?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A national routine pneumococcal pneumonia immunization program started in Japan in 2014. It targeted the population aged ≥65 years and used a 23-valent pneumococcal polysaccharide vaccine; PPSV23. However, its effectiveness was not well defined because of the lack of a comprehensive database on the PPSV23 vaccination status of each subject. We used interrupted time-series analyses to assess the changes in the incidence and prognosis of elderly patients hospitalized for pneumonia before and after initiation of the program. First, we estimated the PPSV23 coverage rates in subjects aged ≥65 years based on the number of shipped PPSV23 syringes and the estimated population in each prefecture. The estimated coverage rates reached around 40% in 2014 for the 3 Tohoku prefectures, while those in the other prefectures remained below 20%. After the national routine immunization program started, the estimated coverage rate increased significantly in every prefecture and exceeded 40% in 2017. Next, we aggregated the data extracted from the Japanese Diagnosis Procedure Combination database from April 2011 through February 2017 for hospitalized pneumonia patients aged ≥65 years. The data included data from 655,746 patients, excluding those in the 3 Tohoku prefectures. Interrupted time-series analyses found no change in the incidence of hospitalized pneumonia patients and in-hospital mortality after the vaccination program, but there was a decrease in the in-hospital mortality of pneumonia patients with severe comorbidities defined by the modified Charlson comorbidity index. These results suggest an association between the vaccination program and an improved outcome in hospitalized elderly pneumonia patients with severe comorbidities in Japan.
Introduction
Pneumonia is the 5th leading cause of death in Japan, which is categorized as a super-aged society,Citation1 and a majority of the cases are aged ≥65 years.Citation1,Citation2 The incidences of community-acquired pneumonia (CAP) and nursing and health care–associated pneumonia (NHCAP) among elderly people in Japan are higher than in other developed countries.Citation2 Those incidences are associated with both increased mortality and a huge economic burden.Citation3 Prophylactic treatment is thought to be one of the most effective strategies for protecting older people as well as alleviating the economic burden by reducing the number of patients hospitalized for pneumonia.Citation3 In Japan, Streptococcus pneumoniae is the most common pathogen of CAP, accounting for approximately 20% of CAP cases,Citation1,Citation4 and it is also one of the main causes of NHCAP.Citation1,Citation5 Prophylaxis targeting Streptococcus pneumoniae in high-risk populations and the elderly is an important challenge.
For prophylaxis of pneumococcal diseases in adults in Japan, the most common agent used is the 23-valent pneumococcal polysaccharide vaccine (PPSV23), which covers approximately 60–80% of the pneumococcal serotypes in Japan.Citation6–8 It is widely accepted that PPSV23 prevents invasive pneumococcal disease (IPD) in immunocompetent adults as well as in immunocompromised adults and is also cost-effective, based on a number of clinical trials and meta-analyses.Citation9–12 Regarding nonbacteremic pneumonia in adults, however, the effectiveness of PPSV23 remains controversial. Prior vaccination with PPSV23 was associated with reduced mortality, complications and length of stay among hospitalized patients with CAP.Citation13 A prospective study showed serotype-specific effectiveness of PPSV23 against pneumococcal pneumonia in the elderly in Japan.Citation14 Targeting of nursing home residents and pneumococcal vaccination in combination with routine seasonal influenza virus vaccination are suggested to be useful strategies for maximizing the benefit of PPSV23 vaccination.Citation15,Citation16 However, there is controversy regarding the effectiveness of PPSV23 vaccination for improving the outcome of nonbacteremic pneumonia in adults.Citation10,Citation12,Citation17,Citation18 A report pointed out the limitations of one RCT studyCitation15 in terms of the internal and external validity.Citation19
There was no routine PPSV23 vaccination program except for those subsidized by local governments until 2014 in Japan. After the Great East Japan Earthquake in March 2011, fully subsidized PPSV23 vaccination for the population aged ≥65 years was initiated in the devastated Tohoku area: Iwate, Miyagi and Fukushima Prefectures. Following the PPSV23 vaccination program held in the Tohoku area, a national routine immunization program targeting the population aged ≥65 years was started in October 2014. It is still unclear whether Japan’s national PPSV23 vaccination program is actually improving the outcomes of elderly pneumonia patients. The effectiveness of that program thus needs to be evaluated. However, to date, there is no nationwide registry containing data for all PPSV23-vaccinated subjects and all pneumococcal pneumonia cases. This is the major obstacle to conducting a nationwide study on the effectiveness of the program. To the best of our knowledge, only one report explored the program’s effectiveness: Jung, et al.Citation20 used the cause-of-death data in Japan to demonstrate reduced pneumonia mortality, especially among those aged ≥90 years.
