ABSTRACT
The non/hypo-response rate of the hepatitis B vaccine among hemodialysis (HD) patients is still high, it is of great significance to explore the influencing factors and their relationships. To study the related factors and their relationships using logistic regression model and Chi-squared Automatic Interaction Detection (CHAID) decision tree model. A randomized controlled trial was conducted between February 2014 and May 2015 in China. HD patients being serologically negative for HBsAg and anti-HBs were randomly assigned to receive three intramuscular injections of the standard dose (20 µg) or high dose (60 µg) of recombinant hepatitis B vaccine at months 0, 1, and 6. Those with anti-HBs concentrations <100 mIU/mL, and ≥100 mIU/mL at month 7 were considered as non/hypo-response and high-level response, respectively. The non/hypo-response was 31.34% (89/284). After adjustment for confounders, logistic analysis showed that males (OR = 2.203, 95%CI: 1.109–4.367) and those with higher dialysis frequency (>4 times per 2 weeks) (OR = 1.918, 95%CI: 1.015–3.626) had a significant risk of non/hypo-response. While the CHAID analysis showed that gender, dose, and dialysis frequency were influencing factors of non/hypo-response, and gender is most important. The interaction between gender and dialysis frequency had the greatest effect on immunization, and followed by the interaction between dialysis frequency and vaccine dose. Taken together, gender, dose and dialysis frequency were influencing factors of hepatitis B vaccine in HD patients.
1. Introduction
Hepatitis B virus (HBV) infection is a global public health issue. It affects approximately 2.57 billion people worldwide of which 73.43% (350 million) is chronic infections Citation1. Vaccination against HBV is an effective and preventive measure. China introduced the hepatitis B vaccine into routine immunization management as comprehensive strategies in 1992 and the prevalence rate of HBV infection dropped precipitously from 9.75% (1992)Citation2 to 7.18% (2006).Citation3 And an ultramodern modeling study in 2016 showed that the prevalence rate of HBV infection was 6.10% (2016).Citation4 Although the hepatitis B vaccine plays a key role in the prevention of HBV infection, HBV infection rate is still high in some special populations.
The hemodialysis (HD) patients are one of the high-risk populations of HBV infection. Although HD can prolong the life of patients with end-stage renal disease, these patients are always accompanied by various immunologic abnormalities of innate and acquired immunity.Citation5 And with immune system damage caused by long-term dialysis,Citation6 the immune effect is not satisfactory after hepatitis B vaccination,Citation7 which resulted in higher non-response rate. Even if weak-response was produced, there is an inability to maintain adequate antibody concentrations over time. Prior studies have shown that many patients with anti-HBs concentrations between 10 and 100 mIU/mL did not retain protective antibody levels 1 year post-vaccination.Citation8 This showed that anti-HBs concentrations ≥100 mIU/mL were considered to be necessary for maintaining protection in immunocompromised patients. Researches showed that only 50 ~ 70% of patients with renal insufficiency developed sufficient immune response, even with high doses of vaccine.Citation9,Citation10 Due to the higher non/hypo-response rate of hepatitis B vaccine in HD patients, multiple strategies have been proposed to improve the response of the hepatitis B vaccine, including vaccine adjuvants,Citation11,Citation12 to re-immunize non/hypo-response, take increasing vaccination into considerationCitation13 and changes in vaccination routes,Citation14 but the non/hypo-response rate is still high and these methods cannot effectively protect patients from HBV infection. Therefore, it is of great significance to explore the influencing factors of the immunological response to hepatitis B vaccine in HD patients.
Prior studies have shown that the influencing factors of immunological response to hepatitis B vaccine are multiple, including male gender, old age, hepatitis B vaccine dose, malnutrition, diabetics, presence of DR3, DR7, and DQ2 HLA antigens, absence of the A2 allele, low albumin level, insufficient urea reduction rate and concomitant HCV infection.Citation7,Citation15,Citation16 But the results of these studies are inconsistent, and most of them only focused on multiple logistic regression analysis which cannot show the comprehensive relationship of the factors. In addition, it is not clear whether their interactions will affect the immunological response of hepatitis B vaccine or even though leads to the non/hypo-response of hepatitis B vaccine. So, it’s worth us paying more attention.
