ABSTRACT
The cholera vaccine can protect patients with inflammatory bowel disease (IBD) against both cholera and travelers’ diarrhea. However, both immunosuppressive treatment and IBD can affect its vaccine immunogenicity. The aim of this study was to assess the immunogenicity and safety of the cholera vaccine in children with IBD. Children older than 6 years with diagnosed IBD were enrolled in this multicenter study. All patients were administered two doses of the oral cholera vaccine (Dukoral®). Anti-cholera toxin B subunit IgA and IgG seroconversion rates were evaluated in a group with immunosuppressive (IS) treatment and a group without IS treatment (NIS). Immunogenicity was assessed in 70 children, 79% of whom received IS treatment. Post-vaccination seroconversion was displayed by 33% of children, for IgA, and 70% of children, for IgG. No statistically significant differences were found in the immune responses between the IS and NIS groups: 35% vs. 27% (p = .90), for IgA, and 68% vs. 80.0% (p = .16), for IgG, respectively. One case of IBD exacerbation after vaccination was reported. The oral cholera vaccine is safe. The immunogenicity of the oral cholera vaccine in children with IBD was lower than previously observed in healthy ones. The treatment type does not seem to affect the vaccine immunogenicity.
Introduction
Inflammatory bowel disease (IBD) is a chronic, lifelong disease that significantly impacts all aspects of every-day life. IBD exacerbations negatively affect quality of life among IBD patients, impacting diet, education, work, and relationships.Citation1–3 Long-term treatment for IBD involves the use of anti-inflammatory agents (such as 5-aminosalicylic acid, antibiotics) and immunosuppressive medications, including steroids, antimetabolites (mainly thiopurines and methotrexate) and biological drugs (in children mostly infliximab and adalimumab). A key goal of IBD treatment is to improve quality of life, to achieve levels similar to those in healthy people, without limiting the possibility of traveling. Nowadays, traveling required for many jobs, allows individuals to maintain contacts with family and friends, and, for many people, is a passion, hobby, or educational endeavor.
Traveling exposes patients with IBD to gastrointestinal infections, both common infections, such as travelers’ diarrhea, which is primarily caused by enterotoxigenic Escherichia coli, and severe infections, such as cholera. Travelers’ diarrhea affects over 15 million individuals, whereas Vibrio cholerae infections has been documented to affect up to 5 million people, worldwide, annually.Citation4,Citation5 However, the risk of travelers’ diarrhea is higher among IBD patients than among the healthy population.Citation6 The risk of experiences a severe course of travelers’ diarrhea is also higher in patients with IBD and in particular for those who receive immunosuppressive (IS) therapy, which is the primary treatment approach in patients with IBD.Citation7–9 Infections, especially those involving the gastrointestinal tract, such as those caused by E. coli, can trigger IBD exacerbation.Citation10 Moreover, many patients worry about travel-related increased risk of worsening IBD symptoms and an urgent need to use the toilet in unfavorable conditions. Therefore, the prevention of any infectious diseases, including travelers’ diarrhea, represents an important challenge associated with IBD therapy.Citation11
Fortunately, effective cholera vaccines are available, including Dukoral® (Valneva Sweden, Stockholm, Sweden), which targets cholera toxin subunit B (CTB). CTB shares structural and antigenic similarities with the heat-labile toxin produced by enterotoxigenic E. coli, which may allow the cholera vaccine to provide additional protection against travelers’ diarrhea.Citation12–14 Theoretically, this combination could be very beneficial for IBD patients. However, Dukoral® is an oral vaccine, which interacts primarily with the intestinal immune system, the function of which is altered in patients with IBD.Citation15 Moreover, IS therapy can limit the immunogenicity of this vaccine.Citation16 Therefore, international authorities recommend not only the vaccination of IBD patients but also the evaluation of vaccine immunogenicity among this group.Citation17 Thus far, only one study has evaluated the immunogenicity of the cholera vaccine in patients undergoing IS treatment; in renal transplant recipients, the post-vaccination response was weaker than that in healthy controls but more than half of the patients seroconverted.Citation16
In addition, because Dukoral® is administered orally, patients and their doctors may fear the risk of IBD exacerbation.
Therefore, we aimed to evaluate the immunogenicity and safety study of an oral cholera vaccine in children with IBD who are treated with IS therapy.
Materials and methods
Study design
This was an open, multicenter, and prospective observational study, assessing the immune response to the cholera vaccinations among children with IBD and comparing between those treated with and without IS therapy (NCT03998449).