As noted above, the effectiveness of Japan’s national PPSV23 vaccination program has not been adequately confirmed based on nationwide clinical data. In the present quasi-experimental study, we aimed to confirm the effectiveness of the national PPSV23 vaccination program based on nationwide clinical data for the first time. We extracted and aggregated data for a large number of pneumonia cases among hospitalized elderly patients in the Japanese Diagnosis Procedure Combination (DPC) database. We then employed interrupted time-series analyses to assess the changes in the incidence of admission for pneumonia and the prognosis among the elderly before and after initiation of the program. This study will provide additional information on which to base future health care policy in Japan.
Methods
Data sources
MSD Japan (Tokyo, Japan), the manufacturer of PPSV23, provided the data on the number of PPSV23 syringes shipped in each prefecture.
We used the DPC database, a national inpatient database in Japan, for this nationwide retrospective observational study. The DPC database includes the patient discharge abstract data from approximately 55% of beds in general hospitals throughout Japan; most of those hospitals provide high-level medical care. The DPC database includes the information about each patient’s age, sex, primary and secondary diagnoses, comorbidities and complications, medications/treatments and discharge status, and the zip code of the hospital. It does not, however, contain information regarding the PPSV23 vaccination status or the causative bacteria of each patient. This study was approved by the Institutional Review Board of Tokyo National Hospital (Tokyo, Japan) and was carried out in accordance with the Declaration of Helsinki. Because of the anonymous nature of the data, informed consent from the study participants was waived.
Estimated PPSV23 coverage rates
As noted above, the DPC database does not include the information regarding the PPSV23 vaccination status of each patient. Therefore, for each prefecture, we estimated the PPSV23 coverage rates for patients aged ≥65 years by dividing the cumulative number of shipped PPSV23 syringes by the estimated population aged ≥65 years in each year.Citation21 We used the number of shipped syringes as a proxy because the number of doses administered to the population aged ≥65 years or those ordered for the national program by each prefecture was not available.
In Japan, PPSV23 is recommended for persons aged ≥65 years and for 60–64 year-old patients with chronic diseases, including respiratory, cardiac or renal dysfunction.
Patient selection and data aggregation
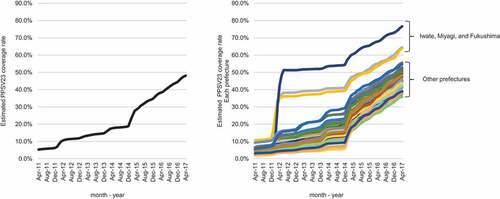
We first identified 933 inpatient facilities that had introduced the DPC system and used it continuously from April 1, 2011, through February 28, 2017. Then we extracted the inpatient data for pneumonia cases admitted to the hospitals during the same period. The patient inclusion criteria were as follows: a) aged 65 years; b) admission because of a confirmed diagnosis of pneumonia, defined as ICD-10 codes: J12, J13, J14, J15, J16, J17 or J18 on the admission date; and c) a record of antibiotic use during the hospital stay. Cases with missing zip codes were excluded, as were cases with zip codes in Iwate, Miyagi and Fukushima prefecture because the PPSV23 coverage rates in those three prefectures were significantly higher than in the other prefectures when the national routine immunization program was started (). The risk of hospitalization for pneumonia was reported to be higher for persons aged ≥85 years than for those aged 65–84 years.Citation22 We therefore categorized the patients into two groups: 65–84 years old and ≥85 years old. The modified Charlson comorbidity index (CCI) is a scoring system for comorbidities that is widely used in health care studies; it weights comorbidities using a scale of 1–6 based on the risk of dying and predicts mortality based on the total score. The CCI was proven to be useful for predicting in-hospital mortality of patients with CAP.Citation23 We classified the patients into three CCI categories: CCI 0–1, CCI 2–3 and CCI ≥4. For the eligible patients, the number of patients in each month was calculated for all patients, in each age category, and in each CCI category, and was used as aggregated data.
