ABSTRACT
Vitiligo is an autoimmune disease in which pigment is lost in patches of the skin. CD4+ T cells are implicated in vitiligo while regulatory T cells (Tregs) could ameliorate vitiligo. Rapamycin together with autoantigen have been shown to induce immunological tolerance and promote Tregs in multiple autoimmune diseases. In the current study, we synthesized nanoparticles containing rapamycin and autoantigen HEL46-61 (NPHEL46-61/Rapa) and investigated their effects on vitiligo. We treated bone marrow-derived dendritic cells (BMDCs) from TrpHEL mice with NPHEL46-61/Rapa and monitored the phenotype of BMDCs. We investigated the effects of NPHEL46-61/Rapa-treated BMDCs on CD4+ T cell proliferation and differentiation. We administrated NPHEL46-61/Rapa to TCR-TrpHEL mice and investigated the effects on vitiligo. We found that BMDCs can uptake the NPHEL46-61/Rapa, which resulted in decreased expression of costimulation molecules CD80 and CD86 in BMDCs. BMDCs treated with NPHEL46-61/Rapa suppressed antigen-specific CD4+ T cell proliferation while promoted the differentiation of these CD4+ T cell to Tregs in vitro. Administration of NPHEL46-61/Rapa to TCR-TrpHEL mice ameliorated vitiligo, promoted Treg production, and suppressed IFN-γ and IL-6 production, while induced IL-10 production. Therefore, our study provides experimental evidence that nanoparticles containing rapamycin and autoantigen could induce antigen-specific immunological tolerance and prevent vitiligo.
Introduction
Vitiligo is an autoimmune disease in which patches of the skin lose their pigment over the body due to the loss of functional melanocytes.Citation1 Vitiligo usually happens in people with dark skin and the patches often begin on skins exposed to the sun.Citation2 Due to the significant role of skin condition in perception of health and desirability, vitiligo could influence the social interactions and even cause social exclusion, which negatively affect patients’ life quality.Citation3
Although Multiple factors including genetic and environmental factors have been implicated vitiligo pathogenesis, the precise mechanisms of vitiligo are not well elucidated.Citation4 Increasing evidences have suggested that autoimmune-mediated destruction of melanocytes is the major cause of vitiligo.Citation5 Current treatments to vitiligo include suppressing the immune system and stimulating the regrowth of melanocyte stem cell, which can survive autoimmune attack because of their hair follicles' location.Citation4 Cytotoxic CD8+ T cells have been detected in vitiligo patients and their cytotoxicity to melanocytes is believed to play essential role in vitiligo.Citation6 Using a TrpHEL mice model of vitiligo, Lambe et al. demonstrated that autoreactive CD4+ T cells are sufficient for vitiligo by destructing melanocytes in a Fas-Fas ligand-dependent manner.Citation7 The regulatory T cells (Tregs) are immunosuppressive T cells which modulate immune system and suppress effector T cells.Citation8 Decreased Tregs have been reported in multiple autoimmune diseases including vitiligo.Citation9 Promoting Tregs proliferation and activity, and adoptive Treg transfer have been shown to ameliorate vitiligo.Citation10
Dendritic cells (DCs) are leukocytes that not only stimulate adaptive immunity but also establish and maintain immunological tolerance.Citation11 Based on the effects on T cells, DCs can be divided into two subsets: the stimulatory DCs which induce effect of T cell response, and tolerogenic DCs which induce tolerance. Three signals including signal 1, antigenic stimulus provided by MHC/peptide, signal 2, costimulatory molecules including CD80 and CD86, and signal 3 cytokine production are required to initiate T cell response.Citation12 The tolergonenicity of tolerogenic DCs is due to the presentation of antigen to T cells without co-stimulation.Citation13
Rapamycin is a macrolide antibiotic with immunosuppressive activity.Citation14 It has been described that rapamycin-treated, alloantigen-pulsed dendritic cells induce antigen-specific Tregs and prolong graft survival.Citation15 Nanoparticles (NPs) are particles with two or more dimensions on the nanometer scale, which are widely used in medical field. Compared to the corresponding bulk materials, NPs have enhanced physical and chemical properties which enable NPs to be used in multiple applications.Citation16 Systemic delivery of nanoparticles containing autoimmune-disease-relevant peptides induces the generation and expansion of antigen-specific regulatory CD4+ T cell.Citation17 In the present study, we utilized a TrpHEL mouse model of vitiligo and investigated the effects of NPs containing rapamycin and HEL46-61 on vitiligo.
