ABSTRACT
Human papillomavirus (HPV) causes >40,000 cancer diagnoses each year, yet vaccination rates remain low because widespread implementation of strategies to increase vaccinations has not occurred. Behavioral nudges have demonstrated efficacy in improving uptake of desired behaviors in health care settings but have not been tested for increasing HPV vaccinations. We assessed the impact of an intervention combining behavioral nudges with other proven strategies (i.e., assessment and feedback, provider communication training) on HPV vaccination rates and parental satisfaction in four Midwestern pediatric, outpatient practices. Practices were randomly assigned to receive either assessment and feedback or assessment and feedback combined with vaccine communication training and behavioral nudges in the form of vaccine commitment posters. Providers (n = 16) completed surveys regarding vaccine policies and parents (n = 215) reported on their child’s vaccine history and satisfaction with the consultation. Three practices increased HPV vaccination rates (1–10%); however, there was no statistically significant difference by study arm. Most parents (M age 41.3; SD 8.1; 85% female, 68% White) indicated their child had previously initiated the HPV vaccine series (61%) and 72% indicated receipt of an HPV vaccine during the study visit. Concerns among HPV vaccine-hesitant parents (28%) included vaccine safety and believing the vaccine is unnecessary (40%). Most parents were satisfied with their consultation. Practices in both intervention groups increased vaccination rates. While some parents continue to harbor concerns about vaccine safety and necessity, parents welcomed discussions about HPV and were satisfied with their provider’s communication regardless of their vaccine decisions.
Introduction
Every year 14 million Americans are infected with human papillomavirus (HPV) and more than 44,000 are diagnosed with HPV-related cervical, oropharyngeal, anal, penile, or vaginal cancer.Citation1,Citation2 While vaccines to prevent HPV infection have been widely available for more than a decade, vaccine initiation rates in the U.S. are only 69.9% for adolescent girls and 66.3% for boys.Citation3 Regional disparities also exist, with some Midwestern states reporting HPV vaccination rates 6% lower than the national average.Citation3 HPV vaccination rates in the U.S. lag behind other developed countries, like Australia whose rates range from 76% to 80% for males and females.Citation4
Effective strategies, including The Centers for Disease Control and Prevention (CDC) AFIX (Assessment, Feedback, Incentive and eXchange) quality improvement program and high-quality provider recommendations that include presumptive announcements, cancer prevention messaging, and urging of same-day vaccination, have been effective in increasing HPV vaccination rates.Citation5–13 However, consistent implementation of these strategies has not been realized with less than 50% of parents receiving a high-quality HPV vaccine recommendation, and some not receiving a recommendation at all.Citation10 Behavioral “nudges,” operationalized as poster-sized commitment displays, have demonstrated success in improving antibiotic prescribing and hand hygiene among providers in clinical care settings.Citation14–17 Nevertheless, no studies to date have examined the potential impact of behavioral nudges on HPV vaccination rates. This pilot study examined the comparative effectiveness of an intervention that combined assessment and feedback with behavioral nudges and provider communication training, to a single intervention of assessment and feedback to increase HPV vaccination rates and assess parent satisfaction.
Methods
Procedures
Community-based practices affiliated with an integrated academic pediatric network were invited to participate in the study. Four practices in urban and suburban areas in a Midwestern region of the United States with lower than national average HPV vaccination rates participated. Practices were matched on baseline HPV vaccination rates (lower = <50% initiation vs higher performing = ≥50% initiation) and randomized to receive either the combined (C) intervention (assessment and feedback, provider communication training, and behavioral nudges) or the single (S) intervention (assessment and feedback alone). One lower and one higher performing practice was randomized to each arm. Baseline and post-intervention vaccination rates were determined through billing claims data submitted by each practice through the Children’s Health Network (CHN) database administered by a local academic children’s hospital.
Participants
A convenience sample of parents or legal guardians (hereinto referred to as parents) was recruited from each practice. Parents were eligible to participate if they were aged 18 or older, English speaking, and presented to one of the four practices with a child aged 9–17 who had not completed the HPV vaccine series. Data collection occurred from October 2018 through September 2019. The study protocol was approved by the hospital’s Institutional Review Board (IRB approval # 00000134).
Measures
Data, including demographics, child’s vaccine history, and parent’s thoughts about HPV vaccine and the visit were collected. The survey was self-administered after the parents’ consultation with a pediatric care provider. All responses were collected and managed using REDCap electronic data capture tools via tablet computers.Citation18 Data collection activities took approximately 5 minutes to complete.