Participants
We enrolled children, older than 6 and younger than 18 years (based on vaccine registration data), under the care of clinical gastroenterology centers in Warsaw and Gdansk for Crohn’s disease (CD) and ulcerative colitis (UC), diagnosed based on standard, clinical, endoscopic, histologic, and radiographic criteria. Disease activity was assessed using the Pediatric Crohn’s Disease Activity Index (PCDAI) scale, for CD, and the Pediatric Ulcerative Colitis Activity Index (PUCAI), for UC.Citation18,Citation19 Values below 10 points were considered to represent remission. Children with severe IBD activity, defined as PCDAI scores ≥ 40 points, for CD, or PUCAI ≥ 65 points, for UC, were excluded, due to the potentially too short exposure time to the vaccine related to diarrhea and the concern that the oral solution may worsen patients’ symptoms.
Based on previous publications and IBD treatment recommendations, IS therapy was defined as treatment with thiopurines (≥ 2.0 mg/kg/day azathioprine or ≥ 1.0 mg/kg/day 6-mercaptopurine), methotrexate, cyclosporine, or biologics, for at least two months, or high-dose corticosteroids (≥ 20 mg/day or ≥ 2 mg/kg/day of prednisone or equivalent), for at least 2 weeks.Citation7,Citation17,Citation20–22 Patients who discontinued or had modified IS treatment less than 3 months prior to the study were excluded.
All children had negative histories of prior vaccination against cholera or of traveling to countries considered to be endemic for cholera infections.Citation23
Intervention
Patients were given two doses of the oral whole-cell Vibrio cholerae (serogroup O1, Inaba, and Ogawa strain), with the recombinant CTB vaccine, Dukoral® (Valneva Sweden, Stockholm, Sweden), with doses separated by 2–3 weeks. The occurrence of adverse reactions, within three days after each dose, and IBD exacerbation, within 4 weeks after each vaccine dose, was assessed by interviewing the patient and caregivers.
Methods
Serum samples were drawn at baseline and 4–6 weeks after the second vaccination dose. The presence of serum anti-CTB IgA and IgG antibodies was assessed by enzyme-linked immunosorbent assay (ELISA, Human Anti-CTB subunit ELISA kit®, Alpha Diagnostic International, San Antonio, Texas, USA). Following the manufacturer’s test recommendations, the positive index was calculated, the achievement of which indicated seroconversion.Citation24
Outcomes
The primary endpoints were the seroconversion rates, for both anti-CTB IgA and IgG, obtained 4–6 weeks after the second vaccination dose. To determine the effects of IS therapy on immunogenicity, the immune response was assessed in two groups, those treated with IS and those without IS treatment (NIS). The secondary endpoints included adverse effects and the occurrence of IBD exacerbation.
Statistical methods
Statistical analyses were performed on two groups, the IS and NIS groups. To determine the statistical significance of demographic dichotomous variables, the χ2 test, with Yates correction for continuity, was used. For the other parameters, the medians were compared with the Mann-Whitney U test. The logistic regression model was used to assess the impacts of therapy and other factors on the immune response. A p-value < .05 was considered significant.
Ethical considerations
This study was approved by the Clinical Research Ethics Committee of the Medical University of Warsaw, Poland (number KB/5/2017). All parents and patients over the age of 16 signed an informed consent form.
Results
Study participants
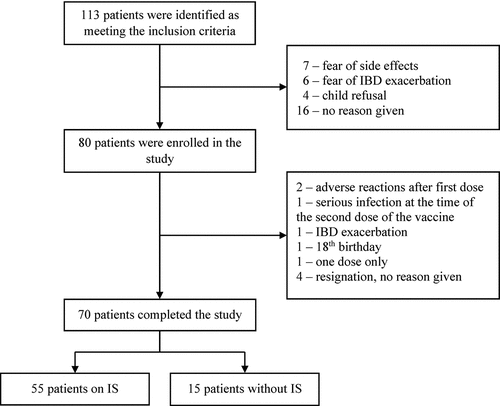
We identified 113 patients that met the inclusion criteria, 80 of whom agreed to participate in the study. Among those patients who did not provide consent to participate and who provided a reason (17/33), the fear of vaccination side effects (41.2%, 7/17) and the fears of exacerbating IBD (35.3%, 6/17) prevailed (). For the reasons presented in , 12.5% (10/80) of patients did not complete the study; of the remaining 70, 78.6% (55/70) were on IS and 21.4% (15/70) were not. Among the IS group, 54.5% (30/55) of children were receiving immunomodulatory drugs, 40.0% (22/55) were treated with the combination of immunomodulatory and biological drugs and only 5.5% (3/55) of children were receiving biological treatments alone. No patients were treated with systemic steroids. No significant demographic differences were identified between the IS and NIS groups, and detailed data are presented in . Due to the selective IgA deficiency, one patient was excluded from the analysis of IgA immune responses.