Study population
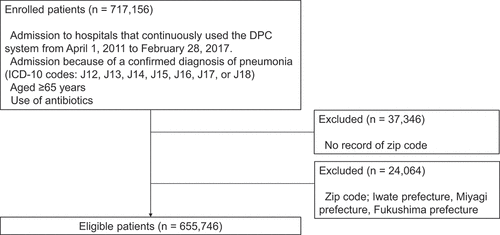
To assess changes in the incidence of pneumonia in the same manner as in sentinel surveillance, we enrolled only patients from facilities that continuously submitted data during the study period. We identified 717,156 patients aged ≥65 years who were hospitalized for pneumonia and treated with antibiotics (). We then excluded patients whose zip code was missing and those whose zip code was for Iwate, Miyagi, and Fukushima prefectures because their PPSV23 coverage rates were significantly higher than in the other prefectures when the national routine immunization program was started (). As a result, 655,746 eligible patients with pneumonia were included in the current study.
Figure 2. Flow-diagram of patient selection. We identified 717,156 patients aged ≥65 years who were hospitalized for pneumonia and treated with antibiotics. Patients without a recorded zip code and those in Iwate, Miyagi and Fukushima prefectures were excluded. Overall, 655,746 eligible pneumonia cases were analyzed
Endpoints
The primary endpoint was the incidence of admissions due to all-cause pneumonia, which was standardized by the estimated population aged 65 years in each given year (Ministry of International Affairs and Communications, Statistics Bureau of Japan, Population Estimates). Subgroups were stratified by age (65–84 years, ≥85 years).
The secondary endpoint was all-cause mortality during the hospital stay. Mortality was calculated based on the records of the status at discharge. The outcomes were calculated and plotted for each month. Subgroups were stratified by age (65–84 years, ≥85 years) or by CCI (0–1, 2–3, ≥4).
Statistical analyses
We employed interrupted time-series (ITS) analysesCitation24 fitting generalized linear model to estimate the effectiveness of the national routine PPSV23 vaccination program. The incidence of admissions for pneumonia was analyzed using a quasi-Poisson segmented regression model. Mortality was assessed using a quasi-binomial segmented regression model. The models included the following variables: the time trend, the level change and the time trend change following the immunization. The quasi-Poisson model also included offset terms of population. To control for seasonality and autocorrelation, we used a harmonic seasonal model with Fourier terms and added autoregressive terms when needed. We defined the appropriate model by likelihood ratio test and checked the independence of the residuals using the Ljung-box test.
We then combined the estimated PPSV23 coverage rates with the aggregated data from the DPC database for further analyses. We set October 1, 2014, as the time point of the beginning of the vaccination program. We did not take into consideration the period of delay in the operation of the vaccination program or a transitional period, because the estimated vaccine coverage rates rose sharply just after the program was started. Because the number of hospitalized elderly patients and deaths due to influenza infection increased significantly from December 2014 through January 2015 compared to the same season in other years,Citation25,Citation26 and because the outbreak of influenza would affect the outcome of pneumoniaCitation27 and thus may act as a confounding factor, we excluded the data from November 1, 2014, through January 31, 2015, from the analyses. Finally, we set the pre-immunization period as from April 1, 2011, through September 30, 2014 (a total of 42 time-points before immunization) and the post-immunization period as from February 1, 2015, through February 28, 2017 (a total of 29 time-points after starting the immunization).
Analyses were performed for all patients and in each age and CCI category. All statistical tests were two-sided. Statistical significance was defined as a P value of less than 0.05. All statistical data were analyzed with SPSS ver. 23 and R Studio v3.6.1.
Results
Estimated PPSV23 coverage rates
The estimated PPSV23 coverage rates in subjects aged ≥65 years in Japan were calculated based on the number of shipped PPSV23 syringes (data not shown) and the estimated population aged ≥65 years (e-) within each prefecture. As noted in the Introduction, there was no routine PPSV23 vaccination program except for subsidy by local governments until 2014 in Japan. In 2011, the estimated PPSV23 coverage rate in the population aged ≥65 years was as low as 10% (). After PPSV23 vaccination was fully subsidized for the population aged ≥65 years in 3 prefectures in the Tohoku area, i.e., Iwate, Miyagi and Fukushima, the PPSV23 coverage rates in those prefectures increased to around 40%, while those in other prefectures remained under 20% (). After the national routine immunization program was launched, the estimated coverage rates increased significantly in every prefecture and exceeded 40% in 2017 ().