Demographics
Demographic information collected included parents' age, gender, race education, and ethnicity. Additionally, parents were asked demographic questions about their child (age, race, gender, health insurance type).
Vaccine history
Ten items assessed adolescents’ prior vaccine history. Parents were asked to indicate whether a health care provider ever recommended tetanus-diphtheria-pertussis vaccine (Tdap/“tetanus shot”), MCV (meningococcal conjugate vaccine/“meningitis shot”) or HPV vaccines. Additionally, parents were asked how old their child was when they received the recommendation and whether their child received each vaccine.
HPV vaccine intentions
Vaccine intentions were assessed by asking parents of children who had not yet completed the HPV vaccine series to indicate the likelihood that their child would receive an HPV vaccine within the next 12 months using a 5-point Likert scale with responses ranging from “not at all likely” to “very likely”. Parents who indicated their child completed the vaccine series that day were not asked about their future vaccine intentions. To analyze future vaccine intentions, parents were classified as HPV vaccine hesitant if they responded with “not too likely”, “not likely at all”, or “not sure/don’t know”, whereas “very likely” and “somewhat likely” responses were considered non-hesitant. Parents who indicated it was unlikely their child would receive an HPV vaccine in the future provided a reason for their hesitation.
Satisfaction with provider
Parent satisfaction with the consultation was assessed with the Engagement with Health Care Provider scale,Citation19 a 13-item scale developed to measure patient’s satisfaction with the services offered by their providers. Reliability of the scale is high (Cronbach’s alpha = .96).Citation19 Parents were asked to indicate their level of satisfaction with their child’s health care provider’s communication about vaccines as well as the overall visit. Response options ranged from very satisfied to very dissatisfied.
Analysis
Categorical frequencies were calculated with differences in proportions compared using chi-square tests (Pearson’s and Fisher’s exact, where appropriate). The Kruskal–Wallis test was used to compare engagement scores across sites and across arm.Citation20 Unadjusted logistic regression models were run when comparing the odds of HPV initiation and same-day vaccinations across sites and across arms. Quantitative analyses were completed using Stata 14.2 software.Citation21 Content analysis strategies were used to identify common themes in parents’ stated reasons for why it was “not likely at all”, “not too likely”, or “not sure/don’t know” if their child would receive an HPV vaccine in the next 12 months.Citation22 Using open coding procedures, two coders independently identified seven themes and assigned parents’ response to one or more theme. Disagreements between raters were resolved through discussion and consensus.
Results
Vaccination rates by practice
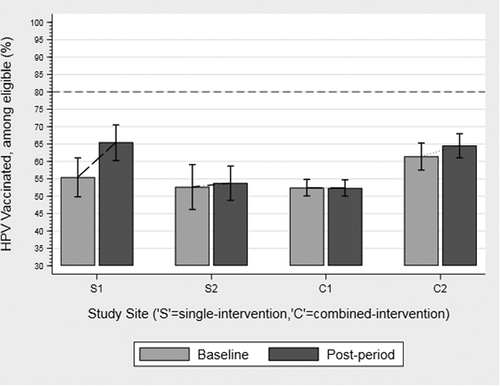
Three of the four practices evidenced an increase in HPV vaccination rates and there was a statistically significant difference by practice; however, there was no significant difference by study arm (. Baseline and Follow-up HPV Vaccination Rates Practice Claims data). The two practices with the highest baseline HPV vaccination rates (S1 and C2), evidenced a 3–10% increase in HPV vaccine initiation rates at 12-month follow-up. Practices with higher baseline vaccination rates had a significant increase in HPV vaccination rates compared to practices with lower baseline vaccination rates (p < .001). The second single-intervention practice (S2) had a slight increase (1%) while the other combined intervention practice (C1) was relatively unchanged. In addition to increased HPV vaccination rates, all practices also showed an increase in Tdap and meningococcal rates (0.8% to 12.1%) ().
Table 1. Baseline and Follow-up of Tdap and MCV Vaccination Rates (Practice Claims data)
Parents’ thoughts about HPV vaccines
Overall, 215 parents were enrolled in the study. Demographic data were analyzed for all parents and patients (). Most parents were, female (85%), White (68%), with a mean age of 41.34 years (SD = 8.05). More than half of the adolescent patients were female (53%) and White (63%), with a mean age of 12.15 years (SD = 1.68).