Materials and methods
Nanoparticle (NP) manufacturing
The nanoparticle containing rapamycin and autoantigen HEL46-61 was prepared using double emulsion method as described previously.Citation18,Citation19 Briefly, PLGA, pegylated polylactic acid (PLA-PEG), with or without rapamycin was dissolved in dichloromethane. After the oil phase formed, the oil phase was emulsified with aqueous solution of Cy5 conjugated HEL46-61 by sonication. After emulsification, an aqueous solution of polyvinylalcohol was added to create a double emulsion and was sonicated a second time. The double emulsion was added to a beaker containing phosphate buffer solution and stirred at room temperature for 2 h to allow the dichloromethane to evaporate. For NPs containing rapamycin but no antigen, a similar oil-in-water single emulsion process was used. The resulting NPs were washed twice and finally suspended in phosphate-buffered saline (PBS). Dynamic light scattering (DLS) is used to calculate the diameter of nanoparticles as described previously.Citation20
Mice treatment
TrpHEL mice described previouslyCitation7 and 3A9 TCR mice were used in the present study. In a certain experiment, TrpHEL mice were crossed to 3A9 TCR mice to get TCR-TrpHEL mice, which had spontaneous vitiligo.Citation7 Four weeks old TCR-TrpHEL mice with six mice per group were i.v. injected with NPs 3 times per week for 4 weeks and vitiligo was scored every week based on criterion described previously:Citation7 0, wild-type; 1, spotting in the ears; 2, ears severely affected; 3, ears severely affected and body affected; and 4, ears and body severely affected. Six weeks post treatment, mice were sacrificed and samples were collected for analysis. All animal studies were approved by the ethics commitment of Weifang Yidu Central Hospital (#2019046).
Bone marrow-derived dendritic cells (BMDCs)
BMDCs were prepared by culturing mouse bone marrow cells in medium containing GM-CSF as described previously.Citation21 Briefly, bone marrow cells were cultured in MEM-F-12 supplemented with 20 ng/mL GM-CSF, 10% heat-inactivated fetal bovine serum, 100 IU/penicillin, 100 µg/ml streptomycin, and 10 mM l-glutamine for 7 d. On d 3, culture medium was replaced with the same volume of fresh medium. The nonadherent cells were removed on d 7, and adherent BMDCs were harvested. For NP uptake, BMDCs were incubated with NP for 24 h at 37°C in 5% CO2 incubator. After wash with PBS, BMDCs were stained for MHCII, CD80 or CD86 and then subjected to immunofluorescence or flow cytometry analysis.
CD4± T cell proliferation
CD4+ T cells were isolated from spleen of 3A9 TCR mice using mouse CD4+ T Cell Isolation Kit (Miltenyi Biotec, Shanghai, China) following manufacturer’s instructions, and then labeled with 10 µM CFSE as described previously.Citation22 BMDCs from TrpHEL mice were transfected with NP. 48 h later, BMDCs (5 × 104) were co-incubated with CFSE labeled CD4+ T cells at the ratio of 1:2 for 72 h. CD4+ T cells were purified and stained with Foxp3 and CD4 for flow cytometry analysis.
Flow cytometry
Cell suspensions from lymph node, spleen and blood were prepared as described previously.Citation7 The cells were pelleted by centrifugation and re-suspended staining buffer (2% fetal bovine serum in PBS) for staining. Antibodies used in the present study included: Brilliant Violet 510™ anti-mouse CD11c, PE-anti-mouse CD80, PE-anti-mouse CD86, FITC-anti-mouse CD45, APC-anti-mouse CD3, PE-anti mouse CD8. PE-Cy5 anti-mouse CD4, Brilliant Violet 510™ anti-mouse Foxp3. FITC-anti-Fas. All antibodies were purchased from Biolegend (Beijing, China). After staining, the samples were subject to BD LSRII flow cytometer and the data were analyzed using FlowJo.
