ABSTRACT
Background
This study was conducted to compare the immunogenicity and safety profile of two quadrivalent influenza vaccines (QIVs) in healthy adults (18–60 years) and elderly (>61 years) participants.
Method
This phase III study was conducted from March 2018 to April 2018 across 12 sites in India. In this randomized, observer-blind, active-controlled study, 480 participants were randomized to receive a single dose of test vaccine (subunit, inactivated influenza vaccine; Influvac® Tetra, Abbott) (n = 240) or reference vaccine (split virion, inactivated influenza vaccine; VaxiFlu-4, Zydus Cadilla Healthcare) (n = 240). The primary objective was to describe and compare the immunogenicity of each vaccination group based on hemagglutination inhibition (HI) assay seroprotection and seroconversion rates, and geometric mean fold increase (GMFI) against four vaccine strains in two age groups. Safety and reactogenicity were also compared for the vaccines in both the age groups.
Results
The pre- and post-vaccination HI titers for both the vaccines were comparable. The GMFI varied from 4.3 – 22.7 in the test and 3.7–21.6 in the reference vaccine group. The seroprotection rates were >90% for the A-strains and ranged between >43% and <60% for B-strains for both the vaccines. Seroconversion rates varied between 41.4% and 78.8%. Overall, the reported adverse events (AEs) for both the vaccines were <1% and comparable. Reported local and systemic reactions were comparable.
Conclusion
Influvac® Tetra elicited an adequate immune response with a favorable safety profile which was comparable with the reference vaccine. (Clinical trial registry number: CTRI/2018/02/012222)
Introduction
Influenza is a major public health concern and occurs in epidemic proportions across the world each year during different seasons. In healthy individuals, the influenza symptoms mostly resolve on their own; however, in case of children (6 − 60 months), elderly (> 65 years), pregnant women, and people with chronic medical conditions, the risk of influenza-related complications such as serious illness, hospitalization, and death is relatively high. Hence, for the aforesaid high-risk groups, preventive intervention in the form of vaccine is warranted.Citation1 Along with these high-risk groups, the World Health Organization (WHO) also recommends vaccines for healthcare workers.Citation2 Though antiviral drugs are available to treat patients, they are effective when administered within 48 hours of the appearance of symptoms, and in majority of the cases, diagnosis of the influenza symptoms is difficult within a stipulated time.Citation3,Citation4 Antiviral drugs are considered second-line defense and not as a substitute to influenza vaccine.Citation5
Vaccines remain the most effective preventive strategy against influenza. The currently available options for the influenza vaccine include trivalent and quadrivalent influenza vaccines. Trivalent influenza vaccines (TIV) contain two Influenza A-strains (A/H1N1, A/H3N2) and one strain of Influenza B, while the quadrivalent influenza vaccine (QIV) includes two B lineages (Victoria and Yamagata), thereby improving the protection against influenza.Citation6 Various other studies have shown that QIV is equivalent to TIV for the common strains and superior for the extra added B strain.Citation7,Citation8 Additionally, QIV may offer an increased range of protection against influenza, thereby, reducing the burden of infections and disease-related costs.Citation8,Citation9
Due to the antigenic drift phenomenon, WHO provides recommendations for updating the composition of the influenza vaccine every 6 months to ensure protection against the strains prevailing in the northern and southern hemispheres.Citation10 In temperate climates, seasonal epidemics of influenza occur mainly during the winter, while in tropical regions, influenza may occur throughout the year causing irregular outbreak.Citation11 Routine annual influenza vaccination of population aged ≥ 6 months, who do not have any contraindications, has been recommended by the Advisory Committee on Immunization Practices (ACIP) since 2010.Citation12
As per the National Center for Disease Control, a total of 28,798 cases of H1N1 influenza have been reported across India in the year 2019, which also included 1218 reported deaths.Citation13 Between 2010 and 2019, about 22,778 cases of H1N1, 6175 cases of H3N2, 248 cases of Yamagata strain, and 526 cases of Victoria strain were reported in India.Citation14 However, this could be only the tip of the iceberg due to underreporting of cases. India presents another unique challenge regarding the peak seasonality of influenza. Based on its geography, India has a wide variation in the climate. Even though the country is located in the northern hemisphere, distinct seasonality ranging from tropical to subtropical is observed. Some northern states face a temperate climate, with moderate to severe winters. This leads to a wide variation in peak influenza seasonality across different states in India, which in turn leads to the differences in ideal influenza vaccination time. Hence, cities with temperate seasonality will benefit from influenza vaccination in September–October, while cities with the monsoon season in July–September will benefit from influenza vaccination in April–May.Citation15 In a resource-limited country like India, where there is already a huge burden of other communicable diseases, influenza can exert pronounce health impact.