Machine data mining, such as decision tree analysis, is a method to explore the relationship between influencing factors. This analysis selects the variables from the database to split the sample into progressively smaller subgroups, resulting in a multilevel structure that resembles a tree. To decide which factor has the strongest association with the dependent variable at each point in the tree structure, the χ2 test is used on the basis of the minimum P value. When the Chi square Automatic Interaction Detection (CHAID) algorithm identifies the most important independent variable, the node divides into two branches until the next best variable is reached. A terminal node or leaf occurs when no remaining independent variable could yield a statistically significant difference (P < .05) or no further split could be made due to the stopping rules previously defined.Citation17 For each of the nodes generated, the decision tree analysis computed the probabilities of the risk expressed as percentages. This process can clearly show the interaction effects and relationships between variables, and can display the role of a variable in each subclass in detail.Citation18
In the CHAID decision tree model, due to the segmentation, the number of samples is shrinking, so it is impossible to support simultaneous testing of multiple variables. But logistic regression pays attention to the fitting of the whole data, so it is grasping the whole situation. And the results of logistic regression analysis fully showed the self-quantitative dependence between variables and dependent variables, the information about the relationship how the independent variables versus the dependent variable is more sufficient than the CHAID decision tree mode.Citation19 Therefore, the purpose of this study was to use the logistic regression and CHAID decision tree model to further explore the influencing factors and their relationship of the non/hypo-response of hepatitis B vaccine, and improve the immune response rate of hepatitis B vaccine, reduce the risk of HBV infection, improve the quality of life of HD patients.
2. Materials and Methods
2.1 Study design
The case control study was based on our previous study which was a multicenter, randomized, double-blind, parallel-controlled trial conducted between February 2014 and May 2015 at 13 hospitals in Shanxi Province, China.Citation20 According to a randomization list generated by a statistician using SAS 9.3., participants with serologically negative for HBsAg and anti-HBs were randomized in a ratio of 1:1 to receive 3 intramuscular injections of the standard dose (20 µg) or high dose (60 µg) of recombinant hepatitis B vaccine at months 0, 1, and 6.
The ready-to-use syringe of recombinant hepatitis B vaccine was provided by designated nurses and delivered to the investigator. And the allocation of participants was masked from participants, investigators, researchers involved in follow-up and data collection, and laboratory technicians. A total of 284 patients were followed up at month 7, and blood samples were collected and tested for anti-HBs using the chemiluminescent microparticle immunoassay (ARCHITECT HBsAg/anti-HBs Reagent Kit, Abbot Ireland Diagnostics Division, Finisklin Business Park, Sligo, Ireland). Patients were included in the case group if anti-HBs concentrations < 100 mIU/mL, and the control group would include those anti-HBs concentrations ≥ 100 mIU/mL.
2.2 Statistical analysis
A sample size of 152 per group is able to provide at least a power of 80% to detect a difference of 15% in terms of seroconversion rates between the standard dose group (20 µg) or high dose group (60 µg), at a significance level of 0.05. The data were double entered using EPI Data software database. After verifying accuracy, statistical analysis of the data was performed using SAS (version 9.4). The quantitative variables were summarized as mean ± standard deviation (SD), and the categorical variables were presented as frequencies and percentages. χ2 test or Fisher-Exact test was used for testing significant differences between two groups and P < .05 was taken as statistical significance. In order to estimate the probability of non/hypo-response and calculate the odds ratio (OR) for the presence of non/hypo-response, unconditional logistic regression and ordinal logistic regression were adopted. Non/hypo-response to hepatitis B vaccine was used as the dependent variable.
Meanwhile, CHAID decision tree was used for identifying possible variable interactions at different levels. Statistical analysis was performed using IBM SPSS Modeler, V.23.0. Classification rules are as follows: (1) Tree growth: the significance level of the growth “branch” segmentation αmerge = αsplit = 0.05; (2) Tree pruning: using the pre-pruning method, the maximum depth of the tree was three layers, the minimum sample number of the parent node was 50, the child node was 50, and the stopping rule was α = 0.05. If the sample size on the node does not reach this required, the node is the terminal node and will not be spilted.
