ABSTRACT
Acute disseminated encephalomyelitis (ADEM) is an autoimmune, central nervous system demyelinating disorder that follows antecedent immunologic challenges, such as infection or vaccination. This study aimed to investigate the potential association between routine childhood vaccinations and ADEM. Children under 7 years of age admitted to the two tertiary level pediatric hospitals in Victoria, Australia with ADEM from 2000–2015 had their clinical information linked to vaccination records from the Australian Childhood Immunization Register. Chart review was undertaken utilizing the Brighton Collaboration ADEM criteria. The self-controlled case-series (SCCS) methodology was employed to determine the relative incidences of ADEM post-vaccination in two risk intervals: 5–28 days and 2–42 days. Forty-six cases were eligible for SCCS analysis with a median age of 3.2 years. Of the forty-six cases, three were vaccine proximate cases and received vaccinations 23, 25 and 28 days before ADEM onset. Two vaccine proximate cases received their 4-year-old scheduled vaccinations (MMR and DTPa-IPV) and one vaccine proximate case the 1-year old scheduled vaccinations (MMR and Hib-MenC). The relative incidence of ADEM during the narrow and broad risk intervals were 1.041 (95% CI 0.323–3.356, p = 0.946) and 0.585 (95% CI 0.182–1.886, p = 0.370) respectively. Sensitivity analyses did not yield any substantial deviations. These results do not provide evidence of an association between vaccinations routinely provided to children aged under 7 years in Australia and the incidence of ADEM. However, these results should be interpreted with caution as the number of ADEM cases identified was limited and further research is warranted.
Introduction
Acute disseminated encephalomyelitis (ADEM) is an uncommon, severe, immune-mediated neurological condition characterized by demyelination of the central nervous system, which has typically affected the pediatric and young adult population (aged under 20 years).Citation1-3 In a pediatric population, incidence rates have been estimated at between 0.07–1.1 per 100,000 person years and recent case-series have described a mortality rate up to 6.5%.Citation2-6 Despite a lack of definitive evidence, vaccinations have been temporally associated with ADEM highlighting the need for further research into this area.Citation2,Citation3,Citation5,Citation7-9 However, because of ADEM’s rarity, research is limited.Citation1 Recent case-series have described that 62–93% of patients with ADEM experience a preceding infection, 2.5–15% a prior vaccination and approximately 30% are cryptogenic.Citation2,Citation3,Citation5,Citation7-9 Although the timing of ADEM onset following infection or vaccination is unclear, multiple studies have suggested most ADEM cases are likely to occur within 6 weeks of immunologic challenge.Citation2,Citation3,Citation7,Citation10 Before the widespread use of vaccines, preceding viral infections (e.g. measles, mumps, rubella, varicella, smallpox and influenza) had been associated with the development of ADEM.Citation7,Citation11-16 More recently, nonspecific respiratory, gastrointestinal and febrile illnesses are the most commonly described.Citation2,Citation3,Citation7,Citation16 ADEM has been reported in temporal association with many vaccines, including influenza,Citation17-19 smallpox,Citation20,Citation21 Japanese B encephalitis,Citation18,Citation22 measles, mumps and rubella (MMR),Citation17,Citation23 human papilloma virus (HPV),Citation18,Citation24 and antiquated rabies vaccines.Citation25,Citation26 There have been several observational studies describing a temporal association between ADEM and vaccination. In the most extensive case series to date, Pellegrino et al.Citation9 explored 404 post-vaccination cases of ADEM from 2005–2012 contained within passive surveillance systems. Despite the large numbers of patients, this case-series was not designed to quantitatively assess the risk of ADEM post-vaccination due to a lack of controls. Furthermore, this study included adults and patients outside of biologically plausible risk intervals (RI). In another descriptive survey, Xiong et al.Citation27 assessed all cases of ADEM in a province of China. There were 47 cases of ADEM, and no cases had received vaccinations in the two months preceding ADEM onset. This study was conducted to explore the association between routine vaccinations provided in the Australian immunization program to children aged under 7 years of age and the incidence of ADEM, utilizing the self-controlled case series (SCCS) methodology.Citation28 Findings from this pilot study would potentially inform future research.