Though numerous global studies are published showcasing the immunogenicity and safety profile of the QIV across various age groups, there is a dearth of comparative immunogenicity and safety data of different QIVs in India. Hence, the primary objective of this randomized clinical study was to describe and compare the immunogenicity (post-vaccination geometric mean HI antibody titers, seroprotection, and seroconversion rates for each of the four vaccine strains) of a subunit versus split QIV in healthy Indian adults (aged 18 to 60 years) and elderly population (≥61 years). The safety (unsolicited AEs) and tolerability (reactogenicity) were also assessed in this population.
Methods
Study design and implementation
This Phase III, randomized, two-arm, observer-blind, parallel-group, active-controlled clinical study (Clinical trial registry number: CTRI/2018/02/012222; DCGI permission letter number: CT-04/2018) evaluating the immunogenicity, safety and reactogenicity of two QIVs in Indian adults (aged 18 years to 60 years) and elderly adults (aged ≥61 years) was conducted between March 2018 and April 2018. The study was conducted across 12 sites (one site each in Nashik, Davangere, Bengaluru, Lucknow, Secunderabad, Srikakulam, Mysuru and Pune and two sites each in Ahmedabad and Varanasi) in India. Written approval for the study was taken from the Independent Ethics Committee/Institutional Review Board registered with the health authority of India (Appendix A)and the study was conducted in compliance with Good Clinical Practices and the applicable national regulations to assure that the rights, safety, and wellbeing of all the participants were protected, consistent with the ethical principles that have their origin in the Declaration of Helsinki. The source data was retained by each center. The data in the eCRF were analyzed and reported by the CRO, IQVIA.
Study participants
Eligible participants were males and females who could provide written informed consent and had stable health. Females of childbearing potential were also enrolled in the study if a) they had practiced highly effective contraception for 30 days prior to study vaccination, b) had a negative urine pregnancy test on the day of vaccination, and c) had agreed to continue highly effective contraception during the entire study period.
Exclusion criteria for the participants included: history of adverse reaction or hypersensitivity to influenza vaccines or its components; history of Guillain-Barré syndrome, other progressive neurological diseases or seizures; immunocompromised, prior receipt of study vaccination or influenza vaccine or laboratory-confirmed influenza infection within the 6 months preceding enrollment; prior receipt of any seasonal or pandemic influenza vaccine; fever and/or acute disease or infection on the day of the study vaccination; any medication that influenced the immune system during 3 months prior to the study vaccination or planned used during the study; receipt of immunoglobulins or any blood products within the 3 months preceding the study vaccination and planned administration during the study period; use of cytotoxic drugs, anticancer chemotherapy or radiation therapy within 36 months before the day of the study vaccination; being a solid organ or bone marrow/stem cell transplant recipient; participation in a placebo-controlled influenza vaccine clinical trial at any time prior to entering this study if the treatment arm was not known; any condition that as per the investigator’s opinion would pose a health risk to the participant or could interfere with the evaluation of the vaccine; receipt of any other investigational agent within 30 days prior to study vaccination or planned exposure during the entire study period; drug/alcohol abuse; planned surgery requiring general anesthesia or inpatient hospitalization for at least 24 hours during the entire study period; being an employee or family member of the sponsor/contract research organization; any applicable contraindication as per the prescribing information of the reference vaccine.