Table 1. Basic characteristics of the study groups
Immune response to vaccination
Post-vaccination seroconversion was achieved by 33.3% (23/69) of children with IBD, for IgA, and 70.0% (49/70), for IgG. The IgA immune response was better in children with CD than in those with UC (42.5% in CD vs. 20.7% in UC; p = .079), and was relatively comparable between the IS and NIS groups (35.2% vs. 26.7%; p = .904). For IgG, immunogenicity was better in the NIS group (80.0% in NIS vs. 67.3% in IS; p = .161) and in those patients who were in remission (76.0% in the remission group vs. 55.0% in patient without remission; p = .055). None of those factors that could potentially affecting the immune response demonstrated a significant effect. Detailed data are presented in .
Table 2. Factors that may potentially influence the immune response
Among children treated with immunomodulatory agents, 33.3% (10/30) achieved seroconversion for IgA, and 76.7% (23/30) achieved seroconversion for IgG, whereas among children treated with combined IS therapy (immunomodulatory and biological drugs), these numbers were 42.9% (9/21) and 59.1% (13/22), respectively. The type of IS treatment had no significant effect on the achievement of seroconversion (p = .792, OR = 0.831 [0.210–3.290] for IgA, p = .195, OR = 0.376 [0.085–1.653] for IgG).
Adverse reactions
The most serious adverse reaction observed was moderate IBD exacerbation, 3 days after the first vaccine dose, in one patient who discontinued the study for this reason. No other cases of IBD exacerbation have been reported by those patients who completed the study, during the 4-week follow-up period.
The most often reported adverse reactions were flatulence or moderate abdominal pain (15.7%, 22/140 of doses), general malaise (10.7%, 15/140), headache (10.0%, 14/140), and nausea (6.4%, 9/140). In two patients, a transient increase in body temperature was observed. No increase in the mean frequency of bowel movements was reported within three days after vaccination, compared with that before vaccination.
Discussion
To date, no studies have been conducted to examine the immunogenicity of oral cholera vaccine in children or adults with IBD. In addition, no studies have examined the response to this vaccine among children treated with IS therapy. The only similar study involved adult renal transplant recipients receiving IS treatment.Citation16 Therefore, identifying an adequate comparison in the literature for our findings has been difficult. However, other vaccine studies performed in patients with IBD may be used as a point of reference.
Immunogenicity
In our study, seroconversion was achieved in 33% of patients, for IgA, and in 70% of patients, for IgG. We did not plan comparisons with a control group, but based on previous publications, post-vaccine anti-CTB seroconversions in healthy children have been estimated to reach as high as 81%–90%, for IgA, and 75%–81%, for IgG, which indicated that the seroconversion rate in IBD patients, especially those for IgA, is lower than those in healthy individuals.Citation16,Citation25,Citation26 This finding contradicts the results of our recently published meta-analysis, which indicated that the immune response in children with IBD was not significantly reduced relative to the response in healthy children.Citation27 However, all of these studies examined parenterally administered, instead of orally administered, vaccines. The oral cholera vaccine was designed to induce an immune response in contact with the gastrointestinal mucosa, which is involved in IBD inflammation. This could potentially explain the reduced immunogenicity observed in our patients compared with that reported in healthy individuals.
This study showed a lower overall seroconversion rate for IgA compared with IgG (33% vs. 70%). The cholera vaccine is administered orally and induces an immune response at the level of the intestinal mucosa, and IgA is considered to be primarily responsible for mucosal immunity.Citation28 However, the presence of IgA in the serum may not reflect the IgA in the gastrointestinal tract; therefore, to increase the credibility of this study, we decided to evaluate both IgA and IgG. However, the evaluation was performed 4–6 weeks after vaccination, when serum IgA levels generally begin to decline and IgG reaches their peak levels, which may explain our results.Citation29 In future studies, an additional checkpoint should be added where antibodies will be measured approximately 10–14 days after each dose, which will increase the reliability of the results, especially for IgA.