Enzyme-linked immunosorbent assay (ELISA)
After treatment, lymph node and spleen were harvested. Total proteins were extracted from lymph node and spleen and subjected for interferon-gamma (IFN-γ), interleukin (IL)-6 and IL-10 detection using commercial ELISA kits (Abcam, Beijing, China).
Statistical analysis
Data were presented as mean ± standard deviation (SD). One- or two-way ANOVA analysis and a Tukey’s or Dunnett’s post hoc test were used to calculate the difference. Experiments were repeated independently at least in triplicate. The statistical difference was considered as significant when p < .05.
Results
PEG-PLA nanoparticles containing autoantigen (HEL46-61) and rapamycin were uptaken by dendritic cells and suppressed expression of costimulation factors
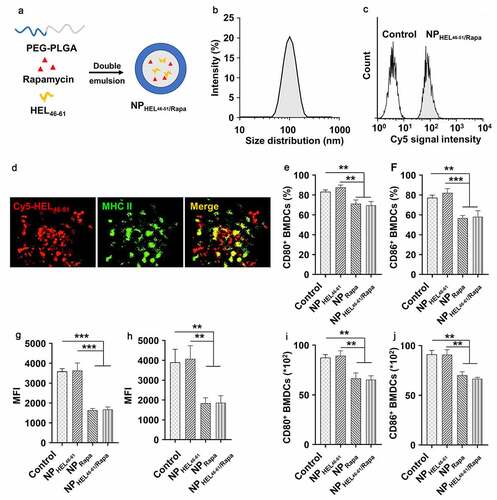
HEL46-61 and rapamycin were constructed following the protocol presented in ). DLS analysis demonstrated that synthesized nanoparticles showed an average size of 100 nm ()). These NPs had similar look under transmission electron microscope (TEM) (Fig.S1). To detect whether BMDCs can successfully uptake the synthesized nanoparticle, we treated BMDCs with NPs and analyzed the BMDCs by flow cytometry. As shown in , we detected Cy5 positive cells, indicating BMDCs engulfed the NPs containing Cy5-labeled HEL46-61. The uptake of NPs by BMDCs was further confirmed by immunofluorescence assay. As shown in , we detected obvious co-localization of MHCII and Cy5-labeled HEL46-61, which indicated the HEL46-61 was loaded on and presented by the MHCII molecules. We further analyzed the effects of NP uptake on CD80 and CD86 expression in BMDCs by flow cytometry. The CD11b+ CD11c+ cells were gated and analyzed (Fig.S2). The uptake of control NPs or NPs only containing HEL46-61 (NPHEL46-61) did not affect the expression of co-stimulation molecules CD80 () and CD86 (). In contrast, uptake of NPs containing rapamycin (NPRapa) and NPs containing rapamycin and HEL46-61 (NPHEL46-61/Rapa) resulted in the decreased expression of CD80 () and CD86 () in BMDCs. Similar trends could be found in the percentages and absolute number of CD80+ (), CD86+ () BMDCs after the treatment with NPRapa and NPHEL46-61/Rapa. In addition, we detected significantly decreased CD80+ and CD86+ cell population in BDMCs which uptake NPRapa or NPHEL46-61/Rapa (Fig.S3). These results indicated that rapamycin inhibited the expression of co-stimulation molecules.