Table 1. Results of segmented linear regression analysis of incidence of hospitalized elderly patients with pneumonia before and after the PPSV23 vaccination program
Incidence of hospitalized pneumonia patients
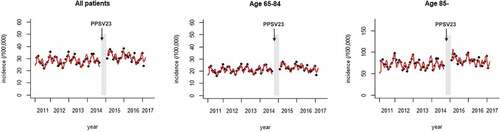
shows the number of patients hospitalized for pneumonia in each facility per 100,000 individuals for each month (plots). The aggregated data from the DPC database that were used in this study are shown in e-–4. As stated in the Methods section, we did not use data from November 1, 2014, through January 31, 2015, when the influenza outbreak in Japan may have affected the number of elderly patients hospitalized for pneumonia. The predicted incidence calculated from the regression model is also shown (solid red line). Before the introduction of the vaccination program, the incidence of hospitalized pneumonia cases was nearly constant, with seasonal fluctuations that peaked in winter. The first-order autocorrelation was also significant, reflecting the transmission of pneumonia in the Japanese population. These findings were also seen in the subgroups stratified by age (). The trend of incidence of hospitalized pneumonia cases was not affected even after the introduction of the vaccination program (trend change following immunization: −0.0039, 95% CI −0.0084 to 0.00064, p = .098; ). No trend change was seen in either of the subgroups stratified by age (65–84 years, trend change following the immunization: −0.0040, 95% CI −0.0085 to 0.00041, p = .081; ≥85 years, trend change following the immunization: −0.0031, 95% CI −0.0082 to 0.0020, p = .24; ) ().
Table 2. Results of segmented linear regression analysis of in-hospital mortality before and after the PPSV23 vaccination program
Figure 3. Incidence of admissions for pneumonia among the elderly. The numbers of inpatients in the registered facilities per 100,000 individuals are plotted by month. The predicted incidence calculated from the regression model is also shown (solid line). The period excluded from the analysis is shown as a gray region. The arrow shows the time-point when routine PPSV23 vaccination was started
In-hospital mortality
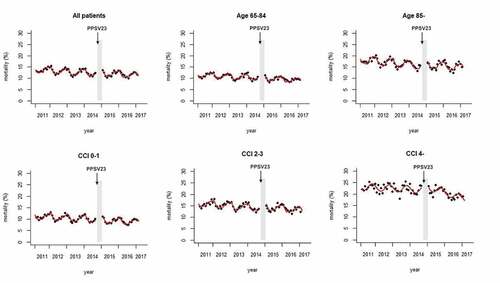
shows the in-hospital mortality for each month (plots). The predicted mortality calculated using the regression model is also shown (solid red line). The mortality gradually decreased over the entire study period (trend in pre-immunization period: ‐0.0039, 95% CI ‐0.0050 to ‐0.0027, p < .01; ), with seasonal fluctuations that peaked in winter (). Autocorrelation was not seen in this model. The trend did not change even after the vaccination program was started (trend change following immunization: 0.00099, 95% CI 0.0036 to 0.0016, p = .46; ). Trends for declining mortality and seasonal fluctuations were also seen in each of the subgroups stratified by age (65–84 years, ≥85 years) or by CCI (0–1, 2–3, ≥4). The trend of mortality in the CCI ≥4 subgroup decreased during the post-immunization period (trend change following immunization: ‐0.011, 95% CI ‐0.015 to ‐0.0064, p < .01; ), but no significant trend change was seen in any of the other subgroups (, ).
Figure 4. In-hospital mortality of elderly pneumonia patients. Mortality is plotted by month. The predicted mortality calculated from the regression model is also shown (solid line). The period excluded from the analysis is shown as a gray region. The arrow shows the time-point when routine PPSV23 vaccination was started
Discussion
In the present study, using the Japanese DPC database, we evaluated the changes in the incidence of hospitalized pneumonia cases and in-hospital mortality in the elderly before and after the launch of the national routine PPSV23 vaccination program in Japan in 2014. To the best of our knowledge, this is the first study using a nationwide in-patient database to estimate the effectiveness of the national PPSV23 vaccination program in Japan. After the vaccination program was started, no improvement was seen in the incidence of admissions for pneumonia or the mortality of all pneumonia cases, but the in-hospital mortality of cases with severe comorbidities (defined by the modified CCI) decreased.
Our method may have overestimated the coverage rate because (1) some vaccinated elderly persons may have died during the study period and (2) PPSV23 is recommended and used not only for persons aged ≥65 years but also for 60–64 year-old patients with chronic diseases such as respiratory, cardiac or renal dysfunction. However, it is likely that the increase in the vaccination rate was a result of the on-going national program, because the growing population of elderly persons aged ≥65 years may off-set deaths of vaccinated elderly people and vaccination of patients aged <65 years. In addition, the proportions of patients who died after vaccination or those of vaccinated patients aged <65 years may not have changed dramatically after the initiation of the program. The impact of any overestimation may therefore be limited, allowing us to properly conduct the subsequent ITS analyses.
