ABSTRACT
Vaccination against COVID-19 may present the most effective strategy to control current viral pandemic. The success of delivering mass vaccination, on the scale of what would be applied to contain COVID-19, largely depends on the compliance of the public to programs mandated by public health officials. This study was aimed to evaluate the perception and possible hesitance of people in Jordan toward a tentative COVID-19 vaccine using self-administrated online survey. During the study period, a total of 1287 agreed to participate in the study. More than half of the participants (n = 734, 57%) were females and the majority (n = 893, 69%) had a University degree. Most of the participants (n = 871, 68%) believed that scientists have adequate tools to develop a safe and efficacious COVID-19 vaccine and two-third of them (n = 861, 67%) believed that developing vaccines would end the pandemic. However, around half of them (n = 665, 52%) reported not having adequate information on the benefits of COVID-19 vaccination. Preference of study participants to achieve immunity against COVID-19 using natural way was the most commonly reported reason to refuse vaccination (n = 826, 64%), followed by their concern about adverse effects associated with the vaccine (n = 781, 61%). In conclusion, the sampled participants showed an overall positive attitude toward receiving a COVID-19 vaccine. Educational campaigns using television and social media are recommended to better inform the public of the benefits of COVID-19 vaccine in reaching a “herd immunity” based strategy to control the current pandemic.
1. Introduction
Immunization through vaccination is considered one of the global health needs that protects public worldwide from several serious illnesses and saves millions of lives yearly through enhancing body’s natural immune systemCitation1. In spite of the availability of more than 20 effective vaccines that protect humanity from more than 20 serious illnesses,Citation2 hesitancy to take vaccines by individuals remains an emerging challenge in several countries.Citation3,Citation4 Vaccination hesitancy and even, refusal, has been documented through history, and has led increased risk for serious viral diseases such as measles and negative/positive perception and hesitancy toward viral vaccination.Citation5 Vaccination, namely, the MMR, has been falsely linked to the development of autism.Citation6 Moreover, prenatal exposure to rubella, and influenza vaccines were linked to increased risk of schizophrenia.Citation7 However, risk/benefits analysis clearly favors the routine administration of influenza vaccines to pregnant women.Citation8
As a consequence of this issue, the World Health Organization (WHO) launched a working group in 2012 to manage and deal with the hesitancy problem.Citation9 This established group defined vaccine hesitancy as “the delay in acceptance or refusal of vaccination despite availability of vaccination services. Vaccine hesitancy is complex and context-specific, varying across time, place and vaccines. It is influenced by factors such as complacency, convenience and confidence”.Citation10
Concurring with the worldwide spread of Coronavirus-19 infection (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the lack of available treatment for the disease so far, international pharmaceutical companies are racing to develop a new vaccine for COVID-19 to prevent the terrifying spread of the virus.Citation11 Till now, more than 100 companies are trying to develop COVID-19 vaccine candidates, and some the vaccines have been successfully approved in several countries.Citation12,Citation13
With all these tremendous efforts exerted to reach a efficacious COVID-19 vaccine, actual success will highly depend on the acceptance of the vaccine by public.Citation3 Especially that the development of new vaccine will take place under exceptional circumstances, where public health policies may be relaxed by the legislature to speed up COVID-19 vaccine clinical trials.Citation14 This, in turn, may lead to an increase in individuals’ vaccine hesitancy more than it is known.
Thus, the current study was conducted to evaluate Jordanian public perception and hesitancy toward any possible new COVID-19 vaccine and to explore reasons and factors affecting their hesitancy. Identifying such factors could help the decision maker to design strategies that could manage the issue of vaccine hesitancy.
