ABSTRACT
We described a 65-year-old male with choroidal metastases (CM) from non-small cell lung cancer (NSCLC). Pembrolizumab (Keytruda) combined with pemetrexed and capecitabine achieved excellent outcomes. After two cycles of pembrolizumab and chemotherapy, blurred vision and left eye pain were significantly relieved. Imaging and ophthalmologic examinations demonstrated complete resolution of the CM, as well as reduction of pulmonary shadow. CM from NSCLC shows complete and durable response to pembrolizumab and chemotherapy. We suggesting that immunotherapy combined with chemotherapy is a promising treatment for CM from NSCLC.
Introduction
Metastasis and invasion is the basic characteristic of malignant tumor, it is also the leading cause of death in cancer patients. Intraocular metastasis occurs in 2–9% of all malignancies, mostly arising from breast cancer (53%), and lung cancer (20%).Citation1 The choroid has a vascular system that supplies rich blood for circulating tumor cells (CTC) or tumor emboli to grow, making it the most common site of intraocular metastasis.Citation2
Clinical manifestations of CM vary from blurred vision (70–81%), flashes and floaters (5–12%), and pain (5–14%), to no symptoms (9–11%).Citation3 The treatment of CM, either systemic or primary, depends on the status, number and location lesion. Current treatments include chemotherapy, radiotherapy, targeted therapy and immunotherapy.Citation4
Among them, immunotherapy is a noval option for malignant tumors. Former US president Jimmy Carter was the first patient to receive the treatment of pembrolizumab plus radiotherapy, after he was diagnosed as malignant melanoma with brain metastases. Pembrolizumab, a common drug in immunotherapy, can activate host immune response to promote the proliferation of T cells that trap and kill tumor cells.Citation5
Here, we reported case presenting with CM from non-small cell lung cancer (NSCLC), and treated with pembrolizumab, pemetrexed and capecitabine as the first-line therapy.
Patient presentation
A 65-year-old male patient, who had a 40-year history of smoking, presented with two months of blurred vision and slight pain in the left eye. Ultrasonography showed that the fundus had developed an amelanotic hemispherical protuberant lesion, located in the superior temporal quadrant of the retina, but the macula was still normal. Hemispherical hyperecho was detected below the retina (). Fundus fluorescein angiography (FFA) demonstrated high fluorescence at the early stage and much fluorescence leakage at the late stage of the superior temporal lesion (). Indocyanine green angiography (IGGA) indicated numerous capillaries in the lesion (). Based on the findings, a primary diagnosis of ocular metastasis was proposed.
Figure 1. Ocular and medical imaging examination of pre-immunotherapy. (a) Ocular ultrasonography. (b) The early stage of FFA. (c) The late stage of FFA. (d) ICGA of fundus. (e) Chest high-resolution CT. (f) MRI of brain
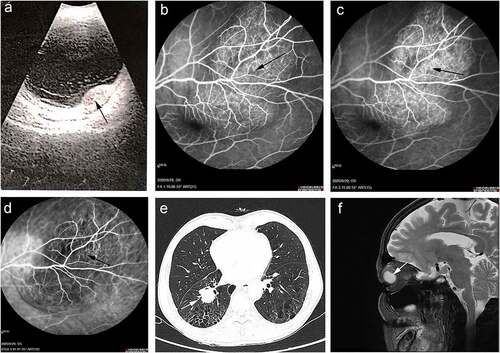
Chest high-resolution computed tomography (CT) showed multiple shadows in the right hilum and the right lower lung, with pulmonary bullae and emphysema (). A roughly circular lesion at the posterior wall of the left eye was scanned through Magnetic Resonance Imaging (MRI) ().
To avoid pneumothorax and iatrogenic metastasis, lung biopsy was not performed due to his poor lung condition. Furthermore, according to his clinical symptoms, medical records, imaging and biochemical indexes, the patient was diagnosed as NSCLC with CM by oncologists and ophthalmologists at our hospital. Thereafter, he was treated with first-line immunotherapy plus chemotherapy based on pembrolizumab (200 mg/cycle), pemetrexed (100 mg/cycle), and capecitabine (100 mg/cycle) since July 2020.
