?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Introduction: World Health Organization has recommended that in healthy persons with category III exposures, who receive wound care and rabies immunoglobulin infiltration, a vaccine regimen consisting of 4 doses administered intramuscularly on days 0, 3, 7, and 14 can be used as an alternative to the 5-dose intramuscular regimen.
Objective: To assess the clinical safety and immunogenicity of rabies vaccine administered as 4-dose Essen intramuscular regimen for post-exposure prophylaxis.
Methods: A non-randomized, comparative, controlled study was conducted at the anti-rabies clinic, KIMS Hospital and Research Center, Bangalore, India. The study subjects were divided into study group i.e., 4-dose intramuscular regimen, and control group i.e., 5-dose intramuscular regimen, and were given post-exposure prophylaxis. All subjects were followed for any adverse drug events. Rabies virus neutralizing antibodies was determined on day 14, 90 & 180 at the WHO collaborating center, NIMHANS, Bangalore, India to assess the immunogenicity.
Results: The present study included 70 adult animal bite victims, 35 each in study group and control group. The incidence of ADEs was 7.8% in 4-dose Essen group and 7.0% in 5-dose Essen group;the difference between them was not significant (P > .05). Similarly, all the subjects in both the groups had protective antibody titers of ≥ 0.5 IU/mL (100% seroprotective) from day 14 till day 180; the difference between two groups was also not significant (P > .05).
Conclusion: The 4-dose intramuscular Essen post-exposure prophylaxis regimen was found to be clinically safe and immunogenic.
Introduction
Rabies is a vaccine-preventable disease which can be controlled and eliminated.Citation1 Early and complete post-exposure prophylaxis (PEP) which includes wound washing with soap/detergent & water, followed by application of virucidal agents to reduce the viral inoculum at the wound site; complete course of post-exposure vaccination to induce antibodies which prevent the risk of virus entering peripheral nerves and wound infiltration with rabies immunoglobulin (RIG)/rabies monoclonal antibodies (RMAb) in all category III exposures to neutralize the virus at the wound site are effective in preventing rabies even after high-risk exposure to animals.Citation2
Every year, more than 15 million people worldwide receive post-exposure vaccination, which is estimated to prevent millions of rabies deaths.Citation3 The rabies vaccine is given as a series of injections to develop an active immunity in the individual i.e., anti rabies antibodies to neutralize the virus. Ever since the cell culture embryonated egg vaccines (CCEEVs) were invented, the number of rabies vaccine doses administered for post-exposure prophylaxis in humans has gradually reduced with improved scientific evidence.Citation4,Citation5 The duration of PEP was initially of 3 months with 6 injections i.e., one dose each on days 0, 3, 7, 14, 30, and 90.Citation6 Later, it was reduced to one month using 5 injections i.e., Gold standard Essen regimen having one dose of vaccine each on days 0, 3, 7, 14, and 28.Citation7
Even after the reduction in the duration of PEP, the compliance for the 5th dose (day 28) of rabies vaccine was found to be only about 60%.Citation8 Hence, there was an emphasis on reducing the long duration PEP by a shorter course that would result in saving vaccine, reducing the number of visits and decreasing travel costs, and therefore ensure better patient compliance.
In this regard, WHO recommended that in healthy, fully immune competent persons, who receive wound care along with high-quality RIG/RMAb and WHO pre-qualified rabies vaccines, a PEP vaccine regimen consisting of four doses administered intramuscularly on days 0, 3, 7 and 14 can be used as an alternative to the 5-dose intramuscular regimen.Citation9 This was originally based on the recommendation given by an expert committee at the Center for Disease Control, USA which determined the potential utility of reducing the current 5-dose intramuscular rabies vaccine to a 4-dose schedule on days 0, 3, 7 & 14 given in conjunction with RIG and proper wound management; based upon the review of literature and available evidence.Citation10
The clinical evidence to support this 4-dose Essen intramuscular schedule was necessary for its implementation in many countries. This study assessed the clinical safety and immunogenicity of rabies vaccine administered as 4-dose Essen intramuscular (IM) regimen that provided the clinical evidence to contribute for the advance in the previously available body of evidence, supporting the use of this regimen.