2. Methods
2.1. Study design, subjects, and settings
The study used a cross-sectional design that was conducted between July and August 2020. The study population was the public at various parts of Jordan. Public were included in the study if they were adult ≥18 years old, Arabic speaking, and willing to participate in the study. A google form survey was distributed to public using social media platforms (Facebook and WhatsApp). Pubic were invited to participate in this study using convenience sampling process. Each potential participant was asked to read a page that included detailed information about the study purpose, benefits, and risks, as well as conditions of participation. They were then asked to agree to an electronic informed consent form as follows “If you do not wish to participate in the research study, please decline participation by clicking on the “disagree” button”, which contained a statement about the anonymity of the survey and voluntary participation. The protocol of this study was approved by the Institutional Review Board of Jordan University of Science and Technology (Approval code: 23/128/2019). It also complied with the World Medical Association Declaration of Helsinki guidance.Citation15
2.2. Sample size calculation
The researcher used the G*Power software version 3.1.9.4 to calculate the sample size. A significance level of 0.05, a power of 0.90 and a small effect size of 0.10 with the minimum number of subjects being 1162. Based on an anticipated a dropout rate of about 10%, the target number of participants was 1300. The researcher performed an analysis of the data on 1287 subjects.
2.3. Study questionnaire
The study survey was based on search of literature using Google Scholar, and Medline/PubMed. Relevant literature relevant to public perception toward vaccination was used. The items included in the study survey were built based on reviewed literature with some modification.Citation16 Then, the developed survey was both face and content validated. At First, an experts group gave feedback on questionnaire items, which were modified as per the comments of this group. Secondly, the questionnaire draft was pilot tested on 30 subjects who provided feedback items comprehensibility and clarity. Data collected from pilot testing was not included in the final analysis. Forward (Arabic) and backward (English) translations were carried out by two bilingual researchers (KZ, OK). The Arabic version was the one used in data collection since Jordan is considered among the Arabic-speaking countries. Finally, the value of the reliability measure Cronbach’s α was 0.882, which indicates acceptable internal consistency.
The final version of the questionnaire consisted of a number of multiple choice questions that were divided into three parts: A) Demographic variables and experiences regarding COVID-19 infection, B) Part 2 examined Public perception toward COVID-19 vaccine development. C) The last part of the survey examined public perceived reasons for hesitancy/refusal to try any new COVID-19 vaccine.
2.4. Measured outcomes
A hesitancy score was calculated for each participant using the following scoring system derived from the Likert scale used in this study: “Strongly agree = 5, agree = 4, neutral = 3, disagree = 2, and strongly disagree = 1”. The average score (out of 5) for the 11 used statements were calculated. These scores were converted (standardized) into Percent of Maximum Possible (POMP) scoreCitation17 using the following equation: POMP = [(score – minimum)/(maximum – minimum)] × 100
Where, score = participant’ average score (out of 5), minimum = the minimum possible score (which is 1 for this l Likert scale), and maximum = the maximum possible score on (which is 5 for the used Likert scale).
2.5. Statistical analysis
The statistical package for social science (SPSS®) version 22 (SPSS® Inc., Chicago, IL, USA) was used for data analysis. The mean ± SD and percentages were used to for continuous and categorical variables, respectively. Normality was checked using the Shapiro-Wilk test.
Univariate and multivariate linear regression were used to screen for factors that affect participants’ hesitancy score toward COVID-19 vaccine. Variables that were found to be significant on a single predictor level (P-value< 0.25) using univariate linear regression analysis were entered into multiple linear regression analysis. Variables were selected after checking their independence, where Pearson’s correlation coefficient (r) less than 0.9 indicates the absence of multicollinearity between the independent variables in regression analysis. In the multiple linear regression analysis, variables that were independently associated with hesitancy score were identified. Statistical significant was considered at P-value <0.05.
3. Results
During the two-month study period, from July to August 2020, 1287 participants agreed to participate in this study. The mean age of participants was 30.1 years (SD = 9.7). More than half of the participants were females (n = 735, 57.0%). The majority of the sample was educated (n = 893, 69.4%), and of Jordanian nationality (n = 1229, 95.5%). Demographic characteristics of the study participants are presented in .