Table 2. Parent and patient Demographics
Vaccine history
When asked “Has a doctor or other health care provider ever recommended your child receive the HPV vaccine?” 95% of parents responded “yes.” However, the proportion of parents that indicated their child had initiated the HPV vaccine series (61%) was significantly lower when compared to Tdap (86%; p < .001) and MCV (69%; p < .001). Many parents were unsure of their child’s prior vaccine history, including 2% who were uncertain about prior HPV vaccination, and 17% who were unsure of previous MCV vaccination. However, 72% of parents indicated their child received an HPV vaccine during the study visit ().
Table 3. Parent reported HPV vaccination uptake
Intentions for future HPV vaccination
Most parents whose child had not completed the vaccine series (70%) indicated their child would likely receive an HPV vaccine within the next 12 months. Nearly 9% of parents were uncertain about their future vaccine intentions. Vaccine hesitancy was similar in the combined and single-intervention arms (27% vs. 33%; p = .51). Vaccine hesitation varied across sites, from 19% to 38%, although these differences were not significant (p = .18).
Reasons for why it was “not likely at all”, “not too likely”, or “not sure/don’t know” that their child would receive an HPV vaccine in the next 12 months were provided by 60 parents. Reasons included parental concerns about vaccine safety (30%), beliefs that the vaccine was unnecessary (15%), and because their child was not sexually active (20%). Less common reasons for hesitancy regarding future HPV vaccine uptake included lack of provider recommendation (2%), no requirement for school entry (5%), lack of insurance or costs of vaccination (2%), and limited knowledge that HPV could cause cancer (2%) and other reasons (e.g. waiting until older to vaccinate-25%).
Parent satisfaction
Most parents were satisfied with the provider’s communication (≥96%), as well as their overall consultation (≥96%). There was no difference in satisfaction between parents of patients who received a same-day HPV vaccination and those who did not (p = .20). Both parents of children who received a vaccine at the visit, and those who did not felt informed about their decision (p = .19) and that their decision was best for their child (p = .69). There was also no difference in satisfaction scores between clinic sites (p = .58) or study arms (p = .42).
Discussion
Three practices evidenced an increase in HPV vaccination rates from baseline to study end. This result was somewhat expected as all four sites received an active intervention. However, despite being randomized to different study arms, the two practices with similarly high baseline vaccination rates had the highest increase in post-intervention vaccination rates. This suggests the impact of interventions may be moderated by clinic policies, procedures, and culture. For example, exit interviews with providers revealed that practices with higher baseline vaccination rates had formal policies requiring every member of the health care team to be aware of a patient’s vaccine status prior to their well-child visit. Other policies included recommending and administering vaccines to eligible patients during any visit (e.g. sick visit, sibling’s well-visit), and excusing parents from the practice who choose not to vaccinate their children (e.g., Tdap and MCV). We also noted that practices with an increase in HPV vaccination rates also had increased rates of Tdap and MCV vaccines. This result confirms results found in previous studiesCitation5,Citation9–13 that suggest parents are more likely to agree to initiate or complete the HPV vaccine series when bundled with other adolescent vaccines. Additionally, some of the sites received CDC and industry-sponsored HPV vaccine promotional items and one single-intervention site decided to appoint a vaccine coordinator shortly after their baseline visit to improve their early childhood and adolescent vaccination rates.
In addition to practice policies, practices with higher baseline and post-intervention HPV vaccination rates tended to be smaller (e.g., 4 or fewer primary care providers), located in suburban communities, and maintained a more stable patient population, increasing the likelihood that the patient’s vaccine history was known to clinic staff and patients would likely return for follow-up care. During parent recruitment activities, research coordinators noted clinic staff often had a long-standing relationship with patients and families (5 or more years) and often provided care for more than one member of the family. Research suggests that parent-provider communication and rapport may impact parent’s willingness to follow the advice of their child’s health care provider.Citation23 Additionally, dissemination of interventions may be more effective in smaller practices with centralized communication channels and a vaccine champion who sets the tone for the practice.
Most parents received HPV vaccine recommendations from their child’s health care providers; however, nearly 40% elected not to vaccinate their child and baseline HPV vaccination rates were significantly lower than those of other adolescent recommended vaccines. This suggests that in addition to training providers to make strong HPV vaccine recommendations that are bundled with other adolescent vaccines, there is a need to incorporate additional parent-focused educational tools to address concerns and dispel myths about HPV vaccines. Specifically, future interventions should highlight HPV vaccine safety, cancer prevention, and a rationale for the recommended vaccine initiation age (most hesitant parents [65%] expressed concerns that the vaccine was unsafe or not necessary or appropriate for their child).