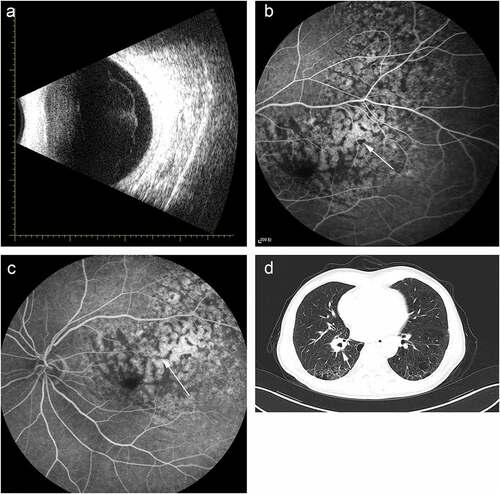
After two cycles of treatment, ocular and pulmonary symptoms were relieved. Meanwhile, ocular ultrasonography showed regression of the lesion (). The exudation on the surface of the lesion decreased significantly (). The best corrected visual acuity (BCVA) was improved from 0.1 to 0.6, and lung shadow diameter reduced from 3 mm to 2 mm (). In addition, during the treatment, the patient did not show the common side-effects of chemotherapy, such as hair loss, nausea and vomiting, except for mild fatigue. Mutation in seven somatic gene, including RB1, TERT, ERBB2, STAT3, BRCA2, TP53, MCL1, was detected by Genetic testing.
Figure 2. Ocular and medical imaging examination of post-immunotherapy. (a) Ocular ultrasonography. (b) The early stage of FFA. (c) The late stage of FFA. (d) Chest high-resolution CT
At this time, the patient is still undergoing the treatment and disease has been well controlled. The patient has provided informed consent for the publication of this case report.
Discussion
Studies have shown that metastatic choroidal tumor is more frequent than primary choroidal melanoma.Citation6 Fifteen percent of CM have an unknown primary, CM originates from the breast in 53%, the lung in 20%, the gastrointestinal tract in 4%, the prostate in 2%, the kidney in 2%, and the skin in 1%.Citation1 Blurred vision occurs in 70–81% of CM cases, often caused by macular or foveal exudative retinal detachment. Other clinical manifestations range from flashes and floaters (5–12%) and pain (5–14%). A small fraction of cases (9–11%) are non-symptomatic, and their metastases are discovered by routine examination.Citation3
During metastasis, tumor cells fall off the extracellular matrix and invade the surrounding tissue. CTCs and tumor emboli break through thin-walled vessels, into the bloodstream to metastasize to target sites. Because of unrestrained cell division, tumor cells are in a high demand of nutrition and oxygen; therefore, proangiogenic factors are released to induce adjacent endothelial cells to form new vascular networks.Citation7 Blood-retinal barrier (BRB), composed of retinal vascular endothelial cells and retinal pigment epithelium (RPE), play a crucial role in maintaining structural integrity, nutritional supply and immune privileges, but this protective mechanism exclude choroid.Citation8 Therefore, tumor cells are highly likely to metastasize here. CM accounts for 88–89% of metastases in the eye, in comparison with those to iris (9%) or ciliary body (2%).Citation4
Platinum-based chemotherapy is the standard first-line treatment for advanced or metastatic NSCLC. In October 2015, the USA FDA approved pembrolizumab for the treatment of metastatic NSCLC in which the programmed cell death protein 1 (PD1) is expressed. PD-L1is mainly expressed on activated CD4+ and CD8 + T-cells, B-cells, natural killer (NK) cells, macrophages and dendritic cells.Citation9 In a physiological condition, the function of PD-1 is to suppress the activation of immune cells and maintain homeostasis. By binding to PD1 on T cells, PD-1 ligands (PDL1) is highly expressed in melanoma, breast cancer, NSCLC and other malignant tumors, which in turn suppresses the activity of T-cells. This is a major mechanism through which tumor cells evade immune surveillance and attack.Citation10 According to previous research, diverse factors, such as tumor microenvironment (TME) and gene mutation, can induce the overexpression of PD-1. TP53 mutation predict the response to Anti–PD-1 in lung adenocarcinoma.Citation11 The expression of immune checkpoints PD-L1 in high-grade serous ovarian carcinoma is interrelated with BRCA status.Citation12 These evidences indicate that gene mutation has great influence on the expression of PD-1. In term of this case, the poor lung condition was not suitable for performing biopsy to identify the expression of PD-1. However, immunotherapy still proved effective.
Pembrolizumab can block the interaction between PD1 and PD-1 L to restore the anti-tumor immunity mediated by T cells.Citation13 Pembrolizumab can be combined with chemotherapy to enhance the anti-tumor effect. In a clinical trial for advanced NSCLC, the median progression-free survival was 10.3 months (95% confidence interval [CI]) in pembrolizumab group, and 6.0 months (95% CI, 4.2 to 6.2) in chemotherapy group (hazard ratio for disease progression or death, 0.50; 95% CI, 0.37 to 0.68; P < .001).Citation14 Another trail about metastatic NSCLC reveals that the estimated rate of overall survival at 12 months was 69.2% (95% CI, 64.1 to 73.8) in pembrolizumab-chemotherapy group, higher than the 49.4% (95% CI, 42.1 to 56.2) in placebo-chemotherapy group (hazard ratio for death, 0.49; 95% CI, 0.38 to 0.64; P < .001).Citation15 The present case received immunotherapy plus chemotherapy that exerted significant therapeutic effect, as manifested by the complete disappearance of ocular lesion and remarkable reduction of pulmonary lesion. Moreover, pembrolizumab strengthened the patient’s autoimmunity against side effects caused by chemotherapy, such as hair loss, nausea and vomiting.
To our knowledge, this is the first case demonstrating that the favorable efficacy and safety of systemic pembrolizumab in combination with pemetrexed and capecitabine for CM.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to the patient for sharing this case.
References
- Arepalli S, Kaliki S, Shields CL. Choroidal metastases: origin, features, and therapy. Indian J Ophthalmol. 2015;63:122–27. doi:10.4103/0301-4738.154380.
- Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104:1265–76. doi:10.1016/S0161-6420(97)30148-1.
- Gozzi E, Angelini F, Rossi L, Leoni V, Trenta P, Cimino G, Tomao S. Alectinib in the treatment of ocular metastases of ALK rearranged non small cell lung cancer: description of 2 case reports. Medicine (Baltimore). 2020;99:e21004. doi:10.1097/MD.0000000000021004.
- Mathis T, Jardel P, Loria O, Delaunay B, Nguyen AM, Lanza F, et al. New concepts in the diagnosis and management of choroidal metastases. Prog Retin Eye Res. 2019;68:144–76.
- Raedler LA. Keytruda (Pembrolizumab): first PD-1 inhibitor approved for previously treated unresectable or metastatic melanoma. Am Health Drug Benefits. 2015;8:96–100.
- Ferry AP, Font RL. Carcinoma metastatic to the eye and orbit. I. A clinicopathologic study of 227 cases. Arch Ophthalmol. 1974;92:276–86. doi:10.1001/archopht.1974.01010010286003.
- Langley RR, Fidler IJ. The seed and soil hypothesis revisited – the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–35. doi:10.1002/ijc.26031.
- Diaz-Coranguez M, Ramos C, Antonetti DA. The inner blood-retinal barrier: cellular basis and development. Vision Res. 2017;139:123–37.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi:10.1146/annurev.immunol.26.021607.090331.
- Kwok G, Yau TC, Chiu JW, Tse E, Kwong YL. Pembrolizumab (Keytruda). Hum Vaccin Immunother. 2016;12:2777–89. doi:10.1080/21645515.2016.1199310.
- Biton J, Mansuet-Lupo A, Pecuchet N, Alifano M, Ouakrim H, Arrondeau J, Boudou-Rouquette P, Goldwasser F, Leroy K, Goc J, et al. TP53, STK11, and EGFR mutations predict tumor immune profile and the response to anti–PD-1 in lung adenocarcinoma. Clin Cancer Res. 2018;24:5710–23. doi:10.1158/1078-0432.CCR-18-0163.
- Wang Z, Sun K, Xiao Y, Feng B, Mikule K, Ma X, Feng N, Vellano CP, Federico L, Marszalek JR, et al. Niraparib activates interferon signaling and potentiates anti-PD-1 antibody efficacy in tumor models. Sci Rep. 2019;9(1):1853. doi:10.1038/s41598-019-38534-6.
- van Vugt MJH, Stone JA, De Greef R, Snyder ES, Lipka L, Turner DC, Chain A, Lala M, Li M, Robey SH, et al. Immunogenicity of pembrolizumab in patients with advanced tumors. J Immunother Cancer. 2019;7:212. doi:10.1186/s40425-019-0663-4.
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi:10.1056/NEJMoa1606774.
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92. doi:10.1056/NEJMoa1801005.