3. Results
3.1 Study patients and follow-up at month 7, and baseline characteristics
Of the 284 HD patients followed up at month 7, 89 patients (31.34%) were non/hypo-response (anti-HBs < 100 mIU/mL), and 195 patients (68.66%) showed high-level response (anti-HBs ≥ 100 mIU/mL). Non/hypo-response group comprised 57 males (64.04%) and 32 (35.96%) females with a mean age of 45.45 years. The primary renal diseases include chronic glomerulonephritis in 38 cases (42.70%), diabetic nephropathy patients in 12 cases (13.48%), nephropathy in 13 cases (14.61%) and the others in 26 cases (29.21%). Lower dialysis frequency was founded in 51.69% of patients. High-level response group consisted of 95 males (48.72%) and 100 females (51.28%), with a mean age of 43.02 years. There were 80 cases of chronic glomerulonephritis (41.03%), 23 cases of diabetic nephropathy patients (11.80%), 30 cases of nephropathy (15.38%) and 62 cases of the others (31.79%). Duration of HD therapy was from 0.09 to 10.07 years, and lower dialysis frequency was founded in 65.13% of patients. The demographic profile of the two groups was similar in terms of age, career, marriage status, primary renal disease and diabetes, and so on ().
Table 1. Univariate analysis of influencing factors of non/hypo-response to hepatitis B vaccine in hemodialysis patients[n(%)]
3.2 Unconditional logistic regression
Non/hypo-response to hepatitis B vaccine was used as the dependent variable. Regarded patients of non/hypo-response as case group, and patients of high-level response as control group. The regression model incorporated with univariate analysis variables and some related variables reported in the literatures. Unconditional logistic regression analysis revealed that the male (OR = 2.203, 95%CI: 1.109–4.367) and higher dialysis frequency (OR = 1.918, 95%CI: 1.015–3.626) were considered as independent risk factors for non/hypo-response to hepatitis B vaccine, after adjusting for confounders ().
Table 2. Unconditional logistic regression of influencing factors of hepatitis B vaccine non/hypo-response in hemodialysis patients
3.3 Ordinal logistic regression
In order to better detect the factors influencing the non/hypo-response to hepatitis B vaccine, we used ordinal logistic regression (the dependent variable divided into non-response, weak response and high-level response) to deeply analyze. And the result showed the same influencing factors with unconditional logistic regression. shows, after adjusting some confounders by means of multivariate analyses, higher dialysis frequency (OR = 1.841, 95%CI: 1.015–3.340) and male (OR = 2.128, 95%CI: 1.089–4.149) were independent predictors for non/hypo-response (). shows the assignment of independent variables.
Table 3. Ordinal logistic regression of influencing factors of hepatitis B vaccine non/hypo-response in hemodialysis patients
Table 4. Variables assignment
3.4 CHAID decision tree model
shows a pruned classification tree model. It had a depth of four levels, with a total of seven nodes, including four terminal nodes (numbered 1, 4, 5 and 6). From CHAID analysis we can see, males, higher dialysis frequency, and 20 μg of hepatitis B vaccine were the risk predictors of hepatitis B vaccine non/hypo-response in HD patients.
Figure 1. Influencing factors of hepatitis B vaccine non/hypo-response based on decision tree model in hemodialysis population
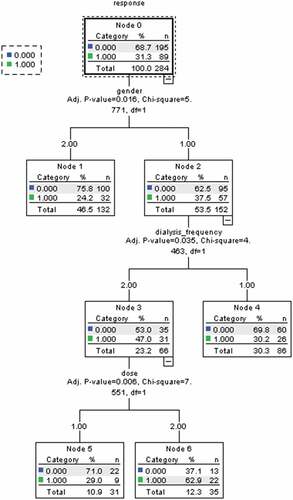
Gender is the first variable selected for the splitting node, meaning that this variable is the best predictor of the non/hypo-response. It divides the population into two groups, of which males had higher rate (37.5%) of non/hypo-response to hepatitis B vaccine than females (24.2%). Among men, the next splitting variable is the dialysis frequency. The incidence of non/hypo-response to hepatitis B vaccine was 47.0% in males with higher dialysis frequency (lower dialysis frequency was 30.2%). The group of men with higher dialysis frequency were further differentiated by the vaccination dose. In the patients of 20 µg hepatitis B vaccine had higher non/hypo-response rate (62.9%) than 60 µg (29.0%). The probability of non/hypo-response to hepatitis B vaccine under the combination of the variables chosen by the decision tree analysis ranged from 24.2% (female) to 62.9% (male, higher dialysis frequency, and 20 μg of hepatitis B vaccine) ( and )
Table 5. The rules of non/hypo-response influencing factors classified by CHAID [n(%)]
Meanwhile, CHAID showed that the interaction among these factors, and could analyze whether a factor is meaningful in each subgroup. Higher dialysis frequency was the influencing factors among male patients of non/hypo-response. And the lower vaccine dose (20 µg) was the risk factor of non/hypo-response of male patients with higher dialysis frequency.