Despite the increased IgG immunogenicity (80% vs. 67%) and slightly lower IgA seroconversion rate (27% vs. 35%) observed for the NIS group of patients compared with the IS group, respectively, the results of our study did not indicate the presence of significant IS therapy effects on vaccine response. This finding is consistent with previous observations, performed for many vaccines in IBD children who receive IS treatment.Citation27 However, a meta-analysis of studies performed in adults obtained the opposite results, with general vaccine response appearing to be reduced among patients with IBD who receive IS therapy.Citation30 Existing data on children who receive IS therapy for other reasons, aside from IBD, have not been conclusive, although, in most cases, they manage to achieve an adequate vaccine response.Citation31–34 The only publication that assessed the immunogenicity of the cholera vaccine in IS-treated adults (renal transplant recipients) showed the significantly reduced immunogenicity of this group (57% in IS vs 81% in healthy ones).Citation16 This result may be due to functional differences in the immune system between children and adults, in addition to the potential effects of disease duration, IS therapy duration, and IS therapy type.Citation35 In our study, we found no significant effect of IBD duration and IS type on immunogenicity. However, it can be assumed that the duration of IBD, as well as its treatment, in adults is longer than in children. Unfortunately, due to the limited size of our present study group, the reliable interpretation of the impacts of specific IS therapy types (e.g., biological) on immunogenicity was difficult.
In our study, we decided to evaluate the development of anti-CTB antibodies in the serum. The oral cholera vaccine primarily causes an intestinal response. Therefore, the presence of antibodies, especially IgA, in the serum represents an imperfect method for the assessment of the vaccine response and may not correspond with protection against cholera.Citation36 A much more precise method of determining the immunogenicity of a cholera vaccine is antibodies in lymphocyte supernatant (ALS) and antibody-secreting cell (ASC) assays.Citation37 However, due to the limited availability of appropriate laboratories and personnel, serum anti-CTB antibodies assessment has been widely used during immunogenicity studies performed for this vaccine, in the past.Citation2,Citation16,Citation26 In addition, no generally accepted cutoff point for the anti-CTB antibody titer has been established. Therefore, we opted to use the positive index threshold, as recommended by the ELISA kit manufacturer.Citation24
It is worth noting that new vaccines against E. coli-induced diarrhea are underway in clinical trials, and the results of studies in Guatemala, Mexico, Bangladesh, and Africa appear very promising.Citation38–40 Which makes the perspective of preventing travelers’ diarrhea even more optimistic.
Safety
We found that the oral cholera vaccine was safe. The reported adverse reactions to the vaccine among our patients were consistent with those described in previous studies, as were the product characteristics.Citation41 The incidence of adverse reactions in healthy people ranges from 1.7% for fever to as much as 57.0% for abdominal bloating.Citation39,Citation42,Citation43 In a study with IS treated patients, the overall incidence adverse reactions was 29.0%.Citation16 The predominantly reported mild symptoms (abdominal bloating and discomfort) were likely to be related with the ingestion of the foaming, gastric acid-neutralizing component of the vaccine.
Only one case of IBD exacerbation was reported during the follow-up period in our study, which is similar to the results of other studies on immunogenicity of vaccination in patients with IBD, although most studies did not evaluate the effect of vaccination on disease activity.Citation44–46
Advantages and limitations
This is the first study to assess the immunogenicity of the cholera vaccine among patients with IBD and is also the first study to assess the immune response to cholera vaccine among children treated with IS. The most important finding identified in this study is that oral vaccine is safe for patients with gut diseases. Moreover, our results suggest that the cholera vaccine may allow IBD patients to travel more safely, allowing them to “explore the world”.
The primary limitation of this study was the relatively small number of participants, especially patients receiving NIS treatments, which results from limited number of patients without IS treatment in the population of children with IBD and the global epidemiological situation (COVID-19 pandemic), which significantly limited patient recruitment. The lack of a healthy control group and randomization can also be considered a limitation. However, the immunogenicity of oral cholera vaccine in healthy children has been previously established in several studies, so we could compare these data with results of our study.Citation16,Citation25,Citation26 Considering relatively low strength of evidence, a study with more participants and possibly a control group is needed in the future.
In addition, the immune response, which was assessed by the presence of antibodies in the serum, may not entirely correspond with that in the intestine.