Figure 1. Nanoparticles containing autoantigen (HEL46-61) and rapamycin induced tolerogenic DCs. (a) PEG-PLA nanoparticles containing autoantigen (HEL46-51) and rapamycin were constructed via double emulsion method. (b) Size distribution of NPHEL46-51/Rapa. (c) Uptake of NPHEL46-51/Rapa by mouse bone marrow dendritic cells (BMDCs). HEL46-51 was labeled with Cy5. (d) Colocalization of Cy5-HEL46-51 peptide (red) and MHC II molecules (green) in BMDCs at 24 h after the transfection of the cells with NPHEL46-51/Rapa. The percentages of CD80+ (e) and CD86+ (f) BMDCs post different treatments. The mean fluorescence intensity (MFI) of CD80 (g) and CD86 (h) on the surface of NPHEL46-51/Rapa-treated BMDCs. The absolute number of CD80+ (i) and CD86+ (j) BMDCs after different treatments. These experiments were repeated three times independently with similar results. Data were presented as mean ± SD and the statistical difference was calculated using one-way ANOVA with a Tukey’s post hoc test **p < .01, ***p < .001
NPHEL46-61/Rapa -treated BMDCs suppressed CD4± T cell proliferation and induced Treg differentiation
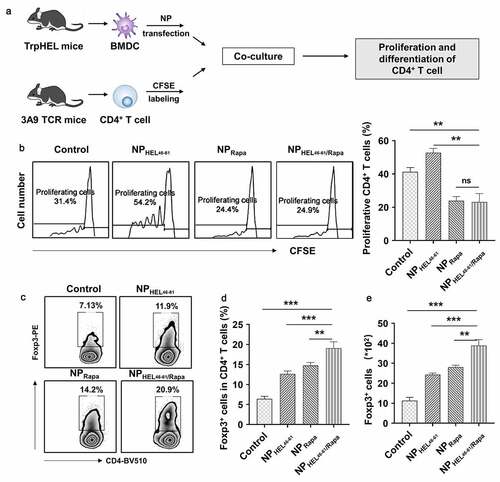
It has been described that rapamycin-treated DCs inhibited CD4+ T cell growth and promoted the induction of Treg cells.Citation23,Citation24 Therefore, we evaluated the effects of NPHEL46-61/Rapa-treated BMDCs on proliferation and differentiation of HEL46-61 specific CD4+ T cell from 3A9 mice (). Co-culture of NPHEL46-51 treated BMDCs from TrpHEL mice with HEL46-61 specific CD4+ T cell from 3A9 TCR mice resulted in increased percentage of proliferating HEL46-61 specific CD4+ T cells (). In contrast, co-culture of NPHEL46-61/Rapa or NPRapa -treated BMDCs from TrpHEL mice with CD4+ T cell from 3A9 TCR mice resulted in significantly decreased percentage of proliferating CD4+ T cell in total BMDC population, indicating NPRapa-treated BMDCs suppressed CD4+ T proliferation (). In addition, co-culture of NP-treated BMDCs from TrpHEL mice with CD4+ T cell from 3A9 TCR mice resulted in significantly increased percentage of Foxp3+ CD4+ T cells (). More importantly, significantly increased percentage () and absolute numbers () of Foxp3+ CD4+ T cells were observed in CD4+ T cell co-cultured with NPRapa-treated BMDCs, or CD4+ T cell co-cultured with NPHEL46-61/Rapa-treated BMDCs, indicating BMDCs treated with NPs rapamycin containing promoted the differentiation of Foxp3+ CD4+ T cells (Tregs). In addition, CD4+ T cells co-cultured with NPHEL46-61/Rapa-treated BMDCs had the lowest level of Fas (Fig.S4A), the lowest MFI of FITC-anti-Fas on BMDCs (Fig.S4B) and the fewest percentages of Fas+ cells (Fig.S4C), when compared to CD4+ T cells co-cultured with other NP-treated BMDCs, suggesting less CD4 T cell-dependent autoimmunity against melanocytes. Taken together, our data demonstrated that NPHEL46-61/Rapa-treated BMDCs suppressed CD4+ T cell proliferation and induced Treg differentiation in vitro.
