ABSTRACT
There has been a considerable controversy about vaccination practices in Children with congenital heart disease (CHD) in China. This study aims to identify the reasons for deferring vaccination among the patient population attending the Vaccination Consultation Clinic in Zhejiang Province and the safety of their vaccination. We analyzed the data of 2442 children with CHD, who visited to our clinic from January 2016 to March 2019. A questionnaire survey was conducted to investigate the reasons for their delayed vaccination. Information about the following vaccination and Adverse Events Following Immunization (AEFI) was collected. Most of the enrolled children did not receive vaccines on time before consultation. The reasons for their deferring vaccination included: 1. Providers in the community health center refused to administer vaccines (77.6%); 2. Parents’ concerns about the safety of vaccines (19.0%); 3. Parents’ doubts about the efficiency of vaccines after certain drug applications (3.4%). According to the evaluation reports issued by the Vaccination Consultation Clinic, 83.7% of CHD children were recommended to be vaccinated on the nationally recommended schedule, 14.4% were recommended to defer some specific vaccination, and 1.9% were recommended to defer all vaccination. Among the group who received vaccines on nationally recommended schedule, the AEFI rate was 33.5/100 000. No rare or serious rare vaccine reactions were observed. Our study provides evidence that routine vaccination is safe in the majority of this patient population.
Introduction
Congenital heart disease (CHD) is one of the most common birth defects in newborns. It affects 6–8 infants in every 1000 live birth,Citation1 with about half of them requiring cardiac surgery or catheter interventions.Citation2 Children with CHD have an increased risk of mortality and morbidity because of their underlying cardiopulmonary compromise. Once they are infected, their symptoms can be more severe than those of healthy children. Despite the improved prognosis, there is still a higher risk of severe infectious disease in these children, which may extend their length of hospitalization for respiratory complications.Citation3 It is reported that infectious diseases like pneumonia, influenza and RSV infections present a major source of morbidity and mortality for children with CHD under age of five,Citation4,Citation5 especially for those awaiting surgery and admitted to the PICU with acute illness.Citation6 Therefore, avoiding infection is vitally important for this patient population.
For public health, vaccination is the most effective means to prevent infectious diseases and their complications. Health-care systems worldwide place great emphasis on the immunization for high-risk population, clearly including children with cardiovascular disease.Citation7 However, the vaccination coverage among them is suboptimal.Citation8,Citation9 For instance, an Israel study reported the influenza vaccination rate was only about 36.6%,Citation10 and a Mexico study reported a 56.3% coverage rate for the pneumococcal conjugate vaccines.Citation11
In China, the public highly concern about the coincidental events after vaccination and the serious/rare adverse events following immunization (AEFI). Safety concerns about vaccination, coupled with some occasional medical disputes led to parents’ and providers’ hesitation on vaccination ultimately affect the timely vaccination rate among children with special health-care needs (CSHCN), including patients with CHD.Citation12,Citation13 So far, no epidemiologic survey has been performed to investigate the vaccination status of children with CHD and the safety of their vaccination. In order to have a better understanding of the attitudes, beliefs, and behaviors of parents and providers influencing vaccination of the target population, we conducted the present study. To our knowledge, this is the first large sample study in China regarding the vaccination among children with CHD. The result of the study can help us identify the critical reasons for the delayed vaccination, and further provide medical evidence for exploring new strategies related to vaccination in this patient population.
Materials and methods
Study setting and design
To provide guidance on vaccination and increase the vaccination rate among CSHCN, the Vaccination Consultation Clinic was set up in Children’s Hospital Zhejiang University School of Medicine, Hangzhou, Zhejiang, China in 201612. Most of the patients visiting the Clinic were introduced by the vaccination providers in the community health centers, some others were introduced by pediatricians or come to the clinic on their own due to their concerns about the vaccination.
In the current study, we mainly focused on children with CHD, expecting to identify the reasons for their vaccination deferral and the safety of their vaccination. As described in our earlier publication,Citation12 the team of pediatricians in the clinic would issue a Vaccination Assessment Report on vaccination recommendation for each patient, based on his/her clinical information and referring to “Chinese Immunization Program for Children Immunization Procedures and Instructions (2016)”Citation14 “Chinese Pharmacopeia”, “Consensus Recommendation on Vaccination for Children with Congenital Heart Disease (China)”Citation15 and “General Best Practice Guidelines for Immunization (2019)”.Citation16 The nationally recommended vaccination schedule in Zhejiang, China is shown in .