4. Discussion
Among persons with normal immune status who respond to hepatitis B vaccine, protection against HBV infection persists even when anti-HBs become undetectable.Citation21 However, protection against HBV infection among HD patients mainly depends on circulating antibodies.Citation8 HD patients with lower anti-HBs concentrations have a relatively high risk of HBV infection. Prior studies provided a background on the immunology of HBV infection, and the possible immunologic mechanisms to explain non/hypo-response to hepatitis B vaccine. Studies adopted strategies to improve vaccine effectiveness, which improved the immune effect of hepatitis B vaccine to a certain extent. However, the non/hypo-response rate is still high and cannot effectively protect patients from HBV infection. It is of great significance to explore the influencing factors and their relationships of the non/hypo-response to hepatitis B vaccine among HD patients. Therefore, two methods were used to investigate the factors affecting the non/hypo-response to hepatitis B vaccine for the first time, in order to provide more precise prevention and control measures of non/hypo-response to hepatitis B vaccine.
The results of decision tree analysis and logistic regression showed that male and higher dialysis frequency were independent risk factors of non/hypo-response to hepatitis B vaccine. In addition, CHAID decision tree model also founded that vaccine dose was an influencing factor of non/hypo-response, which may be due to the different ways of variable selection between two methods. The percentage of men who mounted a protective response to vaccination was lower than that of women, showing that gender difference was a related factor. This finding was in accordance with the literature.Citation5,Citation7,Citation22 It is known that antibody induction in man may in some instances be sex-related. In fact, males in general are more likely than females to become chronic HBsAg carriers, a tendency which is especially marked in dialysis patients. To our knowledge, most men probably smoke, and smoke is likely to affect a wide range of humoral and cell-mediated immune responses,Citation23 which reduce T cells reactive proliferationCitation24 or T-cell-dependent antibody response, so male patients respond more poorly than females. However, another reason is not clear, which still need further research.
Higher dialysis frequency has been reported to possibly negative effect immune responses to hepatitis B vaccine in two ways: first, higher dialysis frequency means high number of blood product transfer, and high number of blood product transfer is well-known factor that is associated with poor response to vaccination.Citation25 It might damage immune system which could play a crucial role in responses to hepatitis B vaccine in HD patients. And patients on dialysis are at an increased risk of viral transmission due to frequent necessity of blood product transfer as well as may use of contaminated dialyzate or dialysis materials. Second, studies have reported that linear macromolecular straight-chain compound can partially be passed by the dialysis membrane.Citation26 The structure of natural antibodies is non-linear, and the antibodies produced by recombinant hepatitis B vaccine may be different from natural antibodies, making it easy to pass through the dialysis membrane, which means the loss of more antibodies.Citation27 In CHAID decision tree model, we also found that intramuscular 60 μg hepatitis B vaccine could significantly improve the immune response in HD patients. It was consistent with our previous studies of hepatitis B vaccine immune effects in HD patients which is the only one study focusing on the immune effect of 60 μg hepatitis B vaccine in HD patients.Citation20 A recent study has evaluated the responses to higher doses of vaccine in various populations. The reported response rate from this study varies widely from 17% to 75%.Citation28 In most studies, individuals with primary renal disease tend to respond favorable to the use of the high dose of vaccine.Citation29–31 This maybe because high dose of vaccination stimulates the activation and proliferation of T and B lymphocytes and the production of cytokines, thereby increasing the immune response level of the vaccine.Citation32,Citation33
Meanwhile, CHAID could analyze the interaction between above influencing factors. First, our study shows that higher dialysis frequency only affects these male patients, which means male patients should avoid higher dialysis. Second, our study finds that lower dose vaccines only affect these patients with higher dialysis frequency, but have no meaning for these patients with lower dialysis frequency. This indicated that HD patients with higher dialysis frequency should be vaccinated with high dose of hepatitis B vaccine. There were interactions between gender and dialysis frequency, as well as between dialysis frequency and vaccine dose, and when they consisted at the same time, the risk of non/hypo-response will significantly increase. These results indicated that male HD patients with higher dialysis frequency were more likely to have non/hypo-response to hepatitis B vaccine than other patients, and should be given high dose hepatitis B vaccination, the implications of which are more prominent than just finding the influencing factors. Male HD patients with higher dialysis frequency were the key population of non/hypo-response to hepatitis B vaccine. These results of our study are of great significance to provide scientific evidence for precise prevention and control measure of non/hypo-response to hepatitis B vaccine in HD patients.