Figure 2. NPHEL46-61/Rapa -treated BMDCs suppressed antigen-specific CD4+ T cell proliferation and induced Treg differentiation. (a) Schematic illustration showing how the ability of NPHEL46-51/Rapa-treated DCs to induce the proliferation and differentiation of CD4+ T cells. (b) The proliferation of CD4+ T cells after coculture with treated BMDCs was examined by CFSE (Carboxyfluorescein diacetate succinimidylester) T cell proliferation assay. Flow cytometric analysis (c) and statistical data obtained in three replicate experiments in which the percentages of Treg cells (CD4+Foxp3+) in CD4+ T cells (d) and the absolute numbers and the percentage of Foxp3+ CD4 T cells (e) after coculture were determined. These experiments were repeated three times independently with similar results and the statistical difference was calculated using one-way ANOVA with a Tukey’s post hoc test. Data were presented as mean ± SD. n = 3, *p < .05, **p < .01, ***p < .001. ns, no significant difference
NPHEL46-61/Rapa ameliorated vitiligo and promoted Treg in TCR-TrpHEL mice
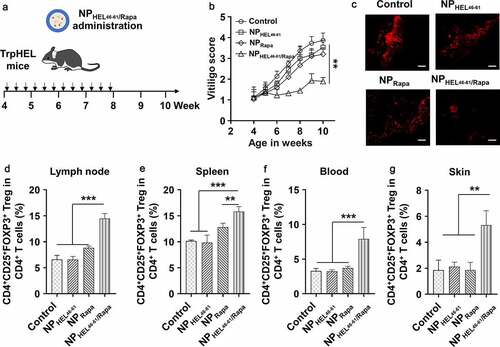
As NPHEL46-61/Rapa suppressed HEL46-61 specific CD4+ T cell proliferation while promoted Treg differentiation in vitro, we continued to investigate the effects of NPHEL46-61/Rapa on vitiligo in vivo using TCR-TrpHEL mice (). For control TCR-TrpHEL mice without NP treatment, increased vitiligo score was observed with time increased () and obvious vitiligo was observed in mice (). NPHEL46-61 or NPRapa did not obviously affect the vitiligo as we detected similar vitiligo scores and vitiligo appearance in mice treated with NPHEL46-61 or NPRapa at each time point. In contrast, mice treated with NPHEL46-61/Rapa had decreased vitiligo score and less vitiligo when compared to control mice or mice treated with NPHEL46-61 or NPRapa (). We further analyzed the Treg population in different tissues of mice after NP treatment using gating strategy described in Fig.S5. We detected significantly increased percentage of CD4+CD25+FOXP3+ Treg in CD4+ T cells from lymph node (), spleen (), blood () and skin () of TCR-TrpHEL mice treated with NPHEL46-61/Rapa, while control mice and mice treated with NPHEL46-61 or NPRapa had similar percentage of Treg cells in CD4+ T cells from lymph node (), spleen (), blood () and skin (). Collectively, our data demonstrated that NPHEL46-61/Rapa ameliorated vitiligo and promoted antigen-specific Tregs in TCR-TrpHEL mice.
Figure 3. NPHEL46-61/Rapa ameliorated vitiligo and promoted Treg in TCR-TrpHEL mice. (a) Therapeutic scheme. TrpTEL mice were treated with NPHEL46-61/Rapa or other formulations at age of four weeks. Mice were treated three times per week for 4 weeks, and the vitiligo scores were recorded. (b) Weekly vitiligo scores of transgenic TCR-TrpHEL mice. (c) The representative staining of destroyed melanocytes for each type of treatment. Representative images of mice with vitiligo post treatment. Active caspase-3 (red; cytoplasmic) as an apoptosis indicator. Scale bar, 50 μm. Percentages of CD4+CD25+FOXP3+ Treg in CD4+ T cells in lymph (d), spleen (e) and blood (f) and skin (g) at 10th week. Data represent means ± SD. Statistical difference was calculated using one-way ANOVA with a Tukey’s post hoc test for the data in C-G, and two-way ANOVA with a Dunnett’s post hoc test for the data in B. n = 6, **p < .01, ***p < .001. ns, no significant difference
NPHEL46-61/Rapa enhanced IL-10 expression and suppressed IFN-γ and IL-6 expression in TCR-TrpHEL mice
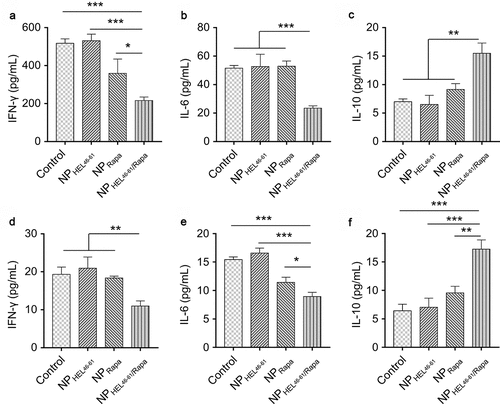
We evaluated the cytokine expression after NPs treatment. There was no significant difference of IFN-γ () and IL-6 () level in lymph node among control mice, mice treated with NPHEL46-61 and mice treated with NPRapa. In contrast, we detected significantly decreased IFN-γ () and IL-6 () in lymph node of mice treated with NPHEL46-61/Rapa when compared to other three groups of mice. Correlatively, the IL-10 level in lymph node of mice treated with NPHEL46-61/Rapa was significantly higher than that in lymph node of control mice, mice treated with NPHEL46-61 or mice treated with NPRapa (). Similarly, we detected significantly decreased IFN-γ () and IL-6 () and significantly increased IL-10 () in spleen of mice treated with NPHEL46-61/Rapa when compared to other three groups of mice. Taken together, our data demonstrated that NPHEL46-61/Rapa enhanced IL-10 expression and suppressed IFN-γ and IL-6 expression in TCR-TrpHEL mice.