Table 1. Vaccination schedule used in Zhejiang, China
The vaccination recommendation included three different types: (1) children who were well grown and had normal cardiac function, or who had recovered well after catheter interventions or cardiac surgery, were recommended to receive vaccines on the nationally recommended schedule; (2) children with immunodeficiency, severe infection, severe malnutrition, or those who had used certain drugs such as antibody containing products or immunosuppressed drugs, were recommended to delay some specific vaccination (e.g. Measles-Mumps-Rubella Vaccine); (3) children with heart failure, severe pulmonary hypertension, or those who had complex lesions and were in need of multiple surgical procedures, were recommended to defer all vaccination.
Study population
In the present study, children with CHD attending at the Consultation Clinic from January 1, 2016 to March 31, 2019 were enrolled. The main diagnostic tool for CHDs is echocardiography. The clinical diagnosis was confirmed by a specialty trained echocardiographic doctor and a pediatric cardiologist based on the Chinese National Criteria of Birth Defects and Tiny Deformities before consultation. CHDs were coded according to the modified codes from the International Classification of Disease, tenth edition (ICD-10: Q20.000–28.000). Children whose parents unwilling to be followed up were excluded from the study.
Data collection
Information of medical records, laboratory results, past medical history, allergic history, family history, as well as their previous vaccination and AEFI history were collected through one-on-one interviews with parents at the first visit. In addition, parents were also asked to complete a questionnaire covering basic demographic information and the reasons for vaccination deferral.
After their first visits to the Consultation Clinic, parents would have their children vaccinated following the individualized vaccination recommendations. Each child was required to have a 6-month follow-up after consultation. For those who did not re-visited on time, a telephone follow-up will be arranged by a designated nurse. Immunization records and data of AEFI for the next 6 months were collected according to the records in the vaccination booklets during the subsequent visits or by telephone interviews. All the information in vaccination booklets is synchronized with the Zhejiang Provincial Immunization Information System and the Chinese National Adverse Event following Immunization Information System. The two systems are the official information systems that contain immunization data of Zhejiang province and information of AEFI cases in mainland China, respectively.
Statistical analysis
Analyses were performed by SPSS for Windows, Version 20.0. Descriptive statistics with simple frequencies and percentages were used. The frequency of the proposed reasons for vaccination deferral and the reported AEFI was calculated. Chi-square was used to compare the proportion of vaccination recommendations between children with different types of CHD, as well as to compare between those with or without cardiac surgeries. The level of statistical significance was set at P < .05.
Ethics statement
The procedures and protocols of this study were approved by the Ethics Committee of the Children’s Hospital Zhejiang University School of Medicine. The Informed Consent was waived by the Ethics Committee of the Children’s Hospital Zhejiang University School of Medicine (2018-IRB-105).
Results
Sample demographics
Data of 2442 children with CHD were collected and used for analysis. 69.0% (1685) of them were under the age of 7 months; half (51.4%, 1256) were males; 27.5% (672) had a history of non-vaccine-related allergy. 81.4% (1987) of the children were screened out to have CHD during the neonatal period. The main type of CHD consulted was left-to-right shunt defect (94.0%, 2295), followed by right-to-left shunt defect (3.0%, 74) and non-shunt defect (3.0%, 73); 0.1% (2) were diagnosed as DiGeorge syndrome. Among them, 7.0% (171) had a history of cardiac surgery. The median value of white blood cell and neutrophils in the last blood routine test were 8.2 × 109(IQR: 6.8–9.8) and 2.1 × 109/L(IQR: 1.5–2.9), respectively ().
Table 2. Sample characteristics (N = 2442)
We checked patients’ vaccination booklets at their first visits to the consultation clinic. Among them, 89.4% had received one dose of hepatitis B (Hep B), 53.7% had two doses, and only 34.2% had three doses. 23.3% had received bacilli Calmette-Guérin vaccine (BCG). 10.6% of them had never been previously vaccinated ().
Table 3. Vaccination of Hep Ba and BCGb before consultation
Reasons for deferring vaccination among CHD children
Among 2442 children, a total of 1979 (81%) parental surveys were eligible and used for analysis, while 463 (19%) were excluded because of parents’ refusal to participate (11.3%, 277) or the information incompletely provided on the questionnaire (7.6%, 186).
As revealed by the analysis, the major reason of the vaccination deferral was that providers in community health center refused to vaccinate children with CHD (77.6%, 1536). Other reasons included parents’ fear of side effects of vaccines (19.0%, 376), as well as their doubt about the efficiency of vaccines after drug applications (3.4%, 67).
Vaccination recommendation among CHD children
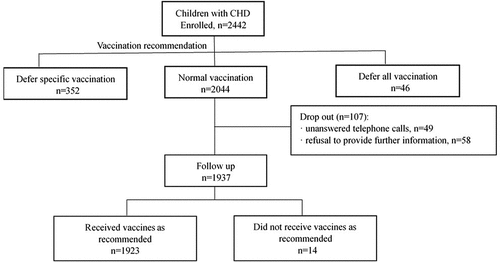
Based on the vaccination assessment reports issued by the consultation clinic, 2044 CHD children (83.7%) were recommended to receive vaccines on the nationally recommended schedule, 352 (14.4%) were recommended to defer some specific vaccination, only 46 (1.9%) were recommended to defer all vaccination.
By further analyzing the part who were recommended to “defer specific or all vaccination”, we found that, in terms of different types of CHD, the proportion of right-to-left shunt defect (53, 71.6%) was the highest among the children who were evaluated to be “defer specific or defer all vaccination”, followed by left-to-right shunt type (336, 14.6%) and non-shunt type (9, 12.3%) (p < .001); In terms of cardiac surgery history, the proportion of CHD children undergone surgery was comparatively higher than that of those without (29.8%, 51 vs. 15.3%, 347, p < .001). ()
Table 4. Vaccination recommendation of different groups
Subsequent vaccination and incidence of AEFI
Among 2044 CHD children who were recommended to receive vaccination on schedule, 107 (5.2%) children dropped-out due to unanswered telephone calls (2.4%, 49) or refusal to provide further information (2.8%, 58). Of the remaining 1937 (94.8%) CHD children, 1923 (99.3%) followed the vaccination recommendation and received vaccines in time; 14 (0.7%) had not been vaccinated as recommended due to some moderate to severe infectious diseases or after using some antibody-containing products ().
For the 1923 valid cases, a total of 32877 vaccine doses were administered. Only 11 cases experienced mild and self-limiting adverse reactions (33.5/100,000), including 1 case of irritability, 5 cases of low-grade fever, 3 cases of endemic induration, and 2 cases of local redness and swelling at the injection site. It was consistent with the national level in 2016 (33.5/100,000 vs. 36.06/100,000).Citation17 No uncommon or serious side effects were reported ().
Table 5. Immunization and occurrence of AEFI after consultation
Discussion
A total of 2442 children with CHD were observed in the cohort analysis. The majority was left-to-right shunt; and mostly had not been timely vaccinated before consultation. Through questionnaire analysis, we found that the vaccine providers’ refusal to vaccinate in community health center was a major reason to the low vaccination rate among these vulnerable children. However, indeed, most of this patient population can be vaccinated safely on the nationally recommended schedule after professional evaluation.
In our sample, more than 2/3 of the children with CHD were up to 7 months of age. By reviewing their medical records, we noted that most of them were diagnosed in the neonatal period, while some were diagnosed during their routine physical examination after birth. The early diagnosis is probably due to the regular application of prenatal ultrasound and neonatal cardiac ultrasound examination in our country. Besides, a majority of children enrolled were left-to-right shunt CHD, such as atrial septal defect, ventricular septal defect and patent ductus arteriosus. They were advised by the cardiologist to follow up in the cardiology clinics regularly with no need for surgery; only 7.0% had a history of cardiac surgery. It implies that, for most of the children consulted, their heart abnormalities were mild and would require little early intervention.
BCG and Hepatitis B vaccines are the first two vaccines to be administered according to the routine vaccination schedule. In our sample, nearly 10% did not receive any vaccines, and only 23.3% received BCG before consultation. Although the coverage of the first dose of hepatitis B vaccine was comparatively high, the following vaccination rates gradually decreased with their age. For other scheduled vaccines, like polio vaccine, acellular pertussis diphtheria vaccine and meningococcal meningitis et.al, the coverage was much lower than that of Hepatitis B vaccine (data not shown). These results indicate the fact that the vaccination coverage among children in our cohort was considerably low. It is similar to the studies from other countries relating to the vaccination of children with cardiac diseases.Citation3,Citation5,Citation10
According to the parental reports, about three quarters of the children with CHD were refused to be vaccinated by providers at community health center. Even those whose defects had self-healed or only had mild valve regurgitation were often refused. Providers naturally classify children with CHD as a group who are unfit to be vaccinated for the sake of safety.Citation12 For one thing, heart disease, as a vaccine contraindication, its definition and classification are not clearly stated in the vaccination instruction. For another, lack of clinical experience on heart diseases also makes providers in the community health centers hesitate to vaccinate these vulnerable children.
Besides, the rest of parents reported that they did not have their children vaccinated due to their concerns on safety and efficiency of vaccines. They believed that CHD is a kind of serious disease and vaccines may aggravate their children’s disease. And they only had providers in the community health centers as the source of information on their children’s vaccination. To some extent, those parents’ ambiguous attitude toward vaccination caused their children not to be vaccinated on time. This is also the case in other chronic diseases cohorts reported in previous studies.Citation18,Citation19
On the basis of vaccination evaluation issued by the specialists of the Vaccination Consultation Clinic, a large part of children with CHD were recommended to receive vaccines on the nationally recommended schedule. Through our clinical review of the results of echocardiogram, electrocardiogram, blood routine test, as well as the previous medical records and family history of each child, we found that most of them did not have any clinical manifestation or family history of immunodeficiency, except for some syndromes that result in immunocompromise, like DiGeorge syndrome. It indicates that children with CHD do not have an increased risk of immunodeficiency.Citation20,Citation21 In addition, only a small number of children were recommended to defer vaccination, including those who had right-to-left shunt or complex lesions accompanied by severe malnutrition or recurrent respiratory infections, as well as those with cardiac dysfunction or severe pulmonary hypertension. Due to the lack of evidence-based medicine support for the safety of vaccination among them, we conservatively recommended them to defer vaccination according to the consensus of domestic experts.Citation15,Citation22 Based on the clinical analysis, we believe that CHD without cardiac dysfunction or immunodeficiency were overestimated as contraindication for vaccination.
After the consultation, we followed up the children who were advised for routine vaccination on nationally recommended schedule. We found that the majority of them were vaccinated as recommended. This result indicates that the recommendation of pediatrician is an important factor in parents’ decision on vaccination. The specialists’ advice and complete explanation helped parents to have a better understanding of vaccination and increase their confidence in the safety of vaccination. As a result, parents would be more likely to have their children vaccinated. With the recommendation and encouragement from experts, the vaccination rate among the vulnerable children can be increased, and the incidence of infectious diseases can be decreased.Citation23,Citation24
Within this cohort, the total estimated AEFI rate was comparable to that of general population in our country in 2016.Citation17 No rare or serious rare vaccine reactions were reported. This result demonstrated that the majority of children with CHD in our cohort can be vaccinated without an increased risk of AEFI, especially for those with left-to-right shunt. This result supports our previous recommendation that many patients without cardiac dysfunction or immunodeficiency were overestimated as contraindication for vaccination; vaccination among children with CHD is safe.
Therefore, it is vitally important to explore interventions on vaccination among children with CHD in our future work. For parents, some education programs can be conducted to help them better understand the knowledge of the disease and the benefits of vaccination for children with CHD. For providers in the community health centers, more trainings are needed on the contraindications and precautions for vaccination. And for pediatricians and cardiologists, it is necessary to raise their awareness of the importance of vaccination in preventing disease in their patients.
The greatest advantage of our study was that it was the first large sample study in China regarding the reasons of the vaccination deferral as well as the safety of immunization among children with CHD. However, there are certain limitations to be considered. Firstly, it was conducted in a single pediatric center, instead of a multicenter study, there may be statistical bias in admission rate. Secondly, despite the large sample size, our study period is relatively short and there was a selection bias in the type of CHD in this study. Therefore, further well-designed longitudinal multicenter follow-up studies will be needed to expand the sample size of children with different types of CHD, to better monitor the long-term safety of vaccination among the target population and ultimately to determine if our results can be generalized.
Conclusion
In summary, most children with CHD who visited our Vaccination Consultation Clinic did not receive nationally recommended vaccines on schedule. The main reason for their delayed vaccination was providers’ refusal to give vaccines in the community health centers. Through our follow-up, we did not observe an increased risk of AEFI after receiving the nationally recommended vaccines. It provides evidence that the majority of the current population can be safely vaccinated according to the nationally recommended schedule. It is essential to continuously educate parents, providers and cardiologists about the importance and benefits of vaccination in children with CHD.
Author contributions
Chai Ji: Conceptualized and designed the study, reviewed and revised the manuscript; Mingyan Li conducted the analysis, interpreted the data, drafted the initial manuscript, and revised the manuscript; Yan Zeng collected data and carried out the initial analyses; Dan Yao and Xia Wang entered data, assisted with the analysis and contributed to the revision of the manuscript; Jie Shao critically reviewed and revised the manuscript. All authors: approved the final content of the manuscript and agree to be accountable for all aspects of the work. None of the authors declared a conflict of interest.
Disclosure of potential conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgments
We are grateful to the study families involved in the study for their effort to complete the surveys and interview, as well as the colleagues at Children’s Hospital of Zhejiang University School of Medicine (Liping Xu, liping Zou, Pingxu Zhang) for their dedication and assistance.
Additional information
Funding
References
- Zhao QM, Ma XJ, Ge XL, Liu F, Yan WL, Wu L, Ye M, Liang X-C, Zhang J, Gao Y, et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014;384:747–54. doi:10.1016/S0140-6736(14)60198-7.
- Blue GM, Kirk EP, Sholler GF, Harvey RP, Winlaw DS. Congenital heart disease: current knowledge about causes and inheritance. Med J Aust. 2012;197:155–59.
- de Souza RP, Ribeiro ALR, de Menezes SAF, Machado LFA. Incidence of respiratory syncytial virus infection in children with congenital heart disease undergoing immunoprophylaxis with palivizumab in Para state, north region of Brazil. BMC Pediatr. 2019;19:299. doi:10.1186/s12887-019-1681-6.
- Fallahzadeh MA, Abdehou ST, Hassanzadeh J, Fallhzadeh F, Fallahzadeh MH, Malekmakan L. Pattern of in-hospital pediatric mortality over a 3-year period at University teaching hospitals in Iran. Indian J Crit Care Med. 2015;19:311–15. doi:10.4103/0972-5229.158257.
- Woodward CS. Keeping children with congenital heart disease healthy. J Pediatr Health Care. 2011;25:373–78. doi:10.1016/j.pedhc.2011.03.007.
- Mohsin SS, Haque A, Shaikh AS, Bano S, Hasan BS. Outcome of infants with unrepaired heart disease admitted to the pediatric intensive care unit: single-center developing country perspective. Congenit Heart Dis. 2014;9:116–21. doi:10.1111/chd.12075.
- Doherty M, Schmidt-Ott R, Santos JI, Stanberry LR, Hofstetter AM, Rosenthal SL, Cunningham AL. Vaccination of special populations: protecting the vulnerable. Vaccine. 2016;34(52):6681–90. doi:10.1016/j.vaccine.2016.11.015.
- Hebert K, Marzouka G, Arcement L, Julian E, Cortazar F, Dias A, Tamariz L. Prevalence of vaccination rates in systolic heart failure: a prospective study of 549 patients by age, race, ethnicity, and sex in a heart failure disease management program. Congest Heart Fail. 2010;16:278–83. doi:10.1111/j.1751-7133.2010.00190.x.
- Rodriguez-Rieiro C, Dominguez-Berjon MF, Esteban-Vasallo MD, Sanchez-Perruca L, Astray-Mochales J, Fornies DI, Ordoñez DB, Jiménez-García R. Vaccination coverage against 2009 seasonal influenza in chronically ill children and adults: analysis of population registries in primary care in Madrid (Spain). Vaccine. 2010;28:6203–09. doi:10.1016/j.vaccine.2010.07.013.
- Livni G, Wainstein A, Birk E, Chodick G, Levy I. Influenza vaccination rate and reasons for nonvaccination in children with cardiac disease. Pediatr Infect Dis J. 2017;36:e268–e71. doi:10.1097/INF.0000000000001579.
- Solórzano-Santos F, Espinoza-García L, Aguilar-Martínez G, Beirana-Palencia L, Echániz-Avilés G, Miranda-Novales G. Pneumococcal conjugate vaccine and pneumonia prevention in children with congenital heart disease. Rev Invest Clin. 2017;69:270–73. doi:10.24875/ric.17002241.
- Li M, Ji C, Wang B, Yao D, Wang X, Zeng Y, Shao J. Incomplete vaccination among children with special health care needs in Zhejiang, China: analysis of retrospective data. Front Pediatr. 2019;7:173. doi:10.3389/fped.2019.00173.
- Liu SJ, Xu EP, Ding H, Liu Y, Du J, Zhang XP, Wang J, Che XR, Gu WW, Xu YY. A survey on the contraindications and precautions in vaccination among children. Prev Med. 2016;28:706–08.
- National Health Commission of the People’s Republic of China. National immunization program vaccine children’s immunization procedures and instructions; 2016. [accessed 2018 Oct 20]. http://www.nhc.gov.cn/jkj/s3581/201701/a91fa2f3f9264cc186e1dee4b1f24084.shtml.
- Lv HT, Zhu YH, Zhang J, Luan L, Wang XJ, Sun JJ, Liu F, Sun XD, Guo X, Ding H, et al. Consensus recommendation on vaccination for Children with special medical condition- congenital heart disease and vaccination. Chin J Pract Pediatri. 2019;34:2–4.
- Ezeanolue E, Hunter P, Kroger A, Pellegrini C General best practice guidelines for immunization. Best practices guidance of the Advisory Committee on Immunization Practices (ACIP). US Department of Health and Human Services, CDC; 2019.
- Xu DS, Li KS, Wu WD, Ye JK, Ji SS, Zheng JS, Cui J, Cao LS, Xiao QY. Analysis of surveillance data of suspected abnormal response to vaccination in China in 2016. Chin J Vaccines Immun. 2018;6:299–309.
- Printza N, Farmaki E, Bosdou J, Gkogka C, Papachristou F. Pandemic influenza A 2009 (H1N1) vaccination in high risk children with chronic renal diseases: acceptance and perceptions. Hum Vaccin. 2010;6:819–22. doi:10.4161/hv.6.10.12846.
- Smith M, Peacock G, Uyeki TM, Moore C. Influenza vaccination in children with neurologic or neurodevelopmental disorders. Vaccine. 2015;33:2322–27. doi:10.1016/j.vaccine.2015.03.050.
- Barry JC, Crowley TB, Jyonouchi S, Heimall J, Zackai EH, Sullivan KE, McDonald-McGinn DM. Identification of 22q11.2 deletion syndrome via newborn screening for severe combined immunodeficiency. J Clin Immunol. 2017;37:476–85. doi:10.1007/s10875-017-0403-9.
- Radford DJ, Thong YH. The association between immunodeficiency and congenital heart disease. Pediatr Cardiol. 1988;9:103–08. doi:10.1007/BF02083708.
- Diao LD. Practical vaccinology[M]. Shanghai (China): Shanghai Scientific and Technical Press; 2015. p. 99.
- Paterson P, Meurice F, Stanberry LR, Glismann S, Rosenthal SL, Larson HJ. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700–06. doi:10.1016/j.vaccine.2016.10.042.
- Takanashi M, Ogata S, Honda T, Nomoto K, Mineo E, Kitagawa A, Ando H, Kimura S, Nakahata Y, Oka N, et al. Timing of Haemophilus influenzae type b vaccination after cardiac surgery. Pediatr Int. 2016;58:691–97. doi:10.1111/ped.12899.