Materials and methods
The study was initiated after obtaining ethical approval from the KIMS Institutional Ethics Committee. The clinical trial was registered in the clinical trial registry, India (CTRI No. 2014/02/006443) and the study was conducted in accordance with ICH – GCP guidelines. It was a non-randomized (1:1), comparative, controlled study conducted at the anti-rabies clinic, Department of Community Medicine, KIMS Hospital & Research Center, Bangalore, India.
Sample size calculation
The incidence of animal exposure was considered as 1.7%.Citation11
Assuming a Power of 90%, Confidence interval of 95% and α = 0.05, the sample size was calculated as follows:
Using the design effect of 2 = 17.13 X 2 = 34.26 = 35
Net sample size was taken as 35 subjects in each group.
The study subjects included dog bite victims, who came for PEP at the study center and signed written informed consent form to participate in the study. The socio-demographic profile of all the study subjects, history of current or past medical problems, concomitant or past medication and allergy to any medicine were noted. All subjects with history of previous anti rabies vaccination were excluded from the study. Pregnant and lactating women and women who intended to conceive during the period of study at the time of recruitment were excluded. General physical and systemic examination of subjects was done to rule out any preexisting illness.
The study subjects were divided into 2 groups:
Study group
4-dose Essen intramuscular regimen group, that consisted of 35 consecutive study subjects, where biting dog was a vaccinated pet and available for observation. These subjects were given 4 doses of vaccine on days 0, 3, 7 & 14.
Control group
5-dose Essen intramuscular regimen group, that consisted of 35 consecutive study subjects, where dog was not available for observation. These subjects were given full course of 5-dose Essen intramuscular PEP regimen on days 0, 3, 7, 14, & 28. The patients were at high risk in this group, hence for ethical reasons, the doses of vaccine were not reduced.
Two WHO pre-qualified vaccines which were available in the market were used in the study viz., Rabipur and Verorab.
Rabipur is a purified chick embryo cell rabies vaccine (PCECV), available in 1 ml vial & manufactured by Chiron Behring Vaccines Pvt. Ltd, India using Flury LEP strain. Market Batch No. 2307 with a potency of > 2.5 IU/dose was used.
Verorab is a purified vero cell rabies vaccine (PVRV), available in 0.5 ml vial & manufactured by Sanofi Pasteur SA – 2, avenue Pont, Pasteur – 69007, Lyon, France; using Wistar rabies PM/WI 38 1503–3 M strain (inactivated). Market Batch No. 2712with a potency of > 2.5 IU/dose was used.
Similarly, two types of rabies immunoglobulin were used in the study viz., human rabies immunoglobulin (Berirab P) and Equine rabies immunoglobulin (Equirab).
Human rabies immunoglobulin (Berirab P), which was available in 2 ml vial and marketed in India by Bharat Serums and Vaccines Ltd. with potency of 150 IU/ml of market Batch No. 12705.
Equine rabies immunoglobulin (Equirab), which was available in 5 ml vial, manufactured and marketed by Bharat Serums and Vaccines Ltd. India with potency of 300 IU/ml of market Batch No. A2712003.
All the study subjects in both the groups were followed up for any adverse drug events (ADEs) following PEP. The subjects were observed for an hour, to rule out any possible immediate solicited ADEs i.e., local reactions such as pain, erythema, pruritus and induration and/or systemic reactions such as shivering, malaise, asthenia, faintness, dizziness, headache, myalgia, arthralgia, nausea, abdominal pain and hypersensitivity or allergic reactions such as urticaria, rash and anaphylaxis; and the reactogenicity was recorded. All subjects were given a follow up card indicating the date of the next dose of vaccination and blood sampling; and also to record unsolicited late ADEs such as pain, induration, erythema, itching, fever, serum sickness, arthralgia and any others. The follow up cards were checked in every visit and the ADEs, if any, were recorded during subsequent visits of subjects on days 3, 7, 14, 28, 90 and180, and the appropriate treatment was provided free of cost.
The sera samples were collected from both the groups for estimation of rabies virus neutralizing antibody (RVNA) titers on days 0, 14, 90 & 180. The serum samples of the study subjects were analyzed by Rapid Fluorescent Focus Inhibition Test (RFFIT) at the Department of Neurovirology, National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, India; which is a World Health Organization (WHO) collaborating center for Reference & Research on Rabies.