Table 1. Demographic characteristics of the study sample at baseline (n = 1287)
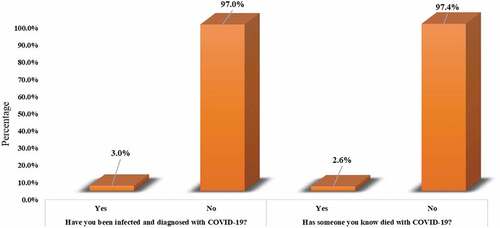
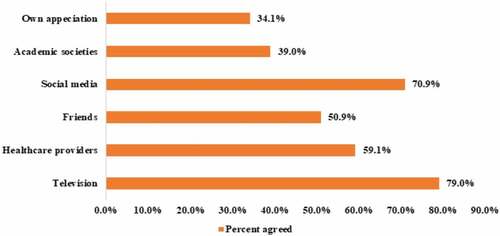
Regarding participants' previous experience with COVID-19 infection (), only 3.0% of them (n = 38) reported that they had a previous exposure/infection with the virus, and around 2.6% of them (n = 34) were acquainted with someone who died with COVID-19. Participants reported that their main source of knowledge about COVID-19 () came from watching television (n = 1017, 79.0%) followed by social media (n = 912, 70.9%) and healthcare providers (n = 761, 59.1%). It is interesting to note that only 39.0% of the participants depend on academic societies as a source for their information about COVID-19.
The perception of the study sample toward a tentative COVID-19 vaccine is described in . In general, the perception of the participants toward the development of such vaccine was positive. More than 60% of the participants agreed/strongly agreed that the scientific community has the tools and potential to develop and effective COVID-19 vaccine (n = 871, 67.7%). In addition, the majority believed that the development of an effective COVID-19 vaccine would put an end to the current pandemic (n = 861, 66.7). Moreover, more than half (64.3%) of the sample (n = 827) agreed/strongly agreed that legislation that governs vaccine manufacturing should be relaxed to expedite the process of vaccine development. However, only 41.0% of them (n = 528) believed that COVID-19 vaccine should be tested on people compared to other less urgent circumstances, even if side-effects are unknown, and 64.2% of them (n = 826) reported that in the event the vaccine passes through a shortened assessment period and approval, it should be subject to a longer than usual post-marketing monitoring.
Table 2. Public perception toward COVID-19 vaccine development (n = 1287)
summarizes the responses of the participants toward statements that evaluated the reasons behind their hesitancy/refusal to receive a COVID-19 vaccine. Our results indicated that the preference of the sampled participants to become immune against COVID-19 using a “more natural” method such as using supplements was the most common reason (n = 826, 64.2%) behind any hesitancy. This was followed by participants’ concern of adverse effects that could be triggered by the COVID-19 vaccine (n = 781, 60.7%). Interestingly, around half (51.7%) of the participants (n = 665) reported that the lack of adequate information on the benefits of immunization against COVID-19 and/or the harms of infection with the virus is the reason behind their refusal to receive any COVID-19 vaccine if it becomes readily available. Noteworthy, less than half (45.8%, n = 589) of the participants indicated financial costs would hinder them from receiving the vaccine and only 20.1% (n = 259) reported that their religious/cultural beliefs were against vaccination and would thus prevent them from receiving any COVID-19 vaccine. Besides, participants showed an overall hesitancy score of 56.2 (SD = 16.0).
Table 3. Public perceived reasons for hesitancy/refusal to try any new COVID-19 vaccine (n = 1287)
Finally, simple and multiple regression models were performed to ascertain which of the variables included in this report significantly predicted hesitancy/refusal of the participants to receive COVID-19 vaccine in the presence of other con-founders (). In this analysis, the variables that significantly predicted hesitancy/refusal in both models were: nationality, previous infection/diagnosis with COVID-19 and acquaintance with someone who succumbed to COVID-19 infection (P-value <0.05). Specifically, Jordanian participants, who had a previous infection with COVID-19 and those who were acquainted with someone who succumbed to COVID-19 were less reluctant to receive a COVID-19 vaccine. The R for the regression was significantly different from zero (P-value<0.001). The highest correlation between independent variables was −0.622 indicating absence of multicollinearity.