Figure 4. NPHEL46-61/Rapa enhanced IL-10 expression and suppressed IFN-γ and IL-6 expression in TCR-TrpHEL mice. (a–c) ELISA analysis of the production of IFN-γ (a), IL-6 (b) and IL-10 (c) in lymph nodes of TrpTEL mice treated with different formulations. (d–f) ELISA analysis of the production of IFN-γ (d), IL-6 (e) and IL-10 (f) in the spleens of TrpTEL mice treated with different formulations. Data represent means ± SD and the statistical difference was calculated using one-way ANOVA) with a Tukey’s post hoc test. n = 6, *p < .05, **p < .01, ***p < .001. ns, no significant difference
Discussion
In the present study, we synthesized NPs containing rapamycin and HEL46-61 peptide (NPHEL46-61/Rapa) and investigated its effects on vitiligo using a TrpHEL mice model. We demonstrated that NPHEL46-61/Rapa could be successfully uptaken by BMDCs from TrpHEL mice, which resulted in decreased expression of costimulation molecules CD80 and CD86 in BMDCs. BMDCs treated with NPHEL46-61/Rapa suppressed the proliferation of HEL46-61 specific CD4+ T cells and promoted Treg differentiation in vitro. Administration of NPHEL46-61/Rapa ameliorated the vitiligo in TrpHEL mice by promoting Treg production. Our data demonstrated that NPHEL46-61/Rapa could be used as a potential therapeutic approach to treat vitiligo.
Vitiligo is an autoimmune disease which results from loss of melanocytes. Multiple components including autoimmunity, intrinsic melanocyte defects and environmental factor have been implicated in vitiligo. T cell-mediated immune responses are strongly implicated in pathogenesis of vitiligo. CD8+ T cells recognize melanocyte antigens and expressed high levels of perforin and granzyme, resulting in melanocyte destruction. Van den Boorn and colleagues isolated the CD8+ T cells directly from lesional vitiligo skin and found these CD8+ T cells migrated into the epidermis of normal skin and induced melanocyte apoptosis, suggesting CD8+ T cells were sufficient to destruct melanocyte in human vitiligo.Citation25 The role of CD4+ T cells in vitiligo was also described. A dominant subset of CD4+IFNγ+ CD4 cells was identified in vitiligo and the secreted IFN-γ affected melanocyte activity and caused apoptosis of melanocyte.Citation26 Lambe et al. established a TrpHEL mice model in which membrane-bound HEL was expressed in melanocytes under the control of TRP-2 promoter. These mice were then crossed to 3A9 CD4+ TCR mice, which had T cells recognizing HEL46-61 peptide. The TCR-TrpHEL mice developed depigmentation of hair. In the present study, we utilized this mice model and observed the depigmentation of hair at age of 4 weeks.
Tregs constitute up to 10% of T cells in the normal human skin and play an important role in immunological tolerance. In contrast, few Tregs were found in vitiligo skin.Citation27 Tregs suppress immune cells and secreting inhibitory cytokines including IL-10 and transforming growth factor-beta.Citation28 Using transgenic mice h3TA2 which carried T cells with a HLA-A2 restricted human tyrosinase reactive TCR and develop spontaneous vitiligo from an early age, Chatterjee et al. reported that adoptive transfer Tregs induced remission of vitiligo. In addition, they found that in IFN-γ knockout h3TA2 mice, the depigmentation was significantly reduced, suggesting a central role for IFN-γ in vitiligo development. Therefore, it could be a potential therapeutic approach for vitiligo treatment via promoting Tregs.
Tolerogenic dendritic cells have been used to efficiently expand antigen-specific Tregs in immunological disorder.Citation13,Citation29 Compared to conventional stimulatory DCs, tolerogenic DCs were without concomitant continuation. Because of inherent capacity to target antigen-presenting cells, nanoparticles have emerged as powerful tools to initiate and modulate immune response. In the present study, we demonstrated that NPs containing rapamycin uptaken by dendritic cells resulted in decreased surface expression of costimulation factors CD80 and CD86. Rapamycin was widely used as an immunosuppressive agent. It was described that human DCs treated with rapamycin had decreased expression of CD80, CD86 and CD40.Citation30 Our findings in the current study were consistent to previous finding, suggesting rapamycin promoted DCs to a tolerogenic phenotype. Correspondingly, dendritic cells treated with NPs containing rapamycin inhibited CD4+ T cell proliferation. Rapamycin was also shown to promote expansion of Tregs,Citation31 which was confirmed in our present study in vitro and in vivo.
Interleukin-10 was an immunoregulatory cytokine which was important in autoimmunity. The IL-10 production was associated with Tregs.Citation32 As we detected increased Tregs in different organs in mice treated with NPHEL46-61/Rapa, significantly increased IL-10 expression was detected correspondingly. Due to the regulatory activity of IL-10,Citation33 decreased IL-6 and IFN-γ were detected.
Taken together, we demonstrated that NPs containing autoantigen and rapamycin could be used as an effective approach to promoted antigen-specific Tregs, which resulted in suppression of autoimmune response and amelioration of autoimmune diseases. However, in the present study, the mice were analyzed 6 weeks after NP treatment, which only reflected the short-term effects of NP. It could be useful to evaluate the long-term effects of NPs on vitiligo. Relative experiments should be carried out for such purpose.
Conclusions
Polymeric nanoparticles containing rapamycin and autoantigen induce antigen-specific immunological tolerance for preventing vitiligo.
Disclosure of potential conflict of interest
The authors declare that they have no conflict of interest.
Supplemental Material
Download MS Word (915.4 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Linthorst Homan MW, Spuls PI, de Korte J, Bos JD, Sprangers MA, van der Veen JP. The burden of vitiligo: patient characteristics associated with quality of life. J Am Acad Dermatol. 2009;61:411–20.
- Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473–91. doi:10.1016/j.jaad.2010.11.061.
- Grimes PE. White patches and bruised souls: advances in the pathogenesis and treatment of vitiligo. J Am Acad Dermatol. 2004;51:S5–7. doi:10.1016/j.jaad.2004.01.007.
- Frisoli ML, Harris JE. Vitiligo: mechanistic insights lead to novel treatments. J Allergy Clin Immunol. 2017;140:654–62. doi:10.1016/j.jaci.2017.07.011.
- Schallreuter KU, Bahadoran P, Picardo M, Slominski A, Elassiuty YE, Kemp EH, et al. Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else? Exp Dermatol. 2008;17:139–40; discussion 41–60.
- Glassman SJ. Vitiligo, reactive oxygen species and T-cells. Clin Sci (Lond). 2011;120:99–120. doi:10.1042/CS20090603.
- Lambe T, Leung JC, Bouriez-Jones T, Silver K, Makinen K, Crockford TL, Ferry H, Forrester JV, Cornall RJ. CD4 T cell-dependent autoimmunity against a melanocyte neoantigen induces spontaneous vitiligo and depends upon Fas-Fas ligand interactions. J Immunol. 2006;177:3055–62. doi:10.4049/jimmunol.177.5.3055.
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–38. doi:10.1038/nature04753.
- Dwivedi M, Kemp EH, Laddha NC, Mansuri MS, Weetman AP, Begum R. Regulatory T cells in vitiligo: implications for pathogenesis and therapeutics. Autoimmun Rev. 2015;14:49–56. doi:10.1016/j.autrev.2014.10.002.