Procedure for RFFIT
It was done as per WHO recommended procedure with some modifications.Citation12 The cell line used was BHK 21 (ATCC CCL 10) and 96 well tissue culture plates (Sigma) and BHK21 adapted CVS 13 strain of rabies virus. The reference serum used was an in house serum calibrated against 2nd international reference standard having a titer of 30 IU/ml (obtained from National Institute of Biological standards, UK). Briefly, doubling dilutions of serum samples and reference serum (after heat inactivation at 56 C for 30 min in a water bath) in duplicate were made in 96 well plates using IMDM (Sigma Cat No.17633). To each 100 µl of serum dilution 100 µl of CVS (100 FFD 50) was added and the plate was incubated at 37°C for 1 hour. A confluent monolayer of BHK 21 cells were trypsinized and re-suspended in 10 ml of IMDM with 10% FCS (Sigma, cat No. F2442). Cell control and virus controls were also included. To each well of the 96 well plate 100 µl of cell suspension was added and the plate was incubated at 37°C in a CO2 incubator (Sanyo, Japan). After 24 h the cells were fixed in cold acetone for 30 minutes and stained by direct FAT using commercially available rabies N conjugate (Light diagnostics USA, Cat No. F199). The plates were then observed under an inverted fluorescence microscope (Nikon Eclipse). The highest dilution of serum showing 50% inhibition of fluorescence foci was taken as end point dilution. The titer was converted to IU/ml in comparison with reference serum.
The data was analyzed statistically by computing rates and percentages for ADEs and the variation in ADEs between the two groups was assessed using Z test. The results obtained for RVNA titers were analyzed by geometric mean concentration (GMC), geometric standard deviation (GSD), 95% confidence interval and the p-value was calculated to find out the difference between the GMCs of RVNA titers between the two groups.
Results
The socio-demographic profile of the two study groups were similar with the mean age ± standard deviation of 37.86 ± 13.33 years and 34.91 ± 12.67 years in 4-dose Essen IM and 5-dose Essen IM respectively.
Majority of the bite victims in both the groups had Cat. III exposure i.e., 85.7% in 4-dose Essen regimen and 91.4% in 5-dose Essen group; the commonest site of bite was on lower limbs i.e., 65.7% in 4-dose Essen regimen and 51.4% in 5-dose Essen group (). All the vaccinated pet dogs were alive and healthy after 10 days of observation and none of the dogs in the control group were traced because of logistical reasons.
Table 1. Details of exposure
In the anti-rabies clinic, all the study subjects were made to wash their wounds with soap and water followed by administration of RIG. The calculated dose of RIG (20 IU/kg body weight for HRIG and 40IU/kg bodyweight for ERIG) was infiltrated around the wound(s) as far as anatomically feasible. In case, there was any RIG remaining after infiltration, it was administered deep intramuscularly at a site away from the anti-rabies vaccination site in the body.
All the subjects received intramuscular rabies vaccine in the deltoid region (). Among the 4-dose Essen group, 33 cases received Rabipur & 2 received Verorab and in the 5 – dose Essen IM group, 34 received Rabipur and only 1 received Verorab as it was out of stock in the country during the study period.
Table 2. Usage of rabies immune-biologicals
None of the study subjects reported immediate ADEs. The common unsolicited late ADEs recorded among the study subjects in both the groups were erythema, itching, pain and induration at the site of injection. In the 4-dose Essen IM regimen group, eleven subjects complained of mild ADEs which resolved with or without any medication and without any complication. The incidence of total ADEs was calculated based on number of adverse events divided by total number of doses i.e., 11/140 and it was found to be 7.8%. Similarly, in the 5 – dose Essen IM regimen group, 12 subjects complained of mild ADEs which resolved with or without any medication and without any complication. The incidence of total ADEs was 12/173 i.e., 7.0%. None of the study subjects in both the groups discontinued the vaccine due to ADEs. The difference between the ADEs among the two groups was not significant (P > .05). ()
Table 3. Adverse drug events among the study groups
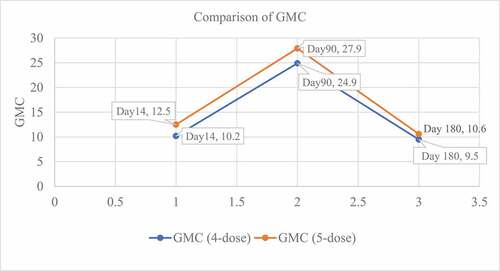
According to WHO recommendation, RVNA titer of ≥ 0.5 IU/ml is considered as protective against rabies. While assessing the immunogenicity; there was 1 dropout in the 4-dose Essen IM regimen group for days 90 & 180; and there was 1 dropout in the 5 – dose Essen IM regimen group for day 14 and 2 dropouts for days 90 & 180. The reasons for dropouts were migration to a different city. All the study subjects in both the groups had protective RVNA titers of ≥ 0.5 IU/ml and sero-conversion rate was 100% from day 14 till day 180. The geometric mean RVNA concentration (GMC) were 10.17 IU/ml, 24.99 IU/ml, and 9.46 IU/ml in 4-dose Essen IM regimen group and 12.54 IU/mL, 27.991IU/mL and 10.60 IU/mL in 5 – dose Essen IM group on days 14, 90, and 180, respectively. The difference between the geometric mean concentrations of RVNAs on all days among the two groups was not significant (P > .05) () ().