Materials and methods
Study design
A retrospective, hypothesis-generating study utilizing the SCCS methodology was undertaken. Patients aged under 7 years who were diagnosed with ADEM between 2000–2015 were identified via automated searches of discharge datasets from the two tertiary pediatric hospitals in Victoria, Australia using International Classification of Diseases, 10th revision, Australian Modification (ICD-10 AM) discharge coding consistent with ADEM (G04.0).Citation29 This age range was selected as the immunization register utilized did not collect data for individuals from their seventh birthday. As major referral centers, it was anticipated that the majority of pediatric ADEM cases in Victoria would be admitted to these hospitals. Patients aged less than 7 years with an admission between 1st January 2000 and 31st December 2015 were included. In 2015, the state of Victoria had a population of approximately 5 million people, with a birth cohort of 73,568 and a 93.02% fully immunized rate in 5 year olds.Citation30,Citation31
Clinical and demographic data were acquired via accessible electronic or hard-copy medical records. Case data were entered into a secure data management platform. Ethical approval was provided by the Monash Health (Ref: 13421L) and Royal Children’s Hospital (Ref: 33258A) Human Research Ethics Committees.
Immunization register, data linkage and vaccine inclusion
The Australian Childhood Immunization Register (ACIR) was a nationwide, population-based, register for recording vaccinations provided to children less than 7 years of age. As of October 2016, ACIR was renamed the Australian Immunization Register and began collecting whole-of-life vaccination data. ACIR is a relatively objective source of vaccination data which is not subject to many biases inherent to other vaccination coverage estimates, such as recall bias in cross-sectional surveys.Citation32,Citation33 Characteristics of ACIR are close to “ideal” with participants enrolled at birth through a unique identifier and information on vaccine administration date, dose and provider collected.Citation32,Citation33 Financial incentives to providers for appropriate notification to ACIR are available, which contributes to excellent documentation coverage for routine childhood vaccinations with 99% of Australian children having an ACIR record.Citation32,Citation33 Vaccinations routinely provided in Victoria to children aged under 7 years in 2015 are noted in with major changes to the program documented online, for instance the introductions of the varicella, HPV and rotavirus vaccinations.Citation34,Citation35
Table 1. Victorian immunization schedule for children up to 7 years of age at January 2015
Vaccination status for each patient was obtained from ACIR by data linkage. Case-matching utilized three domains: full name, date of birth and national health identification number. Patients without consistency in all three matching domains, no vaccinations recorded in ACIR or no ACIR record were excluded. Once records were matched, cases were de-identified and assigned a unique study identifier. All vaccinations recorded in ACIR during the study period, including non-routine vaccinations, were included in the study.
Case-note validation
Case-note validation was undertaken by the primary investigator (TM) and a pediatric neurologist (MF) employing the Brighton Collaboration (BC) ADEM criteria.Citation36 Validation involved a chart review of all accessible medical records to assign a level of diagnostic certainty; these categories were levels 1, 2, 3 and 3A, not ADEM and an additional category of ADEM diagnosed by a pediatric neurologist but not consistent with BC criteria. Any differences in categorization were discussed between investigators to reach consensus. If consensus was not reached, a second pediatrician’s opinion provided a casting vote. Reviewers were blinded to patients’ ACIR records.