The nationwide mortality of pneumonia inpatients decreased over the entire study period, including even before the vaccination program started. Improvement in general health care standards may be one possible explanation for that decreased mortality. Interestingly, the mortality trend in the CCI ≥4 subgroup decreased after the introduction of the vaccination program. These results are compatible with a previous study showing the high efficacy of PPSV23 vaccination, especially in poor-performance status patients.Citation16 Although the PPSV23 vaccine was recommended for patients with chronic medical conditions, the coverage rate of such patients was estimated to remain low; even patients undergoing home oxygen therapy were not fully vaccinated before the national program was implemented.Citation28 Considering this background, the improved outcome in patients with severe comorbidities might be attributable to the increased PPSV23 coverage rate resulting from the vaccination program.
Our results are inconsistent with those reported by Jung, et al., which showed an apparent trend change in mortality in pneumonia patients, especially in those aged ≥90 years.Citation20 In contrast, we found decreased mortality in all patients and also in each age category over the entire study period, including the pre-immunization period, but we found no trend change. A possible explanation for the discrepancy is the different nature of the databases used. That is, death in our study using the DPC database indicates all-cause in-hospital mortality, which is a result of both underlying and contributing causes of death, while death in the cause-of-death data only indicates the contributing cause of death. Because what matters most to patients is all-cause death rather than the contributing cause of death, we consider this difference to be an advantage of our study. Moreover, the definition of the contributing cause of death may vary among physicians and may change with time, e.g., due to new developments in diagnostics.
Our results are also inconsistent with the findings of several other studies, which reported improvement in mortality or decreased incidence of pneumonia following administration of PPSV23,Citation13–16 whereas we observed reduced in-hospital mortality only in a subgroup with severe comorbidities. These discrepancies may be attributable to having evaluated “pneumonia” instead of “pneumococcal pneumonia”. Because Streptococcus pneumoniae is the most common pathogen of CAP and is also one of the main causes of NHCAP, our study included patients with ICD-10 codes corresponding to pneumonia (J12-18). On the other hand, the number of patients diagnosed as “pneumococcal pneumonia” or “invasive pneumococcal disease” in the DPC database was smaller than expected (data not shown) and insufficient for ITS analyses. The following may explain that: 1) attending physicians may not have made a diagnosis of “pneumococcal pneumonia” when Streptococcus pneumoniae was co-infected with other pathogens, which is not uncommon; and 2) a validation study showed relatively low diagnostic sensitivity in the DPC database.Citation29 Furthermore, the database does not include the results of bacteriological examinations. Theoretically, patients with “pneumococcal pneumonia” would be expected to comprise a majority of the patients evaluated in our study, but they may have been diluted with patients with “non-pneumococcal pneumonia”. Our study may thus not have been sensitive enough to detect an improvement in the mortality or a decreased incidence of pneumonia following administration of PPSV23.
We should note that pneumococcal conjugate vaccines (PCVs) may have affected the outcomes observed in our study. PCVs are prophylactic agents for pneumococcal diseases and are administered mainly to children. Routine childhood immunization with a seven-valent PCV (PCV7) significantly reduced the incidences of pneumococcal pneumoniaCitation30 and IPDCitation31 in the targeted subjects. In addition to the direct effect of individual immunization, a herd immunity effect of routine childhood immunization, which depends on PCV coverage rates,Citation32,Citation33 has been recognized in unvaccinated populations, including the elderly. In Japan, following the start of voluntary immunization of infants in 2010, subsidization by local governments increased the coverage rate, and in 2013, PCV7 was included in routine immunizations. Because of the risk of serotype replacement after PCV7 vaccination,Citation31,Citation34 PCV7 was replaced by PCV13 in the same year (2013) and was expected to bring about better outcomes.Citation35 In fact, herd immunity arising from PCVs reportedly decreased genotypic penicillin resistance after the introduction of those vaccines.Citation36 Because of the high coverage rate of PCV13 (>98%) in Japanese childrenCitation37 and because PCV13 and PPSV23 as routine immunization agents were introduced at almost the same time, herd immunity due to PCV13 may have affected the results of our study. Nevertheless, considering that the PCV coverage rate had already reached more than 99% in 2013, when routine PCV immunization was started, the trend shift of mortality among elderly pneumonia patients with severe comorbidities between 2014 and 2015 can be at least partially attributed to the increased PPSV23 coverage rate.
While we need to promote the PPSV23 vaccination program, influenza vaccines are also essential for reducing the incidence and mortality of pneumonia among the elderly. Influenza infection is likely to increase susceptibility to pneumococcal pneumonia,Citation38 and in fact, Streptococcus pneumoniae is the most common cause of secondary influenza pneumonia.Citation39,Citation40 Importantly, the PPSV23 vaccine in combination with seasonal-influenza virus vaccine exhibits significant efficacy in decreasing admissions for pneumonia among the elderly and is also effective for cost-saving.Citation16 Given that the coverage rate of seasonal-influenza virus vaccine is still around 50% in Japan,Citation41 we need to promote routine seasonal influenza virus vaccination in addition to PPSV23.
Our study has a number of limitations. First, although we included only patients diagnosed as pneumonia on the date of admission, we may also have included hospital-acquired pneumonia (HAP) cases. That is because we could not exclude patients transferred from other hospitals or those who were shipped the day before index hospitalization. Since Streptococcus pneumoniae is not the major cause of HAP, the inclusion of HAP patients in our data may have resulted in underestimation of the effectiveness of PPSV23. Second, we were not able to confirm “pneumococcal pneumonia” because the database did not include the results of bacterial culture. Third, we could not analyze deaths from pneumonia or pneumococcal pneumonia, but from all-cause mortality, due to lack of data. Fourth, we were not able to evaluate whether or not the total number of patients with pneumonia, including those treated in the outpatient setting, decreased with time in this study. Fifth, we could not completely adjust for various possible confounders, including epidemiological and social factors. The prevalence of influenza and human metapneumovirus can affect the incidence and prognosis of pneumonia.Citation27,Citation38,Citation42 Recently, when patients at the end of life or in the terminal phase of various diseases also have pneumonia, they are encouraged to move to chronic care facilities or go home as soon as they can be transferred. Thus, in-hospital mortality of the elderly in acute care hospitals may be underestimated. Sixth, as discussed above, evaluating patients with all-cause pneumonia instead of just pneumococcal pneumonia might have led to low sensitivity that was insufficient for detecting improved outcomes in the whole population studied. Finally, because this was an ecological study, causality cannot be determined.
Conclusions
In conclusion, interrupted time-series analyses using aggregated data from the DPC database showed that the PPSV23 vaccination program was associated with decreased mortality of elderly patients with severe comorbidities who were hospitalized for pneumonia. To confirm our findings, the establishment of comprehensive databases for PPSV23 vaccination and pneumococcal pneumonia, and prospective studies using those databases, is needed.
Abbreviations
| CAP | = | Community-acquired pneumonia |
| CCI | = | Charlson comorbidity index |
| DPC | = | Diagnosis Procedure Combination |
| IPD | = | Invasive pneumococcal disease |
| ITS | = | Interrupted time-series |
| NHCAP | = | Nursing and health care-associated pneumonia |
| PCVs | = | Pneumococcal conjugate vaccines |
| PPSV23 | = | 23-valent pneumococcal polysaccharide vaccine |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (72.9 KB)Acknowledgments
The authors thank Dr. Kaoru Watanabe and Dr. Hiroshi Furukawa for serving as scientific advisors by participating in meaningful discussions throughout the research.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Miyashita N, Yamauchi Y. Bacterial pneumonia in elderly Japanese populations. Jpn Clin Med. 2018;9:1179670717751433. doi:10.1177/1179670717751433.
- Morimoto K, Suzuki M, Ishifuji T, Yaegashi M, Asoh N, Hamashige N, Abe M, Aoshima M, Ariyoshi K. The burden and etiology of community-onset pneumonia in the aging Japanese population: a multicenter prospective study. PLoS ONE. 2015;10:e0122247. doi:10.1371/journal.pone.0122247.
- Konomura K, Nagai H, Akazawa M. Economic burden of community-acquired pneumonia among elderly patients: a Japanese perspective. Pneumonia (Nathan). 2017;9:19. doi:10.1186/s41479-017-0042-1.
- Ishiguro T, Takayanagi N, Yamaguchi S, Yamakawa H, Nakamoto K, Takaku Y, Miyahara Y, Kagiyama N, Kurashima K, Yanagisawa T, et al. Etiology and factors contributing to the severity and mortality of community-acquired pneumonia. Int Med. 2013;52:317–24. doi:10.2169/internalmedicine.52.8830.