Table 4. RVNA response to post exposure vaccination
Discussion
Rabies is a zoonotic disease which occurs in more than 150 countries and is a public health concern to over three billion people worldwide. Dogs are the main source of human rabies, contributing to 99% of all rabies transmissions to humans.Citation13,Citation14 The magnitude and epidemiological pattern differs from country to country and it is a disease of poverty, affecting vulnerable population and children.Citation15 Majority of cases occur in Africa and Asia; a combination of large human and dog population in habitable areas has led to more exposures in WHO’s South East Asia region than in any other part of the world. More than 1.4 billion people in this region are at the risk of rabies exposure, mainly dog mediated. Therefore, it continues to be a major public health and economic problem throughout the South East Asian Region.Citation16,Citation17 Improved affordability and access to healthcare services, particularly for vulnerable populations in these rabies-affected countries, can lead to control and eventually elimination of the disease. Prompt post-exposure use of vaccines combined with proper wound management and simultaneous administration of RIG in severe exposures is effective in preventing rabies.
For over a century, the crude nerve tissue vaccine was used for PEP requiring about 14 to 21 daily injections.Citation18 However, with the advent of safer and potent CCEEVs, the duration of immunization which was for 3 months, later reduced to one month (Essen regimen) and 3 weeks (Zagreb regimen), and studies for further reduction in duration of PEP have been encouraged.Citation19–23 In this regard, the expert committee at CDC, USA has recommended shortened 4-dose Essen regimen for intramuscular use of rabies vaccine based on literature review, rabies virus pathogenesis data, animal experiments, clinical studies, epidemiologic surveillance, and health economics.Citation24
The present study provides the clinical evidence with regard to safety and immunogenicity of 4-dose Essen intramuscular PEP regimen and provided the clinical evidence to contribute for the advance in the previously available body of evidence, supporting the use of this regimen.
The study subjects were representative of both sex and various age groups; majority of them had Category III exposure. The incidence of ADEs was found to be 7.8% in 4-dose Essen group and 7% in standard Essen group and the difference between the two groups was not statistically significant (P > .05). All the ADEs were mild and not associated with any complications. The ADEs of study vaccines was similar to other vaccines, studied using standard intramuscular Essen regimen. A study done by Sudarshan et al. with human diploid cell rabies vaccine (HDCV) using Essen regimen showed 8.4% ADEs with local ADEs of 7.2% and systemic ADEs of 1.2%; where pain at site of injection was the common adverse drug reaction recorded. All the ADEs subsided without any complications.Citation25
The objective of vaccination in PEP is to stimulate the immune system to produce antibody titers of at least 0.5 IU/mL by day 14, as recommended by WHO, which should persist for a long time. In the present study, all the study subjects in both the groups had protective antibodies against rabies by day 14 (100% seroprotection). The geometric mean RVNA concentration were 10.17 IU/ml, 24.99 IU/ml and 9.46 IU/ml in 4-dose Essen group and 12.54 IU/ml, 27.99IU/ml and 10.60 IU/ml in Essen group on days 14, 90 and 180, respectively. The difference between the geometric mean concentrations of RVNAs on all days among the two groups was not significant (P > .05).
The immunogenicity in the present study was found to be comparable with other studies using intramuscular Essen regimen for post-exposure prophylaxis. A study conducted by Sudarshan et al., using Essen regimen with HDCV showed GMC of 3.39IU/ml, 7.54IU/ml, and 5.56IU/ml, respectively, on days 14, 28, and 90.Citation25 Similarly, a study by Mahendra et al., using Essen intramuscular regimen with PDEV reported GMC of 10.32IU/ml, 14.31IU/ml, 7.32IU/ml, and 3.70IU/ml on days 14, 28, 90, and 180, respectively.Citation26 In another study by Ashwath Narayana et al, the GMC were reported to be of 6.98 IU/ml for PDEV, 6.65 IU/ml for PVRV and 6.88 IU/ml for PCEC on day 14 followed by 8.64 IU/ml for PDEV, 9.14 IU/ml for PVRV and 8.31 IU/ml for PCEC on day 90 and 3.64 IU/ml for PDEV, 3.45 IU/ml for PVRV and 8.31 IU/ml for PCEC on day 180.Citation27 Thus, the immunogenicity of study vaccines given as intramuscular 4-dose Essen intramuscular regimen for post-exposure prophylaxis was similar to other studies. Similarly, the difference between the geometric mean concentrations of RVNAs on all days among the two groups with or without rabies immunoglobulin was not significant (P > .05).
The limitation of the study was that the biting animals in the control group could not be followed up for their health status because of the logistical issues.
In conclusion, the rabies vaccine administered as 4-dose Essen intramuscular PEP regimen was found to be safe & immunogenic. The present study provides the clinical evidence to contribute for the advance in the previously available body of evidence, supporting the use of this regimen. This will reduce the number of doses, resulting in saving vaccine, reduction in number of visits and travel costs which in turn may increase the patient compliance to complete vaccination, which is important to prevent rabies and ultimately help in eliminating dog mediated human rabies by 2030, which was set in line with the Sustainable Development Goals (SDG) 3.3 to end Neglected Tropical Diseases by 2030.Citation28
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- WHO Expert Consultation on Rabies prevention. Third report. Technical report series no. 1012. Geneva: World Health Organization; 2018.
- World Health Organization. Rabies vaccines: WHO position paper. Weekly Epidemiological Record, No. 16. 2018;93:201–20.
- World Health Organization. Human and dog rabies prevention and control. Report of the WHO/Bill & Melinda Gates Foundation consultation. Annecy (France); 2009. p. 9.
- Knobel DL, Cleaveland S, Coleman PG, Fèvre EM, Meltzer MI, Miranda ME, Shaw A, Zinsstag J, Meslin F-X. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ. 2005;83(5):360–68.
- World Health Organization. Rabies vaccines and immunoglobulins: WHO position,WHO/CDS/NTD/NZD/2018.04. Geneva (Switzerland): World Health Organization; 2018.
- World Health Organization. WHO Expert Consultation on Rabies. Second report. Technical report series no. 931. Geneva (Switzerland): World Health Organization; 2005.
- World Health Organization. WHO Expert Committee on Rabies. 8th report. Technical report series no.824. Geneva (Switzerland): World Health Organization; 1992. p. 10–16.
- Ravish HS, Rachana AR, Veena V, Ashwath Narayana DH. Compliance to anti-rabies vaccination in post-exposure prophylaxis. Indian J Public Health. 2015;59(1):58–60. doi:10.4103/0019-557X.152867.
- World Health Organization. WHO Expert Consultation on Rabies. Second report. Technical report series no. 982. Geneva (Switzerland): World Health Organization; 2013.
- The Morbidity and Mortality Weekly Report, Center for Disease Control and Prevention (CDC). Use of a reduced (4-dose) vaccine schedule for post-exposure prophylaxis to prevent human rabies. Recommend Advisory Committee Immun Prac. 2010;59(RR02):1–10.
- Sudarshan MK, Mahendra BJ, Madhusudana SN, Ashwoath Narayana DH, Rahman A, Rao NS, Meslin X, Lobo F, Ravikumar D, Gangaboraiah K. An epidemiological study of animal bites in India: results of a WHO sponsored national multi-centric rabies survey. J Commun Dis. 2006;38(1):32–39. PMID:17370688
- Zalan E, Wilson C, Pukitis D. A microtest for the quantitation of rabies virus neutralizing antibodies. J Biol Stand. 1979;7(3):213–20. doi:10.1016/S0092-1157(79)80024-4.