Table 4. Assessment of factors affecting participants’ COVID-19 vaccine hesitancy (n = 1287)
4. Discussion
The first case of COVID-19 in Jordan, a third world Middle Eastern country, was officially registered by the WHO on the second of March of 2020.Citation18
In an attempt to suppress the spread of COVID-19 in Jordan, which would overwhelm an already exhausted public health system, the government-mandated a number of strict measures which included among others a complete lockdown of the country for several days.Citation19 The above lockdown was partially lifted but was still implemented for one or two weekdays for the following months. These measures contained the spread of the virus and for several weeks Jordan did not register more than 20 positive cases of COVID-19 per day.Citation20 Unfortunately, after lifting the above measures, the country entered into a second more aggressive wave of the virus with local transmission. Indeed, at the time of writing this report, Jordan registered more than 169,395 cases of COVID-19 with 2,053 total deaths attributed to the virus.Citation18 At this moment, around 25% of COVID-19 tests administered on any single day indicate a positive infection with the virus.Citation20 The current trends of COVID-19 transmission in Jordan and other neighboring countries, the devastating effects a future lockdown would have on the country’s economy, the absence of any efficacious treatment against COVID-19 and the mental health problems associated with sustaining social distancing measures; all indicate that vaccination against COVID-19 would be the most effective measure to control or possibly end COVID-19 pandemic from an economic and health-related standpoint.
The success of delivering mass vaccination, on the scale of what would be applied to contain COVID-19, largely depends on the compliance of the public to programs mandated by public health officials.Citation21 This questionnaire-based study was thus performed to evaluate factors that may affect the hesitancy of Jordanians toward receiving COVID-19 vaccine.
More than half of the sampled participants trusted that scientists have adequate tools and knowledge to develop a COVID-19 vaccine and that the development of such a vaccine would put an end to the current pandemic. The above perceptions reflect an overall positive attitude, in principle, toward a COVID-19 vaccine. Our findings are similar to those published recently by Bell et al., where about 56% of adults were willing to receive a COVID-19 vaccine, and of which 48.2% were even willing to give the vaccine to their own children.Citation22 This general positive attitude toward the COVID-19 vaccine was also reported from multiple other studies, wherein some, 90% of the sampled participants showed willingness to receive a COVID-19 vaccine once it becomes available.Citation23–26
In this report, the positive attitude toward COVID-19 vaccine is further supported by our finding that only a minority of the participants doubts the effectiveness of a COVID-19 vaccine if one is developed. Moreover, the majority do not object to a more relaxed legislature to expedite its development and eventual approval.
An evaluation of the factors that may explain refusal/hesitancy of the public toward receiving a COVID-19 vaccine showed that the most common reason reported by the participants was their preference of a “more natural” way to immunize against COVID-19. This result may be related to another finding of this survey which showed that around two thirds of the participants were concerned of the side effects of a potential COVID-19 vaccine. This result is analogous to the report published by Bell et al. where they found that concerns over a COVID-19 vaccine rank as the second most common reason to refuse the vaccine.Citation22 Concerns over the side effects of a COVID-19 vaccine were also reported from a study that sampled the general population of the United Arab Emirates.Citation27 In this study performed on 1109 participants, 59% of the study population expressed their lack of knowledge toward vaccines and their safety in general.
Several studies found that hesitancy toward the COVID-19 vaccine can be observed clearly among younger adults in many countries, and even among doctors and medical trainees.Citation28–31 In this report, age was a significant predictor of a lower hesitancy score in our univariate regression model indicating that younger individuals were more hesitant to receive the COVID-19 vaccine. This observation may be explained by the fact that younger generations more frequently use the internet and social media as a source of knowledge. These sources of information are under less stringent surveillance by public health officials and may contain a bigger volume of conspiracy theories on COVID-19.Citation30,Citation32
It appears that if health officials in Jordan wish to mandate a COVID-19 vaccine, one of the obstacles they could face would be the notion that a “more natural” way to achieve immunization against COVID-19 is safer and is free of adverse effects. This notion should be challenged prior to starting a COVID-19 vaccine program. Changing the public opinion regarding the above could be achieved by educational campaigns which emphasize that a “more natural way” to achieve immunity against COVID-19 would be to reach “herd immunity”; a state which requires infection of around 60% of the population with COVID-19 according to current epidemiological based models.Citation33 The above educational campaigns should further emphasize that herd immunity means the eventual death of 2% of the patients infected with COVID-19 (around 120,000),Citation34 the potential collapse of the entire health care system in Jordan and the loss of life of patients with other conditions; the lives of which would normally be saved under other circumstances.