The eligible participants were randomized in a 1: 1 ratio to receive either test vaccine (subunit, inactivated influenza vaccine; Influvac® Tetra from Abbott) or reference vaccine (split virion, inactivated influenza vaccine; VaxiFlu-4 from Zydus Cadilla Healthcare) using an interactive web response system (IWRS). A simple randomization technique with a unique number was followed. The randomization scheme was provided by Abbott. The randomization was stratified for age; subjects were randomized 1:1 into two groups: adults 18–60 years and elderly 61 years of age and older. The study investigators, participants and those responsible for evaluating, reviewing or analysis of study data remained blinded. The vaccination was performed by a trained medical site study personnel who did not participate in any of the clinical evaluation. For emergency unblinding, the IWRS could be used. Each participant received one 0.5 mL dose of test or reference vaccine, delivered by intramuscular injection in the deltoid muscle of the upper arm on day 1 of the study. For both the vaccines, active substance contained 15 microgram (mcg) of hemagglutinin of the four vaccine strains (an A/Michigan/45/2015 (H1N1)pdm09-like strain; an A/Hong Kong/4801/2014 (H3N2)-like strain; a B/Brisbane/60/2008-like strain and a B/Phuket/3073/2013-like strain) recommended by the WHO, for the strains recommended for the for the northern hemisphere 2017/2018 season.
Immunological endpoints and assessment
Post-vaccination geometric mean hemagglutination inhibition (HI) antibody titers, seroprotection rate, seroconversion rate, and the geometric mean fold increase (GMFI) against the four vaccine strains (A/H1N1, A/H3N2, B/Yamagata lineage, and B/Victoria lineage) constituted the immunogenicity endpoints. Sampling for serology was performed on visit one (day 1) and visit two (28–33 days after the last vaccination). Antibody titrations were performed by the HI technique by VisMederi srl, Siena, Italy. After sera were separated from the blood sample, it was kept frozen at −20°C until titration. All antibody titrations were done in duplicate. The titer assigned to a sample was the geometric mean of the two measurements. Pre- and post-vaccination sera were titrated in the same experiment. Seroprotection rates were defined as the proportion of participants with HI titers ≥ 40 following vaccination. Seroconversion rates were defined as the proportion of participants with a pre-vaccination titer < 10 and a post-vaccination titer ≥ 40 or a pre-vaccination titer ≥ 10 and at least a fourfold post-vaccination increase.
Seroprotection rates, seroconversion rates, and geometric mean fold increases were compared according to the derived serology criteria that have been used by the Committee for Medicinal Products for Human Use (CHMP) by the European Medicines Agency to define a satisfactory immune response to influenza vaccination in the context of the requirements for annual influenza strain updates. These criteria include seroprotection rate >70% (adults) or >60% (elderly), seroconversion rate >40% (adults) or >30% (elderly) and geometric mean fold increase >2.5 (adults) or >2.0 (elderly). Though our study has used the CHMP criteria, recent changes in EU guidelines replaced the assessment criteria by a diversified approach of measuring and reporting the geometric mean titer (GMT) data, reverse cumulative distribution (RCD) curves and seroconversion rates.Citation16,Citation17
Safety endpoints and assessment
The safety endpoints included a description of the local, systemic reactions and overall inconvenience during the first 7 days after each study vaccination in each age group and overall unsolicited AEs for 28 days. All AEs up to visit two of the study were reported on a per-subject basis. The immediate adverse reactions following vaccination were observed within 30 minutes of vaccine administration. The frequency and severity of any local reactions (injection site pain, redness, swelling) or systemic reactions (fever, headache, malaise, myalgia, arthralgia, fatigue, sweating, and shivering) and overall inconvenience experienced during the first 7 days of vaccination were recorded by participants in home diaries. Participants were further instructed to report influenza-like illness (ILI) until the end of the study by telephone.
Statistical methods
A sample size of 480 participants secured an overall statistical power of 0.9 ensuring that the CHMP criterion for seroconversion was met for all four strains. The sample size allowed for a drop-out rate of up to 7% in the adults and up to 14% in the elderly population. Summary statistics for immunogenicity endpoints were presented for each strain and each age group for each of the received vaccine. The quantifiable primary immunogenicity endpoints were summarized by the number of observations (n), geometric mean, geometric standard deviation and corresponding 95% confidence interval (CI) for the geometric mean for participants with data. For qualitative endpoints, per category, the numbers and proportions of participants with non-missing data (n, %) and corresponding 95% CI for the proportions using the Clopper–Pearson method was presented. The statistical analysis was carried out using the SAS® system version 9.4.