Conclusions
The oral cholera vaccine is safe and well-tolerated in children with IBD. The immunogenicity of the oral cholera vaccine in children with IBD was lower than previously observed in healthy ones. The treatment type does not seem to affect the immunogenicity of the vaccine; however, further studies on larger groups of patients are needed.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflicts of interest.
All authors read and approved the final manuscript.
The datasets generated and analyzed during this study are available from the corresponding author, on reasonable request.
Acknowledgments
We are very grateful to Imed Poland, for facilitating the purchase of Dukoral®. We also thank dr. Marcin Banasiuk for the valuable advice regarding statistical analyses, and dr. Elżbieta Górska and dr. Jan Bukowski for their support during the project.
Additional information
Funding
References
- Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: results of the European Federation of Crohn’s and ulcerative colitis associations (EFCCA) patient survey. J Crohns Colitis. 2007;1(1):10–20. doi:10.1016/j.crohns.2007.06.005.
- Larsson K, Lööf L, Rönnblom A, Nordin K. Quality of life for patients with exacerbation in inflammatory bowel disease and how they cope with disease activity. J Psychosom Res. 2008;64(2):139–48. doi:10.1016/j.jpsychores.2007.10.007.
- Habibi F, Habibi ME, Gharavinia A, Mahdavi S, Akbarpour M, Baghaei A, Emami M. Quality of life in inflammatory bowel disease patients: a cross-sectional study. J Res Med Sci. 2017;22:104. doi:10.4103/jrms.JRMS_975_16.
- Steffen R. Epidemiology of traveler’s diarrhea. Clin Infect Dis. 2005;41:S536‐S540.
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459‐1544.
- Ellul P, Fenech VA, Azzopardi C, Callus L, Delicata N, Muscat J, Azzopardi N, Vassallo M. Diarrhoeal episodes in travellers suffering from IBD. Frontline Gastroenterol. 2013;4(2):120–24.
- Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, de Carpi JM, Bronsky J, Veres G, Aloi M, Strisciuglio C, Braegger CP, et al. Management of paediatric ulcerative colitis, part 1: ambulatory care-an evidence-based guideline from European Crohn’s and colitis organization and European society of paediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr. 2018;67(2):257‐291.
- Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, Amil Dias J, Barabino A, Braegger CP, Bronsky J. European Crohn's and Colitis Organisation; European Society of Pediatric Gastroenterology, Hepatology and Nutrition. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8(10):1179‐270.
- Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155(2):337‐346.e10.
- Axelrad JE, Joelson A, Green PHR, Lawlor G, Lichtiger S, Cadwell K, Lebwohl B. Enteric infections are common in patients with flares of inflammatory bowel disease. Am J Gastroenterol. 2018;113(10):1530‐1539. doi:10.1038/s41395-018-0211-8.
- Orlicka K, Barnes E, Culver EL. Prevention of infection caused by immunosuppressive drugs in gastroenterology. Ther Adv Chronic Dis. 2013;4(4):167‐185. doi:10.1177/2040622313485275.
- Weinke T, Liebold I, Burchard GD, Frühwein N, Grobusch MP, Hatz C, Kollaritsch H, Nothdurft HD, Reisinger E, Rieke B, et al. Prophylactic immunisation against traveller’s diarrhoea caused by enterotoxin-forming strains of Escherichia coli and against cholera: does it make sense and for whom? Travel Med Infect Dis. 2008;6(6):362‐367. doi:10.1016/j.tmaid.2006.05.003.
- Chen WH, Garza J, Choquette M, Hawkins J, Hoeper A, Bernstein DI, Cohen MB. Safety and immunogenicity of escalating dosages of a single oral administration of peru-15 pCTB, a candidate live, attenuated vaccine against enterotoxigenic Escherichia coli and Vibrio cholerae. Clin Vaccine Immunol. 2015;22(1):129‐13. doi:10.1128/CVI.00560-14.
- Ahmed T, Bhuiyan TR, Zaman K, Sinclair D, Qadri F. Vaccines for preventing enterotoxigenic Escherichia coli (ETEC) diarrhoea. Cochrane Database Syst Rev. 2013;2013(7):CD009029.
- Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut. 2013;62(11):1653‐1664. doi:10.1136/gutjnl-2012-303955.