- World Health Organization. Rabies post exposure prophylaxis. [accessed 2017 Jul 20]. http://www.who.int/rabies/human/postexp.
- Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, et al. Global alliance for rabies control partners for rabies prevention. Estimating the global burden of endemic canine rabies. PLoSNegl Trop Dis. 2015 Apr 16;9(4):3709.
- World Health Organization. Rabies. [accessed 2017 Jul 18]. http://www.who.int/mediacentre/factsheets.
- Wilde H, Khawplod P, Khamoltham T, Hemachudha T, Tepsumethanon V, Lumlerdacha B, Mitmoonpitak C, Sitprija V. Rabies control in South and Southeast Asia. Vaccine. 2005 Mar 18;23(17–18):2284–89. doi:10.1016/j.vaccine.2005.01.030.
- WHO South East Asia region: strategic framework for elimination of human rabies transmitted by dogs in the South-East Asia Region: world Health Organization, Regional office for South East Asia; 2012.
- Jackson AC, William HW. Rabies. 2nd ed. London (United Kingdom): Elsevier academic press; 2007. p. 32–56.
- Barth R, Bijok U, Gruschkau H, Smerdel S, Vodopija I. Purified chick embryo cell rabies vaccine for human use. Lancet. 1984;1:7.
- World Health Organization (WHO). Rabies vaccines, WHO position paper. Weekly Epidemiological Record. 2002 Apr 5;14(77):109–20.
- Zang ZF. Rabies and rabies research: past, present and future. Vaccine. 1997;15:20–24. doi:10.1016/S0264-410X(96)00312-X.
- Vodopija I, Sureau P, Lafon M, Baklaic Z, Ljubicic M, Svjetlicic M, Smerdel S. An evaluation of second generation tissue culture rabies vaccines for use in man: a four-vaccine comparative immunogenicity study using a pre-exposure vaccination schedule and an abbreviated 2-1-1 postexposure schedule. Vaccine. 1986;4:245–48. doi:10.1016/0264-410X(86)90138-6.
- Chutivongse S, Wilde H, Fishbein DB, Baer GM, Hemachudha T. One-year study of the 2-1-1 intramuscular postexposure rabies vaccine regimen in 100 severely exposed Thai patients using rabies immune globulin and Vero cell rabies vaccine. Vaccine. 1991;9(8):573–76. doi:10.1016/0264-410X(91)90244-Z.
- Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, Kerr HD, et al. Evidence for a 4-dose vaccine schedule for human rabies post-exposure prophylaxis in previously non-vaccinated individuals. Vaccine. 2009;28(1):1–8.
- Sudarshan MK, Bhardwaj S, Mahendra BJ, Sharma H, Sanjay TV, Ashwathnarayana DH. An immunogenicity, safety and post-marketing surveillance of a novel adsorbed human diploid cell rabies vaccine (Rabivax®) in Indian subjects. Hum Vaccin. 2008;4(4):275–79. doi:10.4161/hv.4.4.5588.
- Mahendra BJ, Madhusudana SN, Ashwathnarayana DH, Sampath G, Datta SS, Sudarshan MK, Venkatesh GM, Muhamuda K, Bilagumba G, Shamanna M et al. A comparative study on the immunogenicity, safety and tolerance of purified duck embryo vaccine (PDEV) manufactured in India (Vaxirab) and Switzerland (Lyssavac-N): A randomized simulated post-exposure study in healthy volunteers. Vaccine. 2007 Dec 5;25(50):8405–09. doi:10.1016/j.vaccine.2007.10.002.
- Ashwath Narayana DH, Madhusudana SN, Sampath G, Sathpathy DM, Mankeshwar R, Haradanhalli S, Ravish, et al. A comparative study on the safety and immunogenicity of purified duck embryo cell vaccine (PDEV, Vaxirab) with purified chick embryo cell vaccine (PCEC, Rabipur) and purified vero cell rabies vaccine (PVRV, Verorab). Vaccine. 2010;28:148–51. doi:10.1016/j.vaccine.2009.09.090.
- Sustainable development goals: 17 goals to transform our world. Goal 3: ensure healthy lives and promote well-being for all at all ages. [accessed 2018 Feb 16]. http://www.un.org/sustainabledevelopment/health.