The results of this survey further support that the above-mentioned campaign could be successful in achieving its goals especially since around half of the participants indicated that they do not have sufficient knowledge of the benefits of vaccination or the harms of COVID-19 infection and have labeled this gap in knowledge as a potential cause to refuse a COVID-19 vaccine.
A significant finding of this survey indicated that television followed by social media were the most common platforms used by participants to obtain knowledge on COVID-19. Mukattash et al. reported similar results as more than 53% of sampled participants reported that television and the internet were the primary sources for acquiring information related to COVID-19.Citation27 Accordingly, campaigns which aim to educate the public on the benefits of a COVID-19 vaccine should primarily use the above platforms to deliver their information.
This survey, although important in assessing public opinion toward a COVID-19 vaccine, has several limitations. First, females represented 57% of survey participants, a number that exceeds the female to male ratio in Jordan where females only show a slight predominance in the population. Secondly, the survey did not collect information on the professional background of the participants including the percentage of participants who are currently working in a health-related field. These participants are expected to have more adequate information on the benefits of vaccines and/or harms of a COVID-19 infection and may thus skew the findings of this survey if they are predominantly represented in the study population. Moreover, this is a cross-sectional study where data were collected using electronic survey and using a convenience sample based on the authors’ networks on social media, which could limit the generalizability of the findings. However, there could not be a more appropriate method of data collection during the lockdown period. Finally, this study was conducted before the approval of COVID-19 vaccines, also public knowledge on COVID-19 is continuously and rapidly evolving especially with a second and even third waves of the disease emerging all over the world.Citation25,Citation35 Thus, the viewpoints of people in Jordan may change in response to that. Accordingly, follow-up studies must be conducted to reflect the evolved viewpoints of the public.
In conclusion, the sampled participants showed an overall positive attitude toward receiving a COVID-19 vaccine. Educational campaigns using television and social media are recommended to better inform the public of the benefits of COVID-19 vaccine in adopting a “herd immunity” to control the current pandemic.
Declaration of potential conflicts of interest
The authors declare that they have no competing interests.
References
- Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12:509–17. doi:10.1038/ni.2039.
- WHO. World Health Organization. Vaccines and immunization. 2020. https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1
- Salmon DA, Dudley MZ, Glanz JM, Omer SB. Vaccine hesitancy: causes, consequences, and a call to action. Vaccine. 2015;33:D66–D71. doi:10.1016/j.vaccine.2015.09.035.
- Cooper LZ, Larson HJ, Katz SL. Protecting public trust in immunization. Pediatrics. 2008;122:149–53. doi:10.1542/peds.2008-0987.
- Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association between vaccine refusal and vaccine-preventable diseases in the united states: a review of measles and pertussis. Jama. 2016;315:1149–58. doi:10.1001/jama.2016.1353.
- Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clin Infect Dis. 2009;48:456–61. doi:10.1086/596476.
- Skowronski DM, De Serres G. Is routine influenza immunization warranted in early pregnancy? Vaccine. 2009;27:4754–70. doi:10.1016/j.vaccine.2009.03.079.
- Blanchard-Rohner G, Eberhardt C. Review of maternal immunisation during pregnancy: focus on pertussis and influenza. Swiss Med Wkly. 2017;147:w14526. doi:10.4414/smw.2017.14526.
- Schuster M, Eskola J, Duclos P. Review of vaccine hesitancy: rationale, remit and methods. Vaccine. 2015;33:4157–60. doi:10.1016/j.vaccine.2015.04.035.
- MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33:4161–64. doi:10.1016/j.vaccine.2015.04.036.
- WHO. World Health Organization. The push for a COVID-19 vaccine. 2020.
- FDA. Food and drug administration: COVID-19 vaccines. 2020.
- Burki TK. The Russian vaccine for COVID-19. Lancet Respir Med. 2020;8:e85–e6. doi:10.1016/S2213-2600(20)30402-1.