A majority of parents in this study were satisfied with the communication from their child’s health care provider. These results are similar to previous studiesCitation9–13 that indicate most parents do not object to HPV vaccine discussions. It is important to note that while most parents indicated their child either received an HPV vaccine during the current visit or indicated future intentions to initiate the series, 28% of parents in the current study indicated vaccine hesitancy. Additionally, 15 parents who indicated their child had not initiated the HPV vaccine series during the current visit reported their child would likely initiate the series within the next 12 months. The median age of these children was 12, raising the possibility that some parents may still prefer to delay HPV vaccination until their child is older.
Limitations
This study has several limitations. Parents were asked if they had received an HPV vaccine recommendation and their child’s previous vaccine status, which is subject to recall bias. However, we attempted to mitigate this by providing the survey immediately following the current visit in which vaccines were discussed. We also do not have data on parents who declined to participate, and it is possible that their survey responses to vaccination and satisfaction with the visit may have been different than those that agreed to participate. However, a low percentage of parents (4% of those approached) declined to participate. Finally, while providers in the combined intervention arm were trained to provide a high-quality vaccine recommendation (e.g., presumptive, bundled with other vaccines and indicating a sense of urgency, cancer prevention, and importance), we did not assess whether parents received recommendations that incorporated these elements of a high-quality recommendation. This could partially contribute to the lack of statistically significant difference in vaccination rates between study arms, as previous studies suggest that parents who receive high-quality recommendations are more likely to vaccinate their child than those who receive lower quality recommendations.Citation10 It is unclear from our results if increases in vaccinations displaced other vaccinations. For example, people vaccinated at our clinics may have otherwise received vaccinations at pharmacies or other clinics. However, prior work on vaccinations using nudges has failed to identify displacement as the cause of the vaccination effect.Citation24
Conclusion
This study showed that parents are willing to discuss HPV vaccination with their child’s health care provider and report high levels of satisfaction regardless of their vaccination decisions. This should encourage providers to initiate conversations about HPV vaccines with all their eligible patients. Additionally, clinics with a higher baseline HPV vaccination rate were more likely to increase their vaccination rates regardless of intervention arm. This suggests that the presence of a recommendation alone is not adequate to achieve high rates of vaccination, but other factors (i.e. the strength and quality of the recommendation) may impact parent’s vaccine decisions. A future study will examine the impact of combining all elements of high-quality vaccine recommendations (e.g. presumptive announcement, importance, cancer prevention, urgency, & bundling) on HPV vaccination rates, parent’s vaccine decisions, and visit satisfaction.
Declaration of Interest Statement
We wish to confirm that there are no known conflicts of interest associated with this publication.
Acknowledgments
This study was supported by the Masonic Cancer Alliance (2018-2020). We also acknowledge the support and contributions of the Kansas City Infectious Diseases Community Coalition Board and the Integrated Care Solutions network.
Additional information
Funding
References
- Centers for Disease Control and Prevention Division of Cancer Prevention and Cancer Control. HPV and cancer. [accessed 2020 Jan 13]. https://www.cdc.gov/hpv/hcp/protecting-patients.html.
- Centers for Disease Control and Prevention. Cancers associated with human Papillomavirus, United States-2012-2016. USCS Data Brief, no 10. Atlanta (GA): Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019 [accessed 2020 Jan 13] https://www.cdc.gov/cancer/uscs/about/data-briefs/no10-hpv-assoc-cancers-UnitedStates-2012-2016.htm.
- Walker TY, Elam-Evans LD, Yankey D, Markowitz LE, Williams CL, Fredua B, Singleton JA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(33):718–6. [accessed 2020 Jan 9]. https://www.cdc.gov/vaccines/imz-managers/coverage/teenvaxview/data-reports/hpv/trend/index.html.
- Dyda A, Shah Z, Surian D, Martin P, Coiera E, Dey A, Leask J, Dunn AG. HPV vaccine coverage in Australia and associations with HPV vaccine information exposure among Australian Twitter users. Hum Vaccin Immunother. 2019;15(7–8):1488–95. doi:10.1080/21645515.2019.1596712.