It is a strength that we used two methods to explore influencing factors and their relationship of non/hypo-response to hepatitis B vaccine in HD patients for the first time. Our study, however, has some limitations: (1) There was no index to directly reflect the immune status in this study. However, the number of hemoglobin was detected. The hemoglobin lower than the normal value indicates anemia, which will lead to the decrease of immunity. As a consequence, the value of hemoglobin can indirectly reflect the immune state of the body. Furthermore, there was no significant difference in hemoglobin value between the two groups, thus the effect of different immune status on the results could be excluded. (2) HBV occult infection (OBI) among subjects with single positive anti-HBc problem was not considered in this study. However, on the one hand, there was no significant difference in anti-HBc between the two groups. On the other hand, the proportion of OBI in the positive of anti-HBc patients was about 1.78%.Citation34 And, in this study, the positive rate of anti-HBc in HD population was 6.6%, and thus the proportion of OBI was only 0.12%, which has limited impact on the result. (3) One report has suggested that HD patients should be vaccinated before commencing dialysis.Citation35 However, our study only focused on the HD patients, the next step in research should focus on patients before dialysis. (4) The sample size may be too small to notice a significant difference. Further studies may be needed to base upon more large survey sample to assess the durability of seroprotection.
5. Conclusions
Male, lower dose and higher dialysis frequency were risk factors of hepatitis B vaccine in HD patients. There is an interaction between gender and dialysis frequency, as well as between dialysis frequency and vaccine dose.
Disclosure of potential conflict of interest
All authors have seen and approved the manuscript, contributed significantly to the work. There is no conflict of interest involved in this publication.
Acknowledgments
We thank all HD patients for participating in this study. We gratefully acknowledge the contribution from our colleagues and students, and doctors, nurses of the hospitals in Shanxi Province and personnel who contributed to this study.
Additional information
Funding
References
- WHO. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. World Health Organization; 2015.
- GuoLiang X, ChongBai L, HuiLin C, ShengLi B, MeiYun Z, ChongAo S, JunHua N, XiaoQui Q. Prevalence of hepatitis B and C virus infections in the general Chinese population. Results from a nationwide cross-sectional seroepidemiologic study of hepatitis A, B, C, D, and E virus infections in China, 1992. Inter Hepatol Commun. 1996;5:62–73. doi:10.1016/0928-4346(96)00282-4.
- Xiaofeng L, Shengli B, Weizhong Y, Longde W, Gang C, Fuqiang C, Yong Z, Jianhua L, Xiaohong G, Yuansheng C, et al. Epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27(47):6550–57. doi:10.1016/j.vaccine.2009.08.048.
- Collaborators PO. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403.
- Sari F, Taskapan H. Good response to HBsAg vaccine in dialysis patients is associated with high CD4+/CD8+ ratio. Int Urol Nephrol. 2012;44(5):1501–06. doi:10.1007/s11255-011-0043-6.
- Vaziri ND, Pahl MV, Crum A, Norris K. Effect of uremia on structure and function of immune system. J Ren Nutr. 2012;22(1):149–56. doi:10.1053/j.jrn.2011.10.020.