Self-controlled case series
The SCCS is an epidemiological method to assess associations between a time-varying exposure and an outcome. It has been primarily used to investigate vaccine safety.Citation37-39 Based on a retrospective cohort model, cases are followed for an observation period that is split into RI and control intervals. The analysis is only undertaken in cases resulting in the implicit control of time-independent variables and uses a conditional Poisson regression model.Citation37,Citation39
For this study, the observation period began on the 1st January 2000 or a child’s date of birth, whichever occurred later. It ended on a child’s seventh birthday, or the 31st December 2015, whichever occurred earlier. Two evidence-based and biologically plausible RIs for ADEM were utilized: A narrow RI of 5–28 days and an exploratory, broad RI of 2–42 days post-vaccination.Citation10 These RI were recommended in a study designed to evaluate biologically plausible RI for ADEM to occur in association with an immunological antecedent.Citation10 A literature review, review of a vaccine adverse event database, analysis of immunological pathophysiology and consideration of expert opinion were undertaken to formulate the RI selected. Sensitivity analyses using standard fortnightly intervals and adjusting for seasonality and age were also undertaken, consistent with previous studies.Citation40,Citation41 Subgroup analysis was conducted by calculating a relative incidence ratio comparing the narrow and broad RI.Citation42 Seasonal adjustment involved incorporating a variable for each season (spring, summer, autumn, winter) per vaccination administration date into the model. Statistical analysis utilized a conditional Poisson Regression model and was consistent with the methodology described by Whitaker et al.Citation37,Citation43 A vaccination administration date was defined as a date in which a case received any number of vaccinations. The RIs were applied for every vaccination administration date obtained from ACIR for each case. To minimize the impact of the healthy vaccinee effect (i.e. children are likely to be healthier than usual before receiving vaccination), a 28-day pre-vaccine interval was removed from the analysis. The control interval for each case consisted of all observation time within the study period excluding the RI and pre-vaccine interval.
Data analysis
Data analysis and linkage was performed using Statistical Analysis Software (SAS – Version 9.3, SAS Institute Inc., Cary, NC, USA) and Stata (Version 13, StataCorp, College Station, TX, USA). The primary SCCS analyses included patients with BC level 1 or 2 classifications. Sensitivity analyses were implemented involving the level of diagnostic certainty and adjusting for age and seasonality.
Results
Participant numbers, data linkage and case-note validation
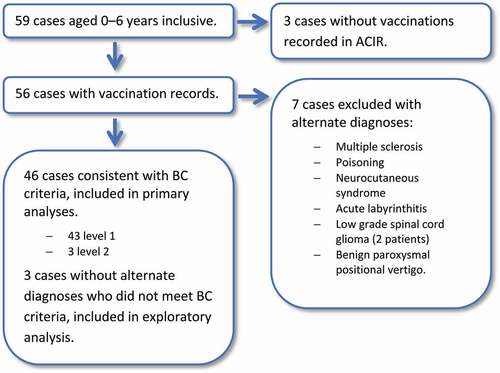
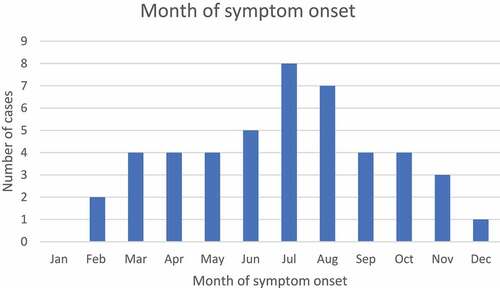
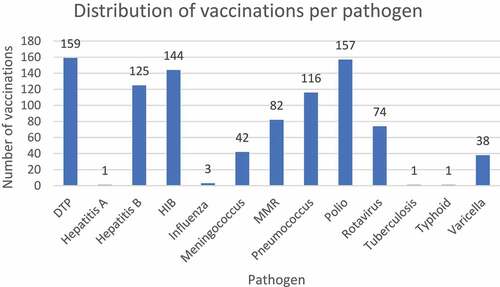
Fifty-nine patients were identified under 7 years of age with a diagnosis code of ADEM within the study period (). Following data linkage, three patients were excluded as no vaccinations were documented in ACIR. Fifty-six eligible patients remained for case-note validation. Seven patients were excluded from analysis due to an alternate diagnosis. Three patients were diagnosed with ADEM but did not meet BC criteria and did not have alternative diagnoses; these cases were included in an exploratory analysis. Forty-six cases had presentations consistent with the BC ADEM criteria with all cases meeting levels 1 or 2; these cases were included in the primary analyses. The median age at admission was 3.2 years (range 4 months–6.7 years) and 23/46 (50.0%) of cases were male. 25/46 (54.3%) were admitted from 2012–2015 and 20/46 (43.5%) cases presented during winter (). 689 vaccinations, over 302 unique vaccination administration dates, were documented to have been provided to the 46 cases over the study period equating to a median of 6 (range 1–9) vaccine administration dates per case. In 2015, Victoria had a birth cohort of 73,568 and there were seven vaccine administration episodes per child in the first 7 years of life within the routine immunization program ().Citation30,Citation34 Five of the vaccinations administered were non-routine: one Bacillus Calmette–Guérin (tuberculosis), one Vivaxim® (hepatitis A, typhoid) and three influenza vaccines. demonstrates vaccination coverage in cases against specific pathogens.