- Jonker EFF, Uijlings MAC, Visser LG, Soonawala D. Comparison of the immunogenicity of Dukoral® oral cholera vaccine between renal transplant recipients on either a calcineurin inhibitor or mycophenolate – a controlled trial. Vaccine. 2019;37(23):3133‐3139. doi:10.1016/j.vaccine.2019.04.010.
- Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, G. D, Ehehalt R, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8(6):443‐468. doi:10.1016/j.crohns.2013.12.013.
- Turner D, Griffiths AM, Walters TD, Seah T, Markowitz J, Pfefferkorn M, Keljo D, Otley A, LeLeiko NS, Mack D, et al. Appraisal of the pediatric Crohnʼs disease activity index on four prospectively collected datasets: recommended cutoff values and clinimetric properties. Am J Gastroenterol. 2010;105(9):2085–92. doi:10.1038/ajg.2010.143.
- Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, Walters TD, Zachos M, Mamula P, Beaton DE, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133(2):423–32. doi:10.1053/j.gastro.2007.05.029.
- Centers for Disease Control and Prevention. Recommendations of the Advisory Committee on Immunization Practices (ACIP): use of vaccines and immune globulins for persons with altered immunocompetence. MMWR Recomm Rep. 1993;42(RR–4):1–18.
- Mamula P, Markowitz JE, Piccoli DA, Klimov A, Cohen L, Baldassano RN. Immune response to influenza vaccine in pediatric patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5(7):851–56. doi:10.1016/j.cgh.2007.02.035.
- Banaszkiewicz A, Targońska B, Kowalska-Duplaga K, Karolewska-Bochenek K, Sieczkowska A, Gawrońska A, Grzybowska-Chlebowczyk U, Krzesiek E, Łazowska-Przeorek I, Kotowska M, et al. Immunogenicity of 13-valent pneumococcal conjugate vaccine in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(7):1607–14. doi:10.1097/MIB.0000000000000406.
- WHO. Cholera Country Profiles. Available at: https://www.who.int/cholera/countries/en. Accessed 3 June 2020.
- Human anti-cholera toxin B subunit ELISA kit® instruction manual M-945-110-CTG, Alpha diagnostic international. [accessed 2020 June 28]. https://www.4adi.com.
- Saha A, Chowdhury MI, Nazim M, Alam MM, Ahmed T, Hossain MB, Hore SK, Sultana GNN, Svennerholm A-M, Qadri F, et al. Vaccine specific immune response to an inactivated oral cholera vaccine and EPI vaccines in a high and low arsenic area in Bangladeshi children. Vaccine. 2013;31(4):647–52. doi:10.1016/j.vaccine.2012.11.049.
- Ahmed T, Svennerholm AM, Al Tarique A, Sultana GN, Qadri F. Enhanced immunogenicity of an oral inactivated cholera vaccine in infants in Bangladesh obtained by zinc supplementation and by temporary withholding breast-feeding. Vaccine. 2009;27(9):1433–39. doi:10.1016/j.vaccine.2008.12.036.
- Ł D, Dziekiewicz M, Banaszkiewicz A. Immune response to vaccination in children and young people with inflammatory bowel disease: a systematic review and meta-analysis [published online ahead of print, 2020 Jun 15]. J Pediatr Gastroenterol Nutr. 2020;71(4):423–32. doi:10.1097/MPG.0000000000002810.
- Li Y, Jin L, Chen T. The effects of secretory IgA in the mucosal immune system. Biomed Res Int. 2020;2020:2032057. Published 2020 Jan 3.
- Uddin T, Harris JB, Bhuiyan TR, Shirin T, Uddin MI, Khan AI, Chowdhury F, LaRocque RC, Alam NH, Ryan ET, et al. Mucosal immunologic responses in cholera patients in Bangladesh. Clin Vaccine Immunol. 2011;18(3):506–12. doi:10.1128/CVI.00481-10.
- Nguyen DL, Nguyen ET, Bechtold ML. Effect of immunosuppressive therapies for the treatment of inflammatory bowel disease on response to routine vaccinations: a meta-analysis. Dig Dis Sci. 2015;60(8):2446–53. doi:10.1007/s10620-015-3631-y.
- Nelson DR, Fadrowski J, Neu A. Immunogenicity of the meningococcal polysaccharide conjugate vaccine in pediatric kidney transplant patients. Pediatr Nephrol. 2018;33(6):1037–43. doi:10.1007/s00467-017-3878-y.