Results
Disposition of participants
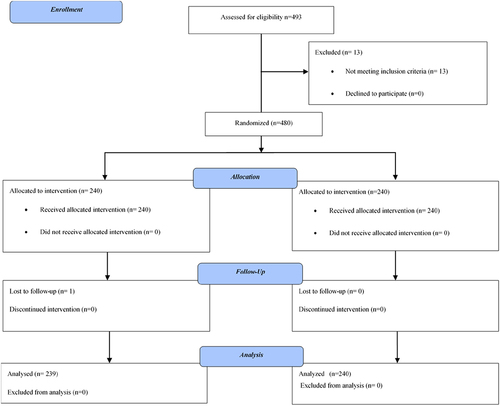
A total of 480 of the consented participants (n = 493) enrolled in the study were randomized to receive test (n = 240) and reference (n = 240) vaccine, respectively. In total, 479 (99.8%) participants completed the study; 239 (99.6%) participants in the test vaccine and 240 (100%) participants in the reference vaccine group. Only one (0.2%) participant in the test vaccine group prematurely terminated the study due to loss to follow-up ().
Demographic and baseline characteristics
The proportion of adults and elderly participants were similar in each vaccine group. Overall, the mean age was 51.6 (±18.1) years with 51.0% (n = 245) males and 49.0% (n = 235) females (). The mean age of the participants in ≥18 years group was 36.0 (± 11.2) years (males: 45.5% [n = 110]; females: 54.5% [n = 132]); mean age of the participants in ≥61 years group was 67.4 (± 5.7) years (males: 56.7% [n = 135]; females: 43.3% [n = 103]). The most commonly reported comorbidities were hypertension (13.8%), followed by post menopause (6.7%) and Type 2 diabetes mellitus (6.5%). The demographics and baseline characteristics were similar across both the vaccine groups ().
Table 1. Demographics and baseline characteristics of participants
Immunogenicity
The GMT and GMFI in HI titers are presented in . Pre-vaccination HI titers were comparable between the two vaccine groups, for both the age groups. The two A-strains showed higher pre-vaccination HI titers than the two B-strains in both the groups. Post-vaccination HI titers were also comparable between the two vaccine groups, for both the age groups. The HI titers were higher for the two A-strains as compared to B-strains. This trend was similar for both adult and elderly adult participants. The GMFIs varied between 4.3 − 22.7 in the test group and between 3.7 − 21.6 in the reference group. The GMFIs were slightly higher in adults as compared to elderly adult participants. Also, GMFI was higher for the A-strains as compared to the B-strains.
Table 2. Geometric mean hemagglutination inhibition (HI) Titer by strain and geometric mean fold increase in HI titer by strain
The seroprotection and seroconversion rates are presented in . Seroprotection rates were comparable between the test and the reference vaccine and were higher in adults as compared to elderly adult participants. Overall, the seroprotection rates were > 90% for the A-strains and ranged between > 43% and < 60% for B-strains for both the vaccine groups. Seroconversion rates varied between 41.4%−78.8% and were lower for the B-strains as compared to the A-strains. In general, the seroconversion rates were slightly higher in adults as compared to elderly adult participants and were comparable between the two vaccine groups.
Table 3. Seroprotection and seroconversion rates based on hemagglutination inhibition titer by strain
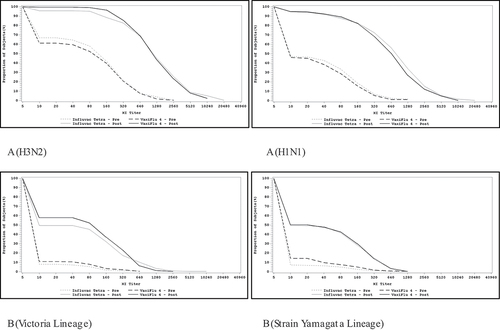
The RCD curves for HI assay for A (H3N2), A (H1N1), B-Strain Victoria lineage, and B-strain Yamagata lineage strains are provided in . Pre and post-vaccination HI RCD curves were comparable for all the four viral strains, for both vaccines. Furthermore, RCD curves between the two vaccine groups across both age groups were comparable.
Safety
Frequency of TEAEs was low in both the vaccine groups (0.8% and 0.4% in the test and reference group, respectively) (). The TEAEs are summarized in . None of the TEAEs were found to have a causal relationship with the study vaccines. Majority of the participants in both the vaccine groups did not experience any inconvenience after the vaccination (96.5% and 97.5% in the test and reference group, respectively). The reported inconvenience was of mild severity. None of the participant in the study reported severe inconvenience after vaccination with test or reference vaccine. The proportion of participants reporting inconvenience after vaccination were similar in adults and the elderly.