Figure and table legend
ACIR: Australian children’s immunization register
ADEM: acute disseminated encephalomyelitis
BC: Brighton Collaboration
DTP: Diphtheria, tetanus, pertussis
HIB: Hemophilus influenzae type B
Infanrix-IPV® (GSK Biological): DTaP-IPV (diphtheria-tetanus-acellular pertussis-inactivated poliovirus vaccine)
Menitorix® (GSK Biological): Hib-MenC (combination Haemophilus influenzae type b and meningococcal serogroup C–tetanus toxoid conjugate vaccine)
MMR: Measles, mumps, rubella.
n/a: not applicable
Priorix® (GSK Biological): MMR (measles, mumps, rubella vaccine)
RI: Risk interval
SCCS: Self-controlled case-series
Varilrix® (GSK Biological): varicella vaccine
Self-controlled case-series (SCCS) analysis
There were three vaccine proximate cases with ADEM symptom onset occurring at 23, 25 and 28 days post-vaccination respectively. Forty-three events occurred in the control interval and zero in the 28-day pre-vaccine interval. In the 43 cases who had ADEM in the control interval, the median number of days between the most recent vaccination administration date and symptom onset was 929 days (range 53–2371 days). The relative incidences of the primary analyses were 1.041 (95% confidence interval (CI) 0.323–3.356, p = 0.946) for the narrow RI and 0.585 (95% CI 0.182–1.886, p = 0.370) for the broad RI (). This analysis included all 46 BC level 1 and 2 cases. Subgroup analysis comparing the narrow and broad risk intervals demonstrated a relative incidence ratio of 1.779 (95% CI 0.379–8.359). Exploratory analyses adjusting for age, season, involving the three cases that did not meet BC criteria and utilizing fortnightly intervals are detailed in .
Table 2. Relative incidences from the SCCS analyses
Antecedent factors in cases
Of the three vaccine proximate cases, two occurred following 4-year old scheduled vaccines and the other following the 12-month scheduled vaccines (). All three patients received Priorix® (measles, mumps and rubella) and two received the inactivated vaccine Infanrix-IPV® (diphtheria, tetanus, acellular pertussis and inactivated poliovirus). Menitorix® (Hemophilus influenzae type b and Meningococcal serogroup C) and Varilrix® (varicella) were also provided on one occasion each.
Table 3. Case details for vaccine proximate cases
34/46 (73.9%) of all cases reported infectious symptoms in the 28 days before ADEM onset, all of which were gastroenteritis, upper respiratory tract infections or viral symptoms. Two of three vaccine proximate cases had infectious symptoms in close temporal association with ADEM. The median number of days between infectious symptom onset and neurological symptom onset was seven days (range 0–22 days).
Discussion
This is the first retrospective observational study conducted exploring the incidence of ADEM following routine childhood vaccination utilizing biologically plausible risk intervals, a rigorous case-validation system and the SCCS methodology. The patient identification approach was designed to detect the majority of ADEM cases over a 15-year period in children aged under 7 years in an Australian state with approximately 5 million residents.Citation30
The primary analyses did not demonstrate a statistically significant increased risk of ADEM post-vaccination in children aged under 7 years who received vaccinations within the routine Australian immunization program from the years 2000–2015. Exploratory analyses adjusting for age, season and time bands post-vaccination did not yield further information. The wide confidence interval of the relative incidence ratio comparing narrow and broad RI did not provide evidence for effect modification.
