL. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7(10):e1000348. doi:10.1371/journal.pmed.1000348.
- Cohen O, Knoll M, O’Brien K, Ramakrishnan M, Constenla D, Privor-Dumm L, Buss-Younkin J, Farrar J, Pilishvili T, Whitney C, et al. Pneumococcal conjugate vaccine (PCV) product assessment. 2017 [accessed 2020 June 3]. https://www.jhsph.edu/ivac/wp-content/uploads/2018/05/pcv-product-assessment-april-25-2017.pdf.
- Poolman JT, Peeters CC, van den Dobbelsteen GP. The history of pneumococcal conjugate vaccine development: dose selection. Expert Rev Vaccines. 2013;12(12):1379–94. doi:10.1586/14760584.2013.852475.
- de Oliveira LH, Camacho LA, Coutinho ES, Martinez-Silveira MS, Carvalho AF, Ruiz-Matus C, Toscano CM. Impact and effectiveness of 10 and 13-valent pneumococcal conjugate vaccines on hospitalization and mortality in children aged less than 5 years in Latin American countries: a systematic review. PLoS One. 2016;11(12):e0166736. doi:10.1371/journal.pone.0166736.
- Cohen O, Knoll M, O’Brien K, Ramakrishnan M, Farrar J, Pilishvili T, Whitney C, Goldblatt D, Moisi J, de Cola M, et al. Pneumococcal conjugate vaccine (PCV) review of impact evidence (PRIME): summary of findings from systematic review. 2017 Oct [accessed 2020 June 3]. https://www.who.int/immunization/sage/meetings/2017/october/3_FULL_PRIME_REPORT_2017Sep26.pdf?ua=1.
- Naucler P, Galanis I, Morfeldt E, Darenberg J, Ortqvist A, Henriques-Normark B. Comparison of the impact of pneumococcal conjugate vaccine 10 or pneumococcal conjugate vaccine 13 on invasive pneumococcal disease in equivalent populations. Clin Infect Dis. 2017;65(11):1780–89. doi:10.1093/cid/cix685.
- Deceuninck G, De Serres G, Boulianne N, Lefebvre B, De Wals P. Effectiveness of three pneumococcal conjugate vaccines to prevent invasive pneumococcal disease in Quebec, Canada. Vaccine. 2015;33(23):2684–89. doi:10.1016/j.vaccine.2015.04.005.
- Gouvernement du Québec. Advice and prevention - pneumococcal conjugate vaccine. 2018 [accessed 2020 June 3]. https://www.quebec.ca/en/health/advice-and-prevention/vaccination/pneumococcal-conjugate-vaccine/.
- Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, Nyquist AC, Gershman KA, Vazquez M, Bennett NM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368(9546):1495–502. doi:10.1016/s0140-6736(06)69637-2.
- Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378(9807):1962–73. doi:10.1016/s0140-6736(10)62225-8.
- Domingues CM, Verani JR, Montenegro Renoiner EI, de Cunto Brandileone MC, Flannery B, de Oliveira LH, Santos JB, de Moraes JC. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. Lancet Respir Med. 2014;2(6):464–71. doi:10.1016/s2213-2600(14)70060-8.
- Rinta-Kokko H, Auranen K, Toropainen M, Nuorti JP, Nohynek H, Siira L, Palmu AA. Effectiveness of 10-valent pneumococcal conjugate vaccine estimated with three parallel study designs among vaccine-eligible children in Finland. Vaccine. 2020;38(6):1559–64. doi:10.1016/j.vaccine.2019.11.049.
- Verani JR, Domingues CM, de Moraes JC. Brazilian Pneumococcal Conjugate Vaccine Effectiveness Study Group. Indirect cohort analysis of 10-valent pneumococcal conjugate vaccine effectiveness against vaccine-type and vaccine-related invasive pneumococcal disease. Vaccine. 2015;33(46):6145–48. doi:10.1016/j.vaccine.2015.10.007.
- Riaz A, Mohiuddin S, Husain S, Yousafzai MT, Sajid M, Kabir F, Rehman NU, Mirza W, Salam B, Nadeem N, et al. Effectiveness of 10-valent pneumococcal conjugate vaccine against vaccine-type invasive pneumococcal disease in Pakistan. Int J Infect Dis. 2019;80:28–33. doi:10.1016/j.ijid.2018.12.007.
- Berman-Rosa M, O’Donnell S, Barker M, Quach C. Efficacy and effectiveness of the PCV-10 and PCV-13 vaccines against invasive pneumococcal disease. Pediatrics. 2020;145(4):e20190377. doi:10.1542/peds.2019-0377.
- Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, Slack M, Ladhani SN, Miller E, Goldblatt D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–46. doi:10.1016/s1473-3099(14)70822-9.
- Weinberger R, van der Linden M, Imohl M, von Kries R. Vaccine effectiveness of PCV13 in a 3+1 vaccination schedule. Vaccine. 2016;34(18):2062–65. doi:10.1016/j.vaccine.2016.02.043.
- Cohen C, von Mollendorf C, de Gouveia L, Lengana S, Meiring S, Quan V, Nguweneza A, Moore DP, Reubenson G, Moshe M, et al. Effectiveness of the 13-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in South African children: a case-control study. Lancet Glob Health. 2017;5(3):e359–e369. doi:10.1016/s2214-109x(17)30043-8.
- Dominguez A, Ciruela P, Hernandez S, Garcia-Garcia JJ, Soldevila N, Izquierdo C, Moraga-Llop F, Diaz A, de Sevilla MF, Gonzalez-Peris S, et al. Effectiveness of the 13-valent pneumococcal conjugate vaccine in preventing invasive pneumococcal disease in children aged 7-59 months. A matched case-control study. PLoS One. 2017;12(8):e0183191. doi:10.1371/journal.pone.0183191.
- Gidding HF, McCallum L, Fathima P, Moore HC, Snelling TL, Blyth CC, Jayasinghe S, Giele C, de Klerk N, Andrews RM, et al. Effectiveness of a 3 + 0 pneumococcal conjugate vaccine schedule against invasive pneumococcal disease among a birth cohort of 1.4 million children in Australia. Vaccine. 2018;36(19):2650–56. doi:10.1016/j.vaccine.2018.03.058.
- Jayasinghe S, Chiu C, Quinn H, Menzies R, Gilmour R, McIntyre P. Effectiveness of 7- and 13-valent pneumococcal conjugate vaccines in a schedule without a booster dose: a 10-year observational study. Clin Infect Dis. 2018;67(3):367–74. doi:10.1093/cid/ciy129.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Holtzman C, Harrison LH, Zansky SM, Rosen JB, Reingold A, Scherzinger K, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med. 2016;4(5):399–406. doi:10.1016/s2213-2600(16)00052-7.
- van der Linden M, Falkenhorst G, Perniciaro S, Fitzner C, Imohl M. Effectiveness of pneumococcal conjugate vaccines (PCV7 and PCV13) against invasive pneumococcal disease among children under two years of age in Germany. PLoS One. 2016;11(8):e0161257. doi:10.1371/journal.pone.0161257.
- Guevara M, Barricarte A, Torroba L, Herranz M, Gil-Setas A, Gil F, Bernaola E, Ezpeleta C, Castilla J. Working Group for Surveillance of the Pneumococcal Invasive Disease in Navarra. Direct, indirect and total effects of 13-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in children in Navarra, Spain, 2001 to 2014: cohort and case-control study. Euro Surveill. 2016;21(14). doi:10.2807/1560-7917.Es.2016.21.14.30186.
- Tomczyk S, Lessa FC, Sanchez J, Peña C, Fernandez J, Gloria Carvalho M, Pimenta F, Cedano D, Whitney CG, Verani JR, et al. Effectiveness of 13-pneumococcal conjugate vaccine (PCV13) against invasive pneumococcal disease in children in the Dominican Republic. BMC Infect Dis. 2018;18(1):152. doi:10.1186/s12879-018-3047-3.
- Palmu AA, Jokinen J, Borys D, Nieminen H, Ruokokoski E, Siira L, Puumalainen T, Lommel P, Hezareh M, Moreira M, et al. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet. 2013;381(9862):214–22. doi:10.1016/s0140-6736(12)61854-6.
- Tregnaghi MW, Saez-Llorens X, Lopez P, Abate H, Smith E, Posleman A, Calvo A, Wong D, Cortes-Barbosa C, Ceballos A, et al. Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in young Latin American children: a double-blind randomized controlled trial. PLoS Med. 2014;11(6):e1001657. doi:10.1371/journal.pmed.1001657.
- Andrews N, Kent A, Amin-Chowdhury Z, Sheppard C, Fry N, Ramsay M, Ladhani SN. Effectiveness of the seven-valent and thirteen-valent pneumococcal conjugate vaccines in England: the indirect cohort design, 2006-2018. Vaccine. 2019;37(32):4491–98. doi:10.1016/j.vaccine.2019.06.071.
- Linley E, Bell A, Gritzfeld JF, Borrow R. Should pneumococcal serotype 3 be included in serotype-specific immunoassays? Vaccines (Basel). 2019;7(1):4. doi:10.3390/vaccines7010004.
- Poolman J, Borrow R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev Vaccines. 2011;10(3):307–22. doi:10.1586/erv.11.8.
- Hausdorff WP, Hanage WP. Interim results of an ecological experiment - conjugate vaccination against the pneumococcus and serotype replacement. Hum Vaccin Immunother. 2016;12(2):358–74. doi:10.1080/21645515.2015.1118593.
- Izurieta P, Bahety P, Adegbola R, Clarke C, Hoet B. Public health impact of pneumococcal conjugate vaccine infant immunization programs: assessment of invasive pneumococcal disease burden and serotype distribution. Expert Rev Vaccines. 2018;17(6):479–93. doi:10.1080/14760584.2018.1413354.
- Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLoS One. 2017;12(5):e0177113. doi:10.1371/journal.pone.0177113.
- Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, Andrews NJ, Miller E, Ramsay ME. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–51. doi:10.1016/s1473-3099(18)30052-5.
- Hernandez S, Moraga-Llop F, Diaz A, de Sevilla MF, Ciruela P, Muñoz-Almagro C, Codina G, Campins M, Garcia-Garcia JJ, Esteva C, et al. Failures of 13-valent conjugated pneumococcal vaccine in age-appropriately vaccinated children 2-59 months of age, Spain. Emerg Infect Dis. 2020;26(6):1147–55. doi:10.3201/eid2606.190951.
- Oligbu G, Collins S, Andrews N, Sheppard CL, Fry NK, Slack MPE, Borrow R, Ladhani SN. Characteristics and serotype distribution of childhood cases of invasive pneumococcal disease following pneumococcal conjugate vaccination in England and Wales, 2006-2014. Clin Infect Dis. 2017;65(7):1191–98. doi:10.1093/cid/cix418.
- Prymula R, Schuerman L. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev Vaccines. 2009;8(11):1479–500. doi:10.1586/erv.09.113.
- Jokinen J, Rinta-Kokko H, Siira L, Palmu AA, Virtanen MJ, Nohynek H, Virolainen-Julkunen A, Toropainen M, Nuorti JP. Impact of ten-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in Finnish children – a population-based study. PLoS One. 2015;10(3):e0120290. doi:10.1371/journal.pone.0120290.
- Rinta-Kokko H, Palmu AA, Auranen K, Nuorti JP, Toropainen M, Siira L, Virtanen MJ, Nohynek H, Jokinen J. Long-term impact of 10-valent pneumococcal conjugate vaccination on invasive pneumococcal disease among children in Finland. Vaccine. 2018;36(15):1934–40. doi:10.1016/j.vaccine.2018.03.001.
- Grant LR, O’Brien SE, Burbidge P, Haston M, Zancolli M, Cowell L, Johnson M, Weatherholtz RC, Reid R, Santosham M, et al. Comparative immunogenicity of 7 and 13-valent pneumococcal conjugate vaccines and the development of functional antibodies to cross-reactive serotypes. PLoS One. 2013;8(9):e74906. doi:10.1371/journal.pone.0074906.
- Su WJ, Lo HY, Chang CH, Chang LY, Chiu CH, Lee PI, Lu CY, Hsieh YC, Lai MS, Lin TY. Effectiveness of pneumococcal conjugate vaccines of different valences against invasive pneumococcal disease among children in Taiwan: a nationwide study. Pediatr Infect Dis J. 2016;35(4):e124–133. doi:10.1097/inf.0000000000001054.
- Nieto Guevara J, Izurieta P, Hoet B. Response to: pneumococcal conjugate vaccines in Latin America: are PCV10 and PCV13 similar in terms of protection against serotype 19A? Expert Rev Vaccines. 2018;17(4):283–84. doi:10.1080/14760584.2018.1459247.
- Kandasamy R, Voysey M, Collins S, Berbers G, Robinson H, Noel I, Hughes H, Ndimah S, Gould K, Fry N, et al. Persistent circulation of vaccine serotypes and serotype replacement after 5 years of infant immunization with 13-valent pneumococcal conjugate vaccine in the United Kingdom. J Infect Dis. 2020;221(8):1361–70. doi:10.1093/infdis/jiz178.
- Desmet S, Lagrou K, Wyndham-Thomas C, Braeye T, Verhaegen J, Maes P, Fieuws S, Peetermans WE, Blumental S. Dynamic changes in paediatric invasive pneumococcal disease after sequential switches of conjugate vaccine in Belgium: a national retrospective observational study. Lancet Infect Dis. 2020;21:127–36. doi:10.1016/s1473-3099(20)30173-0.
- Hausdorff WP, Hoet B, Adegbola RA. Predicting the impact of new pneumococcal conjugate vaccines: serotype composition is not enough. Expert Rev Vaccines. 2015;14(3):413–28. doi:10.1586/14760584.2015.965160.
- Abu Seir R, Azmi K, Hamdan A, Namouz H, Jaar F, Jaber H, Rubin C, Doron D, Rahav G, Abdeen Z, et al. Comparison of early effects of pneumococcal conjugate vaccines: PCV7, PCV10 and PCV13 on Streptococcus pneumoniae nasopharyngeal carriage in a population based study; The Palestinian-Israeli Collaborative Research (PICR). PLoS One. 2018;13(11):e0206927. doi:10.1371/journal.pone.0206927.
- Hammitt LL, Etyang AO, Morpeth SC, Ojal J, Mutuku A, Mturi N, Moisi JC, Adetifa IM, Karani A, Akech DO, et al. Effect of ten-valent pneumococcal conjugate vaccine on invasive pneumococcal disease and nasopharyngeal carriage in Kenya: a longitudinal surveillance study. Lancet. 2019;393(10186):2146–54. doi:10.1016/s0140-6736(18)33005-8.
- Southern J, Andrews N, Sandu P, Sheppard CL, Waight PA, Fry NK, Van Hoek AJ, Miller E. Pneumococcal carriage in children and their household contacts six years after introduction of the 13-valent pneumococcal conjugate vaccine in England. PLoS One. 2018;13(5):e0195799. doi:10.1371/journal.pone.0195799.
- Usuf E, Bottomley C, Bojang E, Cox I, Bojang A, Gladstone R, Kampmann B, Hill PC, Roca A. Persistence of nasopharyngeal pneumococcal vaccine serotypes and increase of nonvaccine serotypes among vaccinated infants and their mothers 5 years after introduction of pneumococcal conjugate vaccine 13 in The Gambia. Clin Infect Dis. 2019;68(9):1512–21. doi:10.1093/cid/ciy726.
- Vissers M, Wijmenga-Monsuur AJ, Knol MJ, Badoux P, van Houten MA, van der Ende A, Sanders EAM, Rots NY. Increased carriage of non-vaccine serotypes with low invasive disease potential four years after switching to the 10-valent pneumococcal conjugate vaccine in The Netherlands. PLoS One. 2018;13(3):e0194823. doi:10.1371/journal.pone.0194823.
- Odutola A, Ota MOC, Antonio M, Ogundare EO, Saidu Y, Foster-Nyarko E, Owiafe PK, Ceesay F, Worwui A, Idoko OT, et al. Efficacy of a novel, protein-based pneumococcal vaccine against nasopharyngeal carriage of Streptococcus pneumoniae in infants: a phase 2, randomized, controlled, observer-blind study. Vaccine. 2017;35(19):2531–42. doi:10.1016/j.vaccine.2017.03.071.
- Pomat WS, van den Biggelaar AHJ, Wana S, Francis JP, Solomon V, Greenhill AR, Ford R, Orami T, Passey M, Jacoby P, et al. Safety and immunogenicity of pneumococcal conjugate vaccines in a high-risk population: a randomized controlled trial of 10-valent and 13-valent pneumococcal conjugate vaccine in Papua New Guinean infants. Clin Infect Dis. 2019;68(9):1472–81. doi:10.1093/cid/ciy743.
- de Oliveira LH, Shioda K, Valenzuela MT, Janusz CB, Rearte A, Sbarra AN, Warren JL, Toscano CM, Weinberger DM, Multinational Study for PCV Impact in Mortality Study Team. Declines in pneumonia mortality following the introduction of pneumococcal conjugate vaccines in Latin American and Caribbean countries. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa614.
- Gisselsson-Solen M. Trends in otitis media incidence after conjugate pneumococcal vaccination: a national observational study. Pediatr Infect Dis J. 2017;36(11):1027–31. doi:10.1097/inf.0000000000001654.
- Edmondson-Jones M, Dibbern T, Hultberg M, Anell B, Medin E, Feng Y, Talarico C. The effect of pneumococcal conjugate vaccines on otitis media from 2005 to 2013 in children aged ≤5 years: a retrospective cohort study in two Swedish regions. Hum Vaccin Immunother. 2020;1–10. doi:10.1080/21645515.2020.1775455.