- WHO. World Health Organization. Accelerating a safe and effective COVID-19 vaccine. 2020.
- World Medical A. World medical association declaration of helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–94.
- Detoc M, Bruel S, Frappe P, Tardy B, Botelho-Nevers E, Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. 2020;38:7002–06. doi:10.1016/j.vaccine.2020.09.041.
- Fischer R, Milfont TL. Standardization in psychological research. Int. J. Psychol. Stud. 2010;3:88–96. doi:10.21500/20112084.852.
- WHO. [accessed 2020 Nov 22]. https://covid19.who.int/region/emro/country/jo.
- Saadeh RA, Alfaqih M, Younis OB, Okour A, Obeidat K. The psychosocial and clinical concerns of physicians treating COVID-19 patients. J Taibah Univ Med Sci. 2020;15(6):544–549.
- Ministry of Health J. [accessed 2020 Nov 22]. https://corona.moh.gov.jo/ar
- Atkeson A. What will be the economic impact of covid-19 in the us? Rough estimates of disease scenarios. Nat Bur Econ Res. 2020.
- Bell S, Clarke R, Mounier-Jack S, Walker JL, Paterson P. Parents’ and Parents’guardians’ views on the acceptability of a future COVID-19 vaccine: A multi-methods study in England. Vaccine. 2020;38:7789–98. doi:10.1016/j.vaccine.2020.10.027.
- Biasio LR, Bonaccorsi G, Lorini C, Pecorelli S. Assessing COVID-19 vaccine literacy: a preliminary online survey. Hum Vaccin Immunother. 2020;1–9. doi:10.1080/21645515.2020.1829315.
- Kreps S, Prasad S, Brownstein JS, Hswen Y, Garibaldi BT, Zhang B, Kriner DL. Factors associated with US adults’ likelihood of accepting COVID-19 vaccination. JAMA Netw Open. 2020;3(10):e2025594. doi:10.1001/jamanetworkopen.2020.25594.
- Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, Kimball S, El-Mohandes A, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2020. doi:10.1038/s41591-020-1124-9.
- Sarasty O, Carpio CE, Hudson D, Guerrero-Ochoa PA, Borja I. The demand for a COVID-19 vaccine in Ecuador. Vaccine. 2020. doi:10.1016/j.vaccine.2020.11.013.
- Muqattash R, Niankara I, Traoret RI. Survey data for COVID-19 vaccine preference analysis in the United Arab Emirates. Data Brief. 2020;33:106446. doi:10.1016/j.dib.2020.106446.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi:10.1016/S0140-6736(20)30566-3.
- Pedersen MJ, Favero N. Social distancing during the COVID-19 pandemic: who are the present and future non-compliers? Public Adm Rev. 2020. doi:10.1111/puar.13240.
- McAteer J, Yildirim I, Chahroudi A. The VACCINES act: deciphering vaccine hesitancy in the time of COVID-19. Clin Infect Dis. 2020;71:703–05. doi:10.1093/cid/ciaa433.
- Grech V, Bonnici J, Zammit D. Vaccine hesitancy in Maltese family physicians and their trainees vis-a-vis influenza and novel COVID-19 vaccination. Early Hum Dev. 2020;105259. doi:10.1016/j.earlhumdev.2020.105259.
- Wilson SL, Wiysonge C. Social media and vaccine hesitancy. BMJ Glob Health. 2020;5. doi:10.1136/bmjgh-2020-004206.
- Gomes MGM, Corder RM, King JG, Langwig KE, Souto-Maior C, Carneiro J, Ferreira MU, Penha-Goncalves C. Individual variation in susceptibility or exposure to SARS-CoV-2 lowers the herd immunity threshold. medRxiv. 2020. doi:10.1101/2020.04.27.20081893.
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, Li X, Peng C, Zhang Y, Zhang W, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. Jama. 2020;324:1–10. doi:10.1001/jama.2020.15543.
- Lin C, Tu P, Beitsch LM. Confidence and receptivity for COVID-19 vaccines: a rapid systematic review. Vaccines. 2020;99(1):16.