Table 4. Overall summary of adverse events
Table 5. Summary of treatment emergent adverse events
Local and systemic reactions within 7 days after vaccination were low (< 5%) and similar between the test and reference vaccine. Local reactions were observed in 2.9% (n = 7) participants in the test and 4.2% (n = 10) in the reference group. The most common local reaction was vaccination site pain (2.5% and 3.0% in test and reference groups, respectively). Overall, all local reaction symptoms lasted for 1 to 2 days for most of the participants and were mild in severity for both the vaccine groups.
Systemic reactions were observed in 4.6% (n = 11) participants in the test and 3.3% (n = 8) participants in the reference group. The most common systemic reaction was fever (2.9% and 1.6% in test and reference groups, respectively). Most of the systemic reactions were mild or moderate in severity. The incidence of systemic and local reactions was slightly higher in adult participants compared to elderly adults’ participants. No deaths were reported in the study. There were no serious adverse events (SAEs) or TEAEs leading to study termination.
Discussion
The result from this Phase III active-controlled clinical study demonstrated that both test and reference QIVs were immunogenic and had an acceptable safety profile in adults and elderly in the Indian population. The study data suggested that the immunogenicity profiles of the two QIVs were comparable and the difference in immune response in the adult and elderly population was consistent with previously published literature findings.Citation18–22 Pre-and post-vaccination HI titers were comparable between both vaccines and age groups. The post-vaccination HI titers were higher for the two A-strains compared to B-strains and the trend was similar in both vaccines and age groups. Overall, the seroprotection rates were > 90% for the A-strains and ranged between43% and 60% for B-strains for both vaccines. Seroprotection rates were similar between the two vaccination groups irrespective of the age. For both vaccines, seroconversion rates were lower for the B-strains (ranging between 41% and 52%) as compared with A-strains (ranging between 71% and 79%) and were slightly higher in adults compared to the elderly, which was in line with previously published literature.Citation23,Citation24 Post-vaccination HI titers, GMFI, and GMT were slightly higher in adults as compared to elderly adults. This difference in immunogenic parameters can be attributed to immunosenescence, since it has been reported that the influenza vaccine becomes < 50% effective in the elderly, even with a well-matched vaccine, leading to a higher susceptibility to influenza and higher mortality.Citation25,Citation26
With regard to safety, both test and reference vaccine reported similar local and systemic reactogenicity profiles. The incidence of systemic and local reactions was slightly higher in adult participants compared to the elderly participants which is in line with previously reported literature.Citation8,Citation27 Overall, no systemic reaction or local reaction symptoms lasted for more than 2 days and majority of participants did not report any inconvenience following vaccination. None of the TEAEs were considered to have a causal relationship with the study vaccine.
Though the study had robust methodology, we duly acknowledge few limitations of our study. The study included healthy participants. With non-inclusion of the high-risk groups and immunocompromised patients in the study, generalizability of the results to the entire population warrants caution. Though, the results cannot be generalized, the immunogenic assessment i.e. HI assay used in our study is known for its universality and accuracy.Citation28,Citation29 Furthermore, the subunit vaccines undergo an additional purification step for removal of the internal sub viral core and contain less protein when compared to the split vaccines. Hence, the former has been reported generally less reactogenic.Citation30–33 However, the above safety correlation could not be demonstrated in our study possibly due to small sample size. Lastly, our study included adult and elderly participants, the current findings cannot be applied to children and adolescents. Hence further studies are warranted to assess the immunogenicity and safety profile of QIVs in children and adolescent population.
In conclusion, this study was uniquely positioned to present the immunogenicity and safety data of a subunit QIV in Indian context. In this study, it was demonstrated that the immunogenicity was comparable between both vaccination groups for all four strains and age groups. The reactogenicity and safety profile for both the vaccines were comparable and there were no unexpected events and none of the adverse events were considered to have a reasonable possibility for a causal relationship with the study vaccine. Collectively, the data from the present study supports the use of QIV for seasonal vaccination of adult and elderly population in the Indian context. This will not only protect the Indian population against influenza but will in turn help in reducing the burden associated with influenza complications.
Data presentation
Presented at the 75th Platinum Jubilee of Annual Conference of the Association of Physicians of India (APICON) at Agra, Uttar Pradesh, India.
Supplemental Material
Download PDF (102.7 KB)Acknowledgments
The authors would like to thank the participating study volunteers, and the research staff at the centers involved in the study. The authors also thank Dr Shalini Nair (Abbott) and Yukti Singh (IQVIA) for support in developing this manuscript.
Disclosure of potential conflicts of interest
Serge van de Witte is an employee of Abbott Healthcare Products B.V. All other authors were the investigators in the study and received grant support from Abbott to conduct this study. No other conflict of interest is declared for the work presented in this article.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1885278.
Additional information
Funding
Notes on contributors
Serge van de Witte
All authors met the International Council of Medical Journal Editors’ criteria for authorship and all those who met those criteria are listed as authors. All authors participated in the design, implementation, analysis, and/or interpretation of the study. All the authors were involved in drafting the manuscript or revising it critically for important intellectual content and provided final approval of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- Immunization Action Coalition. 2020 [Accessed 2020 Feb 6th]; https://www.immunize.org/catg.d/p4208.pdf
- WHO Influenza (Seasonal). 2020 [Accessed 2021 Feb 15]; https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal)
- Centers for Disease Control and Prevention 2020 [Accessed 2021 Feb 15]; https://www.cdc.gov/flu/vaccines-work/vaccineeffect.htm
- National Institute of allergy and Infectious Diseases. 2020 [Accessed 2020 Mar 4]; https://www.niaid.nih.gov/diseases-conditions/influenza-diagnosis
- CDC Antiviral Drugs. 2020 [Accessed 2021 Feb 15]; https://www.cdc.gov/flu/treatment/whatyoushould.htm
- Greenberg DP, Robertson CA, Noss MJ, Blatter MM, Biedenbender R, Decker MD. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine. 2013;31(5):770–76. doi:10.1016/j.vaccine.2012.11.074.
- Kieninger D, Sheldon E, Lin WY, Yu CJ, Bayas JM, Gabor JJ, Esen M, Fernandez Roure JL, Narejos Perez S, Alvarez Sanchez C, et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged >/=18 years. BMC Infect Dis. 2013;13:343. doi:10.1186/1471-2334-13-343.
- Van de Witte S, Nauta J, Montomoli E, Weckx J. A Phase III randomised trial of the immunogenicity and safety of quadrivalent versus trivalent inactivated subunit influenza vaccine in adult and elderly subjects, assessing both anti-haemagglutinin and virus neutralisation antibody responses. Vaccine. 2018;36(40):6030–38. doi:10.1016/j.vaccine.2018.04.043.
- Jamotte A, Chong CF, Manton A, Macabeo B, Toumi M. Impact of quadrivalent influenza vaccine on public health and influenza-related costs in Australia. BMC Public Health. 2016;16(1):630. doi:10.1186/s12889-016-3297-1.
- WHO Vaccine Seasons. 2020 [Accessed 2021 Feb 15]; https://www.who.int/ith/vaccines/si_iAh1n1/en/
- WHO Influenza. 2020 [Accessed 2021 Feb 15]; https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, et al. Centers for disease control and prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices (ACIP), 2010. MMWR Recomm Rep. 2010 Aug 6;59(RR–8):1–62. Erratum in: MMWR Recomm Rep. 2010 Aug 13;59(31):993.Erratum in: MMWR Recomm Rep. 2010 Sep 10;59(35):1147.PMID: 20689501.
- National Centre for disease Control. 2020 [Accessed 2021 Feb 15]; https://ncdc.gov.in/showfile.php?lid=280
- WHO FluNet. 2020 [Accessed 2021 Feb 15]; http://apps.who.int/flumart/Default?ReportNo=12
- Chadha MS, Potdar VA, Saha S, Koul PA, Broor S, Dar L, Chawla-Sarkar M, Biswas D, Gunasekaran P, Abraham AM, et al. Dynamics of influenza seasonality at sub-regional levels in India and implications for vaccination timing. PloS One. 2015;10(5):e0124122. doi:10.1371/journal.pone.0124122.
- Wijnans L, Voordouw B. A review of the changes to the licensing of influenza vaccines in Europe. Influenza Other Respi Viruses. 2016;10(1):2–8. doi:10.1111/irv.12351.