- Maritsi DN, Coffin SE, Argyri I, Vartzelis G, Spyridis N, Tsolia MN. Immunogenicity and safety of the inactivated hepatitis A vaccine in children with juvenile idiopathic arthritis on methotrexate treatment: a matched case-control study. Clin Exp Rheumatol. 2017;35:711–15.
- Hung TY, Kotecha RS, Blyth CC, Steed SK, Thornton RB, Ryan AL, Cole CH, Richmond PC. Immunogenicity and safety of single-dose, 13-valent pneumococcal conjugate vaccine in pediatric and adolescent oncology patients. Cancer. 2017;123(21):4215–23. doi:10.1002/cncr.30764.
- MacIntyre CR, Shaw P, Mackie FE, Boros C, Marshall H, Barnes M, Seale H, Kennedy SE, Moa A, Hayen A, et al. Immunogenicity and persistence of immunity of a quadrivalent human papillomavirus (HPV) vaccine in immunocompromised children. Vaccine. 2016;34(36):4343–50. doi:10.1016/j.vaccine.2016.06.049.
- Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32:e00084–18.
- Bishop AL, Camilli A. Vibrio cholerae: lessons for mucosal vaccine design. Expert Rev Vaccines. 2011;10:79–94.
- Qadri F, Ryan ET, Faruque AS, Ahmed F, Khan AI, Islam MM, Akramuzzaman SM, Sack DA, Calderwood SB. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect Immun. 2003;71(8):4808–14.
- Sack DA, Shimko J, Torres O, Bourgeois AL, Francia DS, Gustafsson B, Kärnell A, Nyquist I, Svennerholm AM. Randomised, double-blind, safety and efficacy of a killed oral vaccine for enterotoxigenic E. Coli diarrhoea of travellers to Guatemala and Mexico. Vaccine. 2007;25(22):4392–400.
- Qadri F, Akhtar M, Bhuiyan TR, Chowdhury MI, Ahmed T, Rafique TA, Khan A, Rahman SIA, Khanam F, Lundgren A, et al. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2020;20(2):208–19.
- Promising preliminary findings from a Phase 2b study of the oral vaccine ETVAX® against travelers’ diarrhea in Finnish travelers to Benin, West Africa. [accessed 2020 Dec 16]. https://scandinavianbiopharma.se/promising-preliminary-findings-from-a-phase-2b-study-of-the-oral-vaccine-etvax-against-travelers-diarrhea-in-finnish-travelers-to-benin-west-africa.
- Dukoral® – product characteristics. [accessed 2020 July 04]. https://www.ema.europa.eu/en/documents/product-information/dukoral-epar-product-information_en.pdf.
- Sanchez JL, Hayashi KE, Kruger HF, Meza R, English CK, Vidal W, Svennerholm AM, Taylor DN. Immunological response to Vibrio cholerae O1 infection and an oral cholera vaccine among Peruvians. Trans R Soc Trop Med Hyg. 1995;89(5):542–45.
- Baik YO, Choi SK, Olveda RM, Espos RA, Ligsay AD, Montellano MB, Yeam JS, Yang JS, Park JY, Kim DR, et al. A randomized, non-inferiority trial comparing two bivalent killed, whole cell, oral cholera vaccines (Euvichol vs Shanchol) in the Philippines. Vaccine. 2015;33(46):6360–65.
- Lee CK, Kim HS, Ye BD, Lee KM, Kim YS, Rhee SY, Kim HJ, Yang SK, Moon W, Koo JS, et al. Korean Association for the Study of Intestinal Diseases (KASID) Study. Patients with Crohn’s disease on anti-tumor necrosis factor therapy are at significant risk of inadequate response to the 23-valent pneumococcal polysaccharide vaccine. J Crohns Colitis. 2014;8(5):384–91.
- Satyam VR, Li PH, Reich J, Qazi T, Noronha A, Wasan SK, Farraye FA. Safety of recombinant zoster vaccine in patients with inflammatory bowel disease. Dig Dis Sci. 2020;65(10):2986–91. doi:10.1007/s10620-019-06016-4.
- Rahier JF, Papay P, Salleron J, Sebastian S, Marzo M, Peyrin-Biroulet L, Garcia-Sanchez V, Fries W, van Asseldonk DP, Farkas K, et al. European Crohn's and Colitis Organisation (ECCO). H1N1 vaccines in a large observational cohort of patients with inflammatory bowel disease treated with immunomodulators and biological therapy. Gut. 2011;60(4):456–62.