- Khalid AS, Sabry A, Zakaria AH, Ismail M. Factors affecting response to hepatitis B vaccine among hemodialysis patients in a large Saudi hemodialysis center. Saudi J Kidney Dis Trans Off Pub Saudi Center Organ Trans Saudi Arabia. 2014;25(1):185–91. doi:10.4103/1319-2442.124572.
- Tong NKC, Beran J, Kee SA, Miguel JL, Sánchez C, Bayas JM, Vilella A, Juanes JR, Arrazola P, Calbo-Torrecillas F, et al. Immunogenicity and safety of an adjuvanted hepatitis B vaccine in pre-hemodialysis and hemodialysis patients. Kidney Int. 2005;68(5):2298–303. doi:10.1111/j.1523-1755.2005.00689.x.
- Jiahui S, Canqing Y, Yu G, Zheng B, Chenxi Q, Ling Y, Yiping C, Li Y, Hui L, Jian L, et al. Chronic hepatitis B virus infection and risk of chronic kidney disease: a population-based prospective cohort study of 0.5 million Chinese adults. BMC Med. 2018;16(1):93. doi:10.1186/s12916-018-1084-9.
- Kara IH, Yilmaz ME, Suner A, Kadiroglu AK, Isikoglu B. The evaluation of immune responses that occur after HBV infection and HBV vaccination in hemodialysis patients. Vaccine. 2004;22(29–30):3963–67. doi:10.1016/j.vaccine.2004.04.001.
- Fabrizi F, Ganeshan SV, Dixit V, Martin P. Meta-analysis: the adjuvant role of granulocyte macrophage-colony stimulating factor on immunological response to hepatitis B virus vaccine in end-stage renal disease. Aliment Pharmacol Ther. 2006;24(5):789–96. doi:10.1111/j.1365-2036.2006.03035.x.
- Saade F, Honda-Okubo Y, Trec S, Petrovsky N. A novel hepatitis B vaccine containing Advax, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine. 2013;31(15):1999–2007. doi:10.1016/j.vaccine.2012.12.077.
- Ramezani A, Eslamifar A, Banifazl M, Ahmadi F, Maziar S, Razeghi E, Kalantar E, Amirkhani A, Aghakhani A. Efficacy and long-term immunogenicity of hepatitis B vaccine in haemodialysis patients. Int J Clin Pract. 2009;63(3):394–97. doi:10.1111/j.1742-1241.2007.01470.x.
- Fabrizi F, Dixit V, Messa P, Martin P. Intradermal vs intramuscular vaccine against hepatitis B infection in dialysis patients: a meta-analysis of randomized trials. J Viral Hepat. 2011;18(10):730–37. doi:10.1111/j.1365-2893.2010.01354.x.
- Kaiming C, Manching L, Chibon L, Cheukchun S, Philipkam-Tao L. Antibody Response to Hepatitis B Vaccine in End-Stage Renal Disease Patients. Nephron Clin Pract. 2006;103(3):c89–93. doi:10.1159/000092016.
- Cordova E, Miglia I, Festuccia F, Sarlo MG, Scornavacca G, Punzo G, Mene P, Fofi C. Hepatitis B vaccination in haemodialysis patients: an underestimated problem. Factors influencing immune responses in ten years of observation in an Italian haemodialysis centre and literature review. Ann Ig. 2017;29(1):27–37. doi:10.7416/ai.2017.2129.
- Saracoglu A, Ozen H. Estimation of traffic incident duration: a comparative study of decision tree models. Arab J Sci Eng. 2020;45(10):8099–110. doi:10.1007/s13369-020-04615-2.
- Gao L, Smielewski P, Li P, Czosnyka M, Ercole A. Signal information prediction of mortality identifies unique patient subsets after severe traumatic brain injury: a decision-tree analysis approach. J Neurotrauma. 2020;37(7):1011–19. doi:10.1089/neu.2019.6631.
- Jian S, Liping L, Yequn C. The role of Decision tree model and Logistic regression in injury influencing factors analysis. Chin J Dis Control Preven. 2015;19:185–89.
- Stevens CE, Alter HJ, Taylor PE, Zang EA, Harley EJ, Szmuness W. Hepatitis B vaccine in patients receiving hemodialysis: immunogenicity and efficacy. N Engl J Med. 1984;311(8):496–501. doi:10.1056/NEJM198408233110803.