- Guideline on Influenza Vaccines Non-clinical and Clinical Module. 2016 [Accessed 2020 May 26]; https://www.ema.europa.eu/en/documents/scientific-guideline/influenza-vaccines-non-clinical-clinical-module_en.pdf
- Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, Nishtala M, Wrammert J, Smith K, James JA, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121(8):3109–19. doi:10.1172/JCI57834.
- Sambhara S, McElhaney JE. Immunosenescence and influenza vaccine efficacy. In: Vaccines for Pandemic Influenza. Atlanta (USA): Springer; 2009. p. 413–29.
- Reber AJ, Chirkova T, Kim JH, Cao W, Biber R, Shay DK, Sambhara S. Immunosenescence and challenges of vaccination against influenza in the aging population. Aging Dis. 2012;3:68.
- Haq K, McElhaney JE. Immunosenescence: influenza vaccination and the elderly. Curr Opin Immunol. 2014;29:38–42. doi:10.1016/j.coi.2014.03.008.
- Dunkle LM, Izikson R, Patriarca PA, Goldenthal KL, Muse D, Cox MMJ. Randomized comparison of immunogenicity and safety of quadrivalent recombinant versus inactivated influenza vaccine in healthy adults 18-49 years of age. J Infect Dis. 2017;216(10):1219–26. doi:10.1093/infdis/jix478.
- Olafsdottir TA, Alexandersson KF, Sveinbjornsson G, Lapini G, Palladino L, Montomoli E, Del Giudice G, Gudbjartsson DF, Jonsdottir I. Age and influenza-specific pre-vaccination antibodies strongly affect influenza vaccine responses in the Icelandic population whereas disease and medication have small effects. Front Immunol. 2018;8:1872. doi:10.3389/fimmu.2017.01872.
- Sharma S, Singh VB, Kumar S, Prajapati V, Patel J, Vukkala R, Jangid SK, Sanmukhani J, Gupta G, Patel P, et al. Immunogenicity and safety of the first indigenously developed Indian tetravalent influenza vaccine (split virion) in healthy adults≥ 18 years of age: a randomized, multicenter, phase II/III clinical trial. Hum Vaccin Immunother. 2018;14(6):1362–69. doi:10.1080/21645515.2018.1441654.
- Beyer WE, McElhaney J, Smith DJ, Monto AS, Nguyen-Van-Tam JS, Osterhaus AD. Cochrane re-arranged: support for policies to vaccinate elderly people against influenza. Vaccine. 2013;31(50):6030–33. doi:10.1016/j.vaccine.2013.09.063.
- Castrucci MR. Factors affecting immune responses to the influenza vaccine. Hum Vaccin Immunother. 2018;14(3):637–46. doi:10.1080/21645515.2017.1338547.
- Pépin S, Donazzolo Y, Jambrecina A, Salamand C, Saville M. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine. 2013;31(47):5572–78. doi:10.1016/j.vaccine.2013.08.069.
- Noah DL, Hill H, Hines D, White EL, Wolff MC. Qualification of the hemagglutination inhibition assay in support of pandemic influenza vaccine licensure. Clin Vaccine Immunol. 2009;16(4):558–66. doi:10.1128/CVI.00368-08.
- Truelove S, Zhu H, Lessler J, Riley S, Read JM, Wang S, Kwok KO, Guan Y, Jiang CQ, Cummings DA. A comparison of hemagglutination inhibition and neutralization assays for characterizing immunity to seasonal influenza A. Influenza Other Respi Viruses. 2016;10(6):518–24. doi:10.1111/irv.12408.
- Raju Shah SP. Advantages of subunit influenza vaccine: an overall perspective. Indian J Clin Practise. 2018;29(3):253-62.
- Beyer W, Palache A, Osterhaus A. Comparison of serology and reactogenicity between influenza subunit vaccines and whole virus or split vaccines. Clin Drug Investig. 1998;15(1):1–12. doi:10.2165/00044011-199815010-00001.
- Palache AM. Influenza vaccines A reappraisal of their use. Drugs. 1997;54:841–56.
- Wood JM, Williams MS. History of inactivated influenza vaccines. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of influenza. London (UK):: Blackwell Science; 1998. p. 317–23.