In this study, all three vaccine proximate cases received the MMR vaccine in the RI, and two the DTaP-IPV (diphtheria-tetanus-acellular pertussis-inactivated poliovirus vaccine) vaccine. It is challenging to draw further conclusions from these findings given the study was designed to examine vaccines in the routine Australian immunization program administered to children under 7 years as a whole. Due to a known background rate of ADEM, there are likely to be non-vaccination related ADEM cases.Citation44 Furthermore, two cases had preceding infectious symptoms and consideration must be given to infection as a possible preceding cause. Some studies have suggested that MMR vaccinations may be most likely to be associated with ADEM.Citation23,Citation45 However, in an extensive, USA-based, cohort study from 2000–2012 involving 708,187 vaccination events comparing measles, mumps, rubella and varicella (MMRV) to MMR plus varicella (MMR+V) vaccination, there was only one case of ADEM in the broad RI (1–42 days) in both vaccination groups.Citation46 The authors concluded that there is unlikely to be an increased risk of ADEM post-MMRV or MMR+V vaccination. In contrast, there are fewer epidemiological studies assessing ADEM in association with DTaP or polio containing vaccines; apart from case reports and case-series.Citation7,Citation8,Citation47-49
In larger case-series of ADEM post-vaccination, ADEM is described more commonly after seasonal influenza and HPV vaccinations. Pellegrino et al.Citation9 found that the most frequently reported vaccines across all age groups were seasonal influenza and HPV vaccines, accounting for almost 35% of post-vaccination ADEM cases. In a 2014 literature review with 71 cases, Karussis and PetrouCitation45 corroborated these findings. These vaccinations were not routinely provided to children aged under 7 years in Australia during the study period with only three influenza and no HPV vaccinations recorded for the included cases. However, Pellegrino et al.Citation9 also demonstrated that the MMR, pneumococcal and DTP vaccines were most commonly reported in the 0–5 age group in the United States Vaccine Adverse Event Reporting System dataset. Causal inference regarding specific vaccines cannot be drawn as many passive surveillance systems are not designed to assign causality, do not consider doses distributed and the data are subject to significant reporting bias.Citation50,Citation51
There have been several studies investigating the risk of ADEM or other adverse events during influenza epidemics and post-influenza vaccination; none of which have demonstrated a statistically increased risk following vaccination. In a retrospective observational study, Jackson et al.Citation15 explored the incidence of ADEM during the 2009–2010 H1N1 influenza pandemic in Canada. There were seven cases of ADEM during the pandemic period, which was 4.7 times the average rate, and only one patient had received an influenza vaccine within a 42-day RI. Persson et al.Citation52 used a population-based cohort model in Sweden to explore narcolepsy, immune and neurological events post-vaccination with a 2009 H1N1 pandemic influenza vaccine and also found no increased risk of ADEM.
In our series, 43.5% of cases presented in winter (), consistent with several other studies.Citation6,Citation53 These findings suggest ADEM may be aligned with the peak viral illness incidence, consistent with the description that nonspecific respiratory, gastrointestinal and febrile illnesses precede ADEM most commonly.Citation2,Citation3,Citation7,Citation16 Our findings were consistent with the previous literature with the majority of cases experiencing infectious symptoms prior to ADEM onset. Seasonal variation is a possible confounder in vaccine safety research broadly, but particularly when using the SCCS methodology, which cannot account for unknown time-varying factors.Citation39 However, in our analyses, seasonal sensitivity analyses did not yield statistically significant differences. Finally, 54.3% of cases presented in the final four years of the study. An increasing prevalence or diagnosis rate of ADEM is consistent with other recent Australian research and may reflect increased ascertainment due to the improved accessibility of magnetic resