ABSTRACT
During the measles epidemic in 2019, in-hospital transmission of measles contributed to more than two-thirds of measles cases in South Korea, where measles is declared eliminated. This study aimed to examine measles seropositivity among healthcare workers (HCWs) in South Korea to help develop an effective measles prevention strategy for hospital settings. Measles IgG titer was tested in 1,579 HCWs working in a university-affiliated hospital and the measles-containing vaccine (MCV) immunization records of 870 HCWs were identified. The overall seropositivity was 92.0%, but the seropositivity and antibody titers were significantly low among HCWs aged 20–25 years (78.6%) and among one-dose vaccine recipients (86.7%). Among two-dose recipients, seropositivity was lower among young HCWs who received two doses during their childhood than among those who received the catch-up vaccination as part of job requirements (70.3% vs. 98.0%). Among 87 seronegative HCWs who received two-dose MMR vaccination, the seroconversion rate was 98.9%. A considerable proportion of young HCWs were potentially susceptible to measles despite receiving the two-dose vaccination during childhood because of the waning immunity against measles in a country with measles-eliminated status. Serological screening for measles of newly employed HCWs and MCV immunization of seronegative HCWs appears to be an effective prevention strategy.
Introduction
Measles is one of the most contagious diseases and is transmitted by airborne droplets.Citation1,Citation2 In 2014, the World Health Organization declared that measles was eliminated in South Korea; however, local outbreaks of measles caused by imported cases have occurred since.Citation3 Currently, healthcare workers (HCWs) have much less experience with measles patients than they did in the past because of the significantly reduced incidence of measles in the community. This has led to delayed diagnosis and isolation of measles patients, resulting in an increased risk of in-hospital transmission of measles to patients and HCWs. During the recent resurgence of measles worldwide, measles outbreaks in hospital settings have been frequently observed, and the incidence of measles among HCWs was higher than that in the general population.Citation2–7 As measles occurs most frequently in adolescents and young adults rather than in children in measles-eliminated countries with a high vaccine coverage during childhood,Citation1 young HCWs who are often in the frontlines are at the highest risk of contracting measles. This situation is particularly concerning in that the seropositivity among young adults (16–24 years of age) in South Korea was reported to be 48.5%-69.6% according to the nationwide seroprevalence survey in 2014.Citation8 This low seropositivity was consistently observed among young Korean HCWs in 2019.Citation9–11 Therefore, an effective preventive strategy for measles needs to be implemented in hospitals. Currently, two-dose measles-mumps-rubella (MMR) vaccination is recommended for HCWs if they do not have evidence of immunity against measles, which includes documentation of vaccination with two doses of a measles-containing vaccine (MCV), laboratory evidence of immunity, laboratory confirmation of disease, or proof of birth before 1967 in South Korea. However, a recent survey showed that only 24.5% of Korean hospitals had a measles prevention program as of 2018.Citation12
Between January and October 2019, measles outbreaks occurred in 12 areas in South Korea, and 68.5% of the cases were attributed to nosocomial transmission.Citation3 In response, our hospital conducted a serological survey of all HCWs and offered free MMR vaccines for seronegative HCWs. This study aimed to analyze the serological results of HCWs and to establish an infection control and prevention strategy for measles that can be applicable to hospitals in countries with measles-eliminated status.
Materials and methods
The results of the serological tests for measles of HCWs working in Daejeon St. Mary’s Hospital, Daejeon, South Korea, between January and August 2019, were retrospectively reviewed. Daejeon St. Mary’s Hospital is a 630-bed, university-affiliated hospital. Serological tests were performed using serum samples collected for the annual regular health check of employed HCWs and for the recruitment health check of new employees. HCWs were divided into seven age groups: 20–25, 26–29, 30–34, 35–39, 40–44, 45–49, and ≥50 years. In South Korea, a nationwide measles outbreak occurred between 2000 and 2001, and the government launched the school-based catch-up vaccination program with a measles-rubella vaccine targeting students aged between 8 and 16 years from May to June 2001.Citation8 The HCWs in the 26–29 and 30–34 years age groups in this study were recipients of the catch-up vaccination in 2001. History of MCV immunization before the serological test was reviewed using the electronic National Immunization Registry Information System operated by the Korea Disease Control and Prevention Agency. The HCWs were categorized into seropositive and seronegative groups based on serological test results, and the demographics, occupational group, and previous history of MCV immunization were compared between the two groups. The seropositivity and median titer of measles IgG according to the number of MCV doses were compared among all HCWs and within each age group. In 2014, our hospital experienced in-hospital transmission of measles in the pediatric ward. Since then, one-dose MMR vaccination has been recommended for HCWs at increased risk for measles exposure, but with no documentation of two-dose MCV immunization. For newly hired HCWs, completion of two-dose MCV immunization has been recommended before starting work. Thus, seropositivity in HCWs who received MCV immunization while adults-as recommended by the hospital’s policy-was compared to seropositivity in those who received one- or two-dose MCV immunization only in childhood.
HCWs who were detected as seronegative by the baseline serological survey were offered two-dose MMR vaccination regardless of MCV immunization history. They underwent repeat serological tests ≥4 weeks after completing vaccination. Median titers of measles IgG after vaccination were compared according to demographics, occupation, and previous MCV immunization.
Measles IgG titers were measured by a chemiluminescence immunoassay (CLIA) using the LIAISON® system (LIAISON® Measles IgG assay with LIAISON®XL analyzer, DiaSorin S.p.A., Saluggia, VC, Italy). The detection range for measles IgG with the LIAISON® system was 5.0–300.0 AU/mL. All IgG titers <5.0 AU/mL and >300.0 AU/mL were regarded as 2.5 AU/mL and 300 AU/mL, respectively. Serostatus was classified as positive (≥16.5 AU/mL), equivocal (13.5–16.4 AU/mL), and negative (<13.5 AU/mL) according to the manufacturer’s recommendations, and equivocal results were considered seronegative.
The seropositive and seronegative groups were compared using a chi-squared test. Variables with a P value < .1 following the univariable analysis were included in a multiple logistic regression analysis to identify factors significantly associated with measles seropositivity. The trends of seropositivity according to age group and number of MCV doses were analyzed using linear-by-linear association. Continuous variables were compared using the Mann-Whitney or Kruskal-Wallis test based on the number of compared groups. The SPSS 21 program (IBM Corporation, Amork, New York, USA) was used for statistical analyses, and statistical significance was defined as a two-tailed P value < .05. This study was approved with a waiver of informed consent by the Institutional Review Board of the Daejeon St. Mary’s Hospital (Approval number: DC20RISI0083).
Results
A total of 1,663 HCWs were subjected to serological tests for measles. Among them, 84 HCWs did not undergo serological tests, including 53 (3.2%) who quit work, three (0.2%) who were on leave, and 28 (1.7%) without documented reasons. Finally, the serological test results of 1,579 (94.9%) HCWs were analyzed in this study.
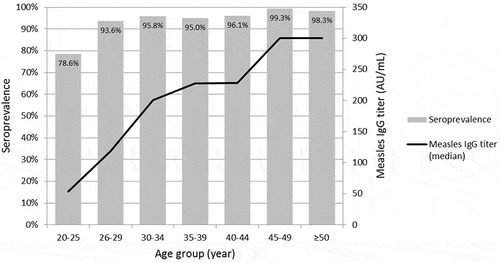
Among the tested HCWs, measles seropositivity was 92.0% (n = 1,453, ). The median age of the tested HCWs was 32 years (range: 20–68 years). Seropositive HCWs were significantly older than seronegative ones (p < .001, ), and the seropositivity and median titer of measles IgG tended to increase with age (p < .001, ). There were differences in seropositivity according to sex, occupation, and previous history of vaccination (). Documented records of MCV immunization were identified for 870 (55.1%) HCWs. HCWs without documented records of MCV immunization were more likely to be older (mean 44.5 ± 9.3 years vs. 27.7 ± 5.5, p < .001) and seropositive (96.2% vs. 88.6%, p < .001) than those with documented records of MCV immunization. Among 870 HCWs whose records of MCV immunization were documented in the registry, seropositivity increased significantly with the number of previous MCV immunizations (p = .003). In a multivariate analysis, measles seropositivity was significantly associated with old age (p < .001) and ≥2-dose MCV immunization (p = .002, ).
Table 1. Comparison between healthcare workers who were seropositive and seronegative to measles
Among 870 HCWs with documented records of MCV immunization, 632 (72.6%), 204 (23.4%), 33 (3.8%), and one (0.1%) received one, two, three, and four doses of vaccination, respectively. The seropositivity and the measles IgG titer increased significantly with the number of MCV doses in the 20–25 year age group (). However, in other age groups, the relationship between dose and seropositivity was not significant (). The majority (84.5%, 201/238) of ≥2 doses recipients received the last dose MMR at the workplace or before employment in accordance with the hospital’s policy, and the seropositivity was significantly higher (98.0%, 197/201) in these HCWs compared to those who received one (84.4%. 450/533), or two doses of MCV (70.3%, 26/37) during their childhood. Only 66.7% (20/30) of HCWs aged 20–25 years who received two-dose vaccination during childhood remained seropositive.
Table 2. Seroprevalence against measles and measles IgG titer according to the number of previous MCV immunizations in each age group of the healthcare workers with a previous history of MCV immunization
For HCWs with equivocal or negative results for baseline serological tests, the MMR vaccine was offered and seroconversion after vaccination was determined. Among the 29 HCWs with equivocal results, 19 (65.5%) received one dose of MMR vaccination, and serological tests were not repeated after vaccination. Among 97 seronegative HCWs, 96 (99.0%) received two doses of MMR, and 87 of them underwent serological tests ≥4 weeks after completing vaccination. Seroconversion was detected in 86 (98.9%) and equivocal results in one (1.1%). For the 86 seroconverted HCWs, the median titer of measles IgG was not significantly different according to age, sex, occupational group, or doses of previous MCV immunization ().
Table 3. Measles IgG titer after two-dose MMR vaccination among seronegative healthcare workers
Discussion
In this study, the overall seropositivity for measles was 92.0% among HCWs, but the seropositivity in HCWs aged 20–25 years was only 78.6% and the workplace catch-up MMR vaccination effectively increased the seropositivity in young HCWs.
In South Korea, there was a wide variation in the overall seropositivity for measles among HCWs across hospitals (71.7%−93.1%), which was driven by the substantial differences in the seropositivity among young HCWs (47%-95.7%).Citation9–11 These differences appeared to be determined by the hospital’s vaccination policy for HCWs. At our hospital, for HCWs at risk of exposure to measles without documented two-dose immunization, one free dose of MMR vaccine is offered without serological tests, and for newly employed HCWs, documentation of two-dose MMR vaccination has been required since 2014. If newly employed HCWs do not have this documentation, catch-up MMR vaccination is recommended before beginning work. This policy can explain the relatively high seropositivity among HCWs aged 20–25 years (born in 1994–1999) compared to the same birth cohorts in the general population (48.5%).Citation8 However, catch-up MMR vaccination is not mandatory, and this level of seropositivity among frontline HCWs is not sufficient for preventing measles transmission to HCWs because measles is highly infectious with a basic reproductive number of 12–18.Citation13 Therefore, measles vaccination status should be up-to-date among young HCWs, and mandatory MCV immunization should be considered for HCWs.
Although two-dose MCV immunization is assumed to provide long-term protection against measles,Citation2 there is limited information on the durability of protective immunity offered by the MCV in countries with measles-eliminated status and vaccine-induced immunity may wane rapidly because the exogenous boosting effect resulting from exposure to circulating measles virus would decline with a decrease in measles incidence.Citation14–17 In South Korea, two-dose MMR immunization during childhood was recommended in 1997, and its verification at school entry has been mandatory since 2002. Thus, most HCWs aged 20–25 years in this study should have received at least two doses of MMR vaccines. However, their seropositivity was lower than that of HCWs aged 25–34 years who received one-dose school-based catch-up vaccination in 2001. In South Korea, before 2001, nationwide measles outbreaks occurred every 4–6 years.Citation18 After the catch-up vaccination campaign for school-age children in 2001, the measles incidence markedly decreased to <1 case/100,000 population and has remained stable since 2002.Citation8 Thus, vaccine-induced immunity among HCWs aged 20–25 years (born in 1994 and later) may wane more rapidly due to the reduced exogenous boosting effect. The Korean seroprevalence study in 2014 also showed rapid waning of vaccine-induced immunity in adolescents. Measles seropositivity began to decrease from 10 years of age and dropped to 48.5% among those aged 16–19 years, despite high MMR coverage.Citation8
Documentation of immunization with ≥2 doses of MCV is currently considered presumptive evidence of measles immunity regardless of the serological test results.Citation2 However, 11.7% (11/94) of measles patients aged 10–29 years had documented records of two-dose MMR vaccination during their childhood in the 2019 measles epidemic in South Korea.Citation3 In one report in the US, 34.5% of 29 HCWs infected at work were two-dose recipients.Citation6 Thus, childhood two-dose vaccination may not provide sufficient immunity against measles among HCWs who are at increased risk of exposure to measles in the post-elimination period. Laboratory evidence of immunity should be verified by serological tests for HCWs, whether or not the HCW has documentation of receiving two-dose MCV vaccines. If HCWs are identified as seronegative, they should receive subsequent two-dose MMR vaccination.
This study had some limitations. The National Immunization Registry Information System was launched in 2000 and became widely utilized in 2011.Citation19 Therefore, records of MCV immunization might not be complete for some HCWs. Second, the presence of measles IgG was regarded as the presence of immunity against measles; however, the development and durability of cell-mediated immunity (CMI) against measles and its protective effects in seronegative vaccine recipients have not been defined.Citation20–23 In this study, neutralizing antibody titers were not measured, and a commercial immunoassay kit was used to measure the measles IgG titers. The CLIA used in this study performed well, with a sensitivity of 97% and specificity of 93% compared to the enzyme immunoassay (Enzygnost, Dade Behring, Germany).Citation24 This immunoassay kit showed an excellent ability to detect measle immunity compared to the plaque reduction neutralization test.Citation25 However, most commercial immunoassay kits exhibited low agreement with the neutralization test in low-titer ranges.Citation26 Thus, HCWs with low-range titers might have been misclassified as equivocal or seronegative. Furthermore, commercial immunoassay kit results may not correlate with true protective immunity against measles.Citation26 Therefore, further studies on determining CMI and measuring neutralizing antibody titers will help establish MMR vaccination strategies for HCWs.
In summary, seropositivity for measles was not sufficient to prevent transmission among young HCWs, although the two-dose vaccination rate was high among them. This indicates that vaccine-induced immunity wanes in young adults who are less likely to experience immunity boosting resulting from exposure to natural measles infection, and the number of HCWs susceptible to measles is expected to increase with time in South Korea. Active prevention strategies for measles should be implemented for young HCWs who are at high risk of contracting measles. As of now, universal serological tests for measles for newly employed HCWs and additional MMR vaccination for seronegative HCWs seem to be appropriate preventive measures.
Disclosure of potential conflicts of interest
The authors have declared no potential conflicts of interest.
Additional information
Funding
References
- Moss WJ. Measles. Lancet. 2017;390:2490–502. doi:10.1016/S0140-6736(17)31463-0.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS. Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2013;62:1–34.
- Choi S, Cho EH. Analysis of the occurrence of measles in Korea, 2019. Public Health Wkly Rep. 2020;13:2445–58.
- Maltezou HC, Dedoukou X, Vernardaki A, Katerelos P, Kostea E, Tsiodras S, Mentis A, Saroglou G, Theodoridou M, Georgakopoulou T. Measles in healthcare workers during the ongoing epidemic in Greece, 2017-2018. J Hosp Infect. 2018;100:e261–3. doi:10.1016/j.jhin.2018.06.007.
- Coppeta L, Pietroiusti A, Morucci L, Neri A, Ferraro M, Magrini A. Workplace vaccination against measles in a teaching hospital of Rome. J Hosp Infect. 2019;101:364–65. doi:10.1016/j.jhin.2018.11.022.
- Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61:615–18. doi:10.1093/cid/civ387.
- Botelho-Nevers E, Cassir N, Minodier P, Laporte R, Gautret P, Badiaga S, Thiberville DJ, Ninove L, Charrel R, Brouqui P. Measles among healthcare workers: a potential for nosocomial outbreaks. Euro Surveill. 2011;16:19764.
- Kang HJ, Han YW, Kim SJ, Kim YJ, Kim AR, Kim JA, Jung HD, Eom HE, Park O, Kim SS. An increasing, potentially measles-susceptible population over time after vaccination in Korea. Vaccine. 2017;35:4126–32. doi:10.1016/j.vaccine.2017.06.058.
- Jung J, Kim SK, Kwak SH, Hong MJ, Kim SH. Seroprevalence of measles in healthcare workers in South Korea. Infect Chemother. 2019;51:58–61. doi:10.3947/ic.2019.51.1.58.
- Chang HH, Kim SW, Kwon KT, Kim HI, Kim MJ, Ryu SY, Kim HA, Hur J, Kwon HH, Hong HL. Preliminary report of seroprevalence of anti-measles immunoglobulin G among healthcare workers of 6 teaching hospitals of Daegu, Korea in 2019. Infect Chemother. 2019;51:54–57. doi:10.3947/ic.2019.51.1.54.
- Kwak YG, Song JE, Oh GB, Jeong IH, Cho CR, Kim N, Yoo HM, Yoo GM, Lee MJ, Kim BN. Comparison of the seroprevalence of measles antibodies among healthcare workers in two Korean hospitals in 2019. Infect Chemother. 2020;52:93–97. doi:10.3947/ic.2020.52.1.93.
- Park SH, Lee MS, Kim SR, Kwak YG. A nationwide survey on the hospital vaccination policies in Korea. J Korean Med Sci. 2020;35:e76. doi:10.3346/jkms.2020.35.e76.
- Guerra FM, Bolotin S, Lim G, Heffernan J, Deeks SL, Li Y, Crowcroft NS. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis. 2017;17:e420–8. doi:10.1016/S1473-3099(17)30307-9.
- Davidkin I, Jokinen S, Broman M, Leinikki P, Peltola H. Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. J Infect Dis. 2008;197:950–56. doi:10.1086/528993.
- Whittle HC, Aaby P, Samb B, Jensen H, Bennett J, Simondon F. Effect of subclinical infection on maintaining immunity against measles in vaccinated children in West Africa. Lancet. 1999;353:98–102. doi:10.1016/S0140-6736(98)02364-2.
- Dai B, Chen ZH, Liu QC, Wu T, Guo CY, Wang XZ, Fang HH, Xiang YZ. Duration of immunity following immunization with live measles vaccine: 15 years of observation in Zhejiang Province, China. Bull World Health Organ. 1991;69:415–23.
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–15. doi:10.1056/NEJMoa066092.
- Kang JH. Review of measles in Korea: quarantine and elimination. Infect Chemother. 2020;52:113–22. doi:10.3947/ic.2020.52.1.113.
- Kim CS, Park O, Kim MY, Kim MJ, Lee SG, Jung HK. A study on registration data analysis of national immunization registry information system. J Korean Inst Info Commun Eng. 2015;19:1151–56. doi:10.6109/jkiice.2015.19.5.1151.
- Naniche D, Garenne M, Rae C, Manchester M, Buchta R, Brodine SK, Oldstone MB. Decrease in measles virus-specific CD4 T cell memory in vaccinated subjects. J Infect Dis. 2004;190:1387–95. doi:10.1086/424571.
- Kennedy RB, Ovsyannikova IG, Thomas A, Larrabee BR, Rubin S, Poland GA. Differential durability of immune responses to measles and mumps following MMR vaccination. Vaccine. 2019;37:1775–84. doi:10.1016/j.vaccine.2019.02.030.
- Fiebelkorn AP, Coleman LA, Belongia EA, Freeman SK, York D, Bi D, Kulkarni A, Audet S, Mercader S, McGrew M, et al. Measles virus neutralizing antibody response, cell-mediated immunity, and immunoglobulin G antibody avidity before and after receipt of a third dose of measles, mumps, and rubella vaccine in young adults. J Infect Dis. 2016;213:1115–23. doi:10.1093/infdis/jiv555.
- Haralambieva IH, Ovsyannikova IG, O’Byrne M, Pankratz VS, Jacobson RM, Poland GA. A large observational study to concurrently assess persistence of measles specific B-cell and T-cell immunity in individuals following two doses of MMR vaccine. Vaccine. 2011;29:4485–91. doi:10.1016/j.vaccine.2011.04.037.
- de Ory F, Minguito T, Balfagon P, Sanz JC. Comparison of chemiluminescent immunoassay and ELISA for measles IgG and IgM. APMIS. 2015;123:648–51. doi:10.1111/apm.12413.
- Ratnam S, Gadag V, Wet R, Burris J, Oates E, Stead F, Bouilianne N. Comparison of commercial enzyme immunoassay kits with plaque reduction neutralization test for detection of measles virus antibody. J Clin Microbiol. 1995;33:811–15. doi:10.1128/JCM.33.4.811-815.1995.
- World Health Organization. The immunological basis for immunization series: module 7: measles. Update 2020. Geneva (Switzerland): World Health Organization; 2020.