- Le Poole IC, Mehrotra S. Replenishing regulatory T cells to halt depigmentation in vitiligo. J Investig Dermatol Symp Proc. 2017;18:S38–S45. doi:10.1016/j.jisp.2016.10.023.
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi:10.1146/annurev.immunol.21.120601.141040.
- Cronin SJ, Penninger JM. From T-cell activation signals to signaling control of anti-cancer immunity. Immunol Rev. 2007;220:151–68. doi:10.1111/j.1600-065X.2007.00570.x.
- Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–65.
- Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–40. doi:10.1016/S0009-9120(98)00045-9.
- Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce ag-specific T cell regulation and prolong graft survival. Am J Transplant. 2005;5:228–36. doi:10.1046/j.1600-6143.2004.00673.x.
- Mauricio MD, Guerra-Ojeda S, Marchio P, Valles SL, Aldasoro M, Escribano-Lopez I, Herance JR, Rocha M, Vila JM, Victor VM, et al. Nanoparticles in medicine: a focus on vascular oxidative stress. Oxid Med Cell Longev. 2018;2018:6231482. doi:10.1155/2018/6231482.
- Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, Wang J, Garabatos N, Izquierdo C, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530(7591):434–40. doi:10.1038/nature16962.
- Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, Griset AP, O’Neil C, Altreuter DH, Browning E, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci U S A. 2015;112:E156–65. doi:10.1073/pnas.1408686111.
- Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. J Biomater Sci Polym Ed. 2006;17:247–89. doi:10.1163/156856206775997322.
- Lim J, Yeap SP, Che HX, Low SC. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res Lett. 2013;8:381. doi:10.1186/1556-276X-8-381.
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi:10.1084/jem.176.6.1693.
- Lee WT, Pasos G, Cecchini L, Mittler JN. Continued antigen stimulation is not required during CD4(+) T cell clonal expansion. J Immunol. 2002;168:1682–89. doi:10.4049/jimmunol.168.4.1682.
- Dong M, Wang X, Liu J, Zhao YX, Chen XL, Li KQ, Li G. Rapamycin combined with immature dendritic cells attenuates obliterative bronchiolitis in trachea allograft rats by regulating the balance of regulatory and effector T cells. Int Arch Allergy Immunol. 2015;167:177–85. doi:10.1159/000437207.
- Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–31. doi:10.4049/jimmunol.178.11.7018.
- van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, Vyth-Dreese FA, Luiten RM. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009;129(9):2220–32. doi:10.1038/jid.2009.32.
- Ala Y, Pasha MK, Rao RN, Komaravalli PL, Jahan P. Association of IFN-gamma: IL-10 cytokine ratio with nonsegmental vitiligo pathogenesis. Autoimmune Dis. 2015;2015:423490. doi:10.1155/2015/423490.
- Klarquist J, Denman CJ, Hernandez C, Wainwright DA, Strickland FM, Overbeck A, Mehrotra S, Nishimura MI, Le Poole IC. Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res. 2010;23:276–86. doi:10.1111/j.1755-148X.2010.00688.x.
- Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Front Immunol. 2012;3:51. doi:10.3389/fimmu.2012.00051.
- Yamazaki S, Inaba K, Tarbell KV, Steinman RM. Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity. Immunol Rev. 2006;212:314–29. doi:10.1111/j.0105-2896.2006.00422.x.
- Monti P, Mercalli A, Leone BE, Valerio DC, Allavena P, Piemonti L. Rapamycin impairs antigen uptake of human dendritic cells. Transplantation. 2003;75:137–45. doi:10.1097/00007890-200301150-00025.
- Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–47. doi:10.4049/jimmunol.177.12.8338.
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi:10.1146/annurev.immunol.19.1.683.
- Hempel L, Korholz D, Bonig H, Schneider M, Klein-Vehne A, Packeisen J, et al. Interleukin-10 directly inhibits the interleukin-6 production in T-cells. Scand J Immunol. 1995;41:462–66. doi:10.1111/j.1365-3083.1995.tb03593.x.