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: A randomized trial. Pediatrics. 2017;139(1):e20161764. [accessed 2017 Jan 23]. http://pediatrics.aappublications.org/content/139/1/e20161764.long.
- Matheson EC, Derouin A, Gagliano M, Thompson JA, Blood-Siegfried J. Increasing HPV vaccination series completion rates via text message reminders. J Pediatr Health Care. 2014;28(4):e35–e39. doi:10.1016/j.pedhc.2013.09.001.
- Fiks AG, Grundmeier RW, Mayne S, Song L, Feemster K, Karavite D, Hughes CC, Massey J, Keren R, Bell LM, et al. Effectiveness of decision support for families, clinicians, or both on HPV vaccine receipt. Pediatrics. 2013;131(6):1114–24. doi:10.1542/peds.2012-3122.
- Chao C, Preciado M, SLezak J, Xu L. A randomized intervention of a reminder letter for human papillomavirus vaccine series completion. J Adolesc Health. 2015;56(1):85–90. doi:10.1016/j.jadohealth.2014.08.014.
- Gilkey MB, Dayton AM, Moss JL, Sparks AC, Grimshaw AH, Bowling JM, Brewer NT. Increasing provision of adolescent vaccines in primary care: a randomized controlled trial. Pediatrics. 2014;134(2):e346–53. doi:10.1542/peds.2013-4257.
- Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: the impact of recommendation quality. Vaccine. 2016;34(9):1187–92. doi:10.1016/j.vaccine.2016.01.023.
- Perkins RB, Zisblatt L, Legler A, Trucks E, Hanchate A, Gorin SS. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine. 2015;33(9):1223–29. doi:10.1016/j.vaccine.2014.11.021.
- Malo TL, Gilkey MB, Hall ME, Shah PD, Brewer NT. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiology Biomarkers & Prevention. 2016;25(10):1383–91. doi:10.1158/1055-9965.EPI-16-0224.
- Opel DJ, Mangione-Smith R, Robinson JD, Heritage J, DeVere V, Salas HS, Zhou C, Taylor JA. The influence of provider communication behaviors on parental vaccine acceptance and visit experience. Am J Public Health. 2015;105(10):1998–2004. doi:10.2105/AJPH.2014.302425.
- Meeker D, Knight TK, Friedberg MW, Linder JA, Goldstein NJ, Fox CR, Rothfeld A, Diaz G, Doctor JN. Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med. 2014;174(3):425–31. doi:10.1001/jamainternmed.2013.14191.
- Tannenbaum D, Doctor JN, Persell SD, Friedberg MW, Meeker D, Friesema EM, Goldstein NJ, Linder JA, Fox CR. Nudging physician prescription decisions by partitioning the order set: results of a vignette-based study. J Gen Intern Med. 2015;30(3):298–304. doi:10.1007/s11606-014-3051-2.
- Auriemma CL, Greyson SR. Nudging providers to improve sleep for hospitalized patients. J Hosp Med. 2019;14(1):65–66.
- Caris MG, Labuschagne HA, Dekker M, Kramer MHH, van Agtmael VA, Vandenbroucke-Grauls CMJE. Nudging to improve hand hygiene. J Hosp Infect. 2018;98(4):352–58. doi:10.1016/j.jhin.2017.09.023.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzales N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi:10.1016/j.jbi.2008.08.010.
- Bakken S, Holzemer WL, Brown MA, Powell-Cope GM, Turner JG, Inouye J, Nokes KM, Corless IB. Relationship between perception of engagement with health care provider and demographic characteristics, health status, and adherence to therapeutic regiment in persons with HIV/AIDS. AIDS Patient Care STDS. 2000;14(4):189–97. doi:10.1089/108729100317795.
- Kruskal WH, Wallis WA. Wallis WA: use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47(260):907–11. 583–621 and errata, ibid. 48. doi:10.1080/01621459.1952.10483441.
- StataCorp. Stata statistical software: release 14. College Station (TX): StataCorp LLC; 2015.
- Weber RP Basic content analysis. Quantitative applications in the social sciences. Sage University Paper 49. Beverly Hills (CA): SAGE Publications, 1985.
- Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Sources of perceived credibility of vaccine-safety information for parents. Pediatrics. 2011;27(Supplement 1):S107–S112. doi:10.1542/peds.2010-1722P.
- Chapman GB, Li M, Leventhal H, Leventhal EA. Default clinic appointments promote influenza vaccination uptake without a displacement effect. Behav Sci Policy. 2016;2:40–50.