- Campos Nizama J. Humoral immunity level and factors associated with the response to the vaccine against the hepatitis B virus in health personnel of the case-essalud national hospital, arequipa september 1995-march 2002. Rev Gastroenterol Peru. 2005;25:141–49.
- Sopori ML, Kozak W, Savage SM, Geng Y, Soszynski D, Kluger MJ, Perryman EK, Snow GE. Effect of nicotine on the immune system: possible regulation of immune responses by central and peripheral mechanisms. Psychoneuroendocrinology. 1998;23(2):189–204. doi:10.1016/S0306-4530(97)00076-0.
- Scharf P, Da Rocha GHO, Sandri S, Heluany CS, Pedreira Filho WR, Farsky SHP. Immunotoxic mechanisms of cigarette smoke and heat-not-burn tobacco vapor on Jurkat T cell functions. Environ Pollut. 2020;268(Pt B):115863. doi:10.1016/j.envpol.2020.115863.
- Sit D, Esen B, Atay AE, Kayabasi H. Is hemodialysis a reason for unresponsiveness to hepatitis B vaccine? Hepatitis B virus and dialysis therapy. World J Hepatol. 2015;7(5):761–68. doi:10.4254/wjh.v7.i5.761.
- Zhimao C, Bo H. Study on permeability of dialysis membranes to macromolecular straight-chain compounds. Chin Pharm J. 2000;35:120.
- Yanlin S, Xinjie W, Liaolin L. Causes of rapid decrease of hepatitis B surface antibody titer in hemodialysis patients. J Clin Intern Med. 2016;33:277.
- Yongliang F, Xiaohong S, Jing S, Linying G, Guangming L, Yanpeng C, Minghu P, Li C, Jun W, Xuxia G, et al. Immunogenicity, antibody persistence, and safety of the 60 µg hepatitis B vaccine in hemodialysis patients: a multicenter, randomized, double-blind, parallel-controlled trial. Expert Rev Vaccines. 2017;16(10):1045–52. doi:10.1080/14760584.2017.1367667.
- Aziz A, Aziz S, Li DS, Murphy L, Leone N, Kennedy M, Dhillon S, Van Thiel DH. Efficacy of repeated high-dose hepatitis B vaccine (80 micron) in patients with chronic liver disease. J Viral Hepat. 2006;13(4):217–21. doi:10.1111/j.1365-2893.2005.00674.x.
- Dhillon S, Moore C, Li SD, Aziz A, Kakar A, Dosanjh A, Beesla A, Murphy L, Van Thiel DH. Efficacy of High-Dose Intra-dermal Hepatitis B Virus Vaccine in Previous Vaccination Non-responders with Chronic Liver Disease. Dig Dis Sci. 2012;57(1):215–20. doi:10.1007/s10620-011-1996-0.
- Oguz Y, Doganci L, Vural A. Seroconversion rates of two different doses of hepatitis B vaccine in Turkish haemodialysis patients. Cent Eur J Public Health. 2001;9:44–45.
- Cuiyu W, Jinghua S, Bei Z, Steven L, Rongbin Y, Jianqing W, Weihong Z. Hepatitis B virus infection and related factors in hemodialysis patients in China - systematic review and meta-analysis. Ren Fail. 2010;32(10):1255–64. doi:10.3109/0886022X.2010.517354.
- Sabry R, Mohamed ZAZ, Abdallah AM. Relationship between Th1 and Th2 cytokine serum levels and immune response to Hepatitis B vaccination among Egyptian health care workers. J Immunoassay Immunochem. 2018;39(5):496–508. doi:10.1080/15321819.2018.1509871.
- Pileggi C, Papadopoli R, Bianco A, Pavia M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine. 2017;35(46):6302–07. doi:10.1016/j.vaccine.2017.09.076.
- Xiaofeng L, Fang J, Fuchu Q, Weihong W. Molecular characterization of occult HBV infection among subjects with single positive HBcAb. Chin J Health Lab Tec. 2020;30:578–80.
- Soi V, Soman S. Preventing hepatitis b in the dialysis unit. Adv Chronic Kidney Dis. 2019;26(3):179–84. doi:10.1053/j.ackd.2019.03.003.