- Maruyama T, Niederman MS, Kobayashi T, Kobayashi H, Takagi T, D’Alessandro-Gabazza CN, Fujimoto H, Gil Bernabe P, Hirohata S, Nakayama S, et al. A prospective comparison of nursing home-acquired pneumonia with hospital-acquired pneumonia in non-intubated elderly. Respir Med. 2008;102:1287–95. doi:10.1016/j.rmed.2008.03.027.
- Ubukata K, Chiba N, Hanada S, Morozumi M, Wajima T, Shouji M, Iwata S. Invasive pneumococcal diseases surveillance study group. Serotype changes and drug resistance in invasive pneumococcal diseases in adults after vaccinations in children, Japan, 2010-2013. Emerging Infect Dis. 2015;21:1956–65. doi:10.3201/eid2111.142029.
- Oishi K, Yoshimine H, Watanabe H, Watanabe K, Tanimura S, Kawakami K, Iwagaki A, Nagai H, Goto H, Kudoh S, et al. Drug-resistant genes and serotypes of pneumococcal strains of community-acquired pneumonia among adults in Japan. Respirology. 2006;11:429–36. doi:10.1111/j.1440-1843.2006.00867.x.
- Fukusumi M, Chang B, Tanabe Y, Oshima K, Maruyama T, Watanabe H, Kuronuma K, Kasahara K, Takeda H, Nishi J, et al. Invasive pneumococcal disease among adults in Japan, April 2013 to March 2015: disease characteristics and serotype distribution. BMC Infect Dis. 2017;17:2. doi:10.1186/s12879-016-2113-y.
- Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–60. doi:10.1056/NEJM199111213252101.
- Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, Hanson CA, Mahoney LD, Shay DK, Thompson WW. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–55. doi:10.1056/NEJMoa022678.
- Ogilvie I, El Khoury A, Cui Y, Dasbach E, Grabenstein JD, Goetghebeur M. Cost-effectiveness of pneumococcal polysaccharide vaccination in adults: A systematic review of conclusions and assumptions. Vaccine. 2009;27:4891–904. doi:10.1016/j.vaccine.2009.05.061.
- Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422. doi:10.1002/14651858.CD000422.pub3.
- Fisman DN, Abrutyn E, Spaude KA, Kirchner AKC, Daley J. Prior pneumococcal vaccination is associated with reduced death, complications, and length of stay among hospitalized adults with community-acquired pneumonia. Clin Infect Dis. 2006;42:1093–101. doi:10.1086/501354.
- Suzuki M, Dhoubhadel BG, Ishifuji T, Yasunami M, Yaegashi M, Asoh N, Ishida M, Hamaguchi S, Aoshima M, Ariyoshi K, et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17:313–21. doi:10.1016/S1473-3099(17)30049-X.
- Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, D’Alessandro-Gabazza C, Nakayama S, Nishikubo K, Noguchi T, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004. doi:10.1136/bmj.c1004.
- Kawakami K, Ohkusa Y, Kuroki R, Tanaka T, Koyama K, Harada Y, Iwanaga K, Yamaryo T, Oishi K. Effectiveness of pneumococcal polysaccharide vaccine against pneumonia and cost analysis for the elderly who receive seasonal influenza vaccine in Japan. Vaccine. 2010;28:7063–69. doi:10.1016/j.vaccine.2010.08.010.
- Diao W-Q, Shen N, Yu P-X, Liu B-B, He B. Efficacy of 23-valent pneumococcal polysaccharide vaccine in preventing community-acquired pneumonia among immunocompetent adults: a systematic review and meta-analysis of randomized trials. Vaccine. 2016;34:1496–503. doi:10.1016/j.vaccine.2016.02.023.
- Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180:48–58. doi:10.1503/cmaj.1090018.
- Gessner BD, Theilacker C, Jodar L. Rethinking results from the Japanese 23-valent pneumococcal polysaccharide vaccine randomized clinical trial. Vaccine. 2019;37:4853–57. doi:10.1016/j.vaccine.2019.07.040.
- Jung S-M, Lee H, Nishiura H. The impact of pneumococcal vaccination on pneumonia mortality among the elderly in Japan: a difference-in-difference study. PeerJ. 2018;6:e6085. doi:10.7717/peerj.6085.
- Ministry of International Affairs and Communications. Statistics Bureau of Japan, population estimates. [accessed 2019 Dec 7]. https://www.stat.go.jp/english/index.html.
- Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988-2002. JAMA. 2005;294:2712–19. doi:10.1001/jama.294.21.2712.
- Tokgoz Akyil F, Yalcinsoy M, Hazar A, Cilli A, Celenk B, Kilic O, Sayiner A, Kokturk N, Sakar Coskun A, Filiz A, et al. Prognosis of hospitalized patients with community-acquired pneumonia. Pulmonology. 2018;24:164–69. doi:10.1016/j.rppnen.2017.07.010.
- Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–55. doi:10.1093/ije/dyw098.
- National Institute of Infectious Diseases. [accessed 2019 Dec 7].https://www.niid.go.jp/niid/images/idsc/disease/influ/fludoco1415-2.pdf.
- National Institute of Infectious Diseases. [accessed 2019 Dec 7]. https://www.niid.go.jp/niid/images/idsc/disease/influ/fludoco1516.pdf.
- Shrestha S, Foxman B, Berus J, van Panhuis WG, Steiner C, Viboud C, Rohani P. The role of influenza in the epidemiology of pneumonia. Sci Rep. 2015;5:15314. doi:10.1038/srep15314.
- The Japanese Respiratory Society. [accessed 2019 Dec 7]. http://www.jrs.or.jp/uploads/uploads/files/photos/1096.pdf.
- Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol. 2017;27:476–82. doi:10.1016/j.je.2016.09.009.
- Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369:1179–86. doi:10.1016/S0140-6736(07)60564-9.
- Feikin DR, Kagucia EW, Loo JD, Link-Gelles R, Puhan MA, Cherian T, Levine OS, Whitney CG, O’Brien KL, Moore MR, the Serotype Replacement Study Group. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med. 2013;10:e1001517. doi:10.1371/journal.pmed.1001517.
- Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio. 2011;2:e00309–10. doi:10.1128/mBio.00309-10.
- Tsaban G, Ben-Shimol S. Indirect (herd) protection, following pneumococcal conjugated vaccines introduction: a systematic review of the literature. Vaccine. 2017;35:2882–91. doi:10.1016/j.vaccine.2017.04.032.
- Suga S, Chang B, Asada K, Akeda H, Nishi J, Okada K, Wakiguchi H, Maeda A, Oda M, Ishiwada N, et al. Nationwide population-based surveillance of invasive pneumococcal disease in Japanese children: effects of the seven-valent pneumococcal conjugate vaccine. Vaccine. 2015;33:6054–60. doi:10.1016/j.vaccine.2015.07.069.
- Alicino C, Paganino C, Orsi A, Astengo M, Trucchi C, Icardi G, Ansaldi F. The impact of 10-valent and 13-valent pneumococcal conjugate vaccines on hospitalization for pneumonia in children: A systematic review and meta-analysis. Vaccine. 2017;35:5776–85. doi:10.1016/j.vaccine.2017.09.005.
- Ubukata K, Takata M, Morozumi M, Chiba N, Wajima T, Hanada S, Shouji M, Sakuma M, Iwata S, the Invasive Pneumococcal Diseases Surveillance Study Group. Effects of pneumococcal conjugate vaccine on genotypic penicillin resistance and serotype changes, Japan, 2010–2017. Emerging Infect Dis. 2018;24:2010–20. doi:10.3201/eid2411.180326.
- Number of routine immunizations. Ministry of Health. Tokyo (Japan): Labour and Welfare. [accessed 2014 Oct 21].
- Shrestha S, Foxman B, Weinberger DM, Steiner C, Viboud C, Rohani P. Identifying the interaction between influenza and pneumococcal pneumonia using incidence data. Sci Transl Med. 2013;5:191ra84. doi:10.1126/scitranslmed.3005982.
- Centers for Disease Control and Prevention (CDC). Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) - United States, May-August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1071–74.
- Cillóniz C, Ewig S, Menéndez R, Ferrer M, Polverino E, Reyes S, Gabarrús A, Marcos MA, Cordoba J, Mensa J, et al. Bacterial co-infection with H1N1 infection in patients admitted with community acquired pneumonia. J Infect. 2012;65:223–30. doi:10.1016/j.jinf.2012.04.009.
- OECD. Vaccinations, in Health at a Glance 2019: OECD Indicators. Paris: OECD Publishing; 2019.
- Shafagati N, Williams J. Human metapneumovirus - what we know now. Version 1. F1000Res. 2018;7:135. doi:10.12688/f1000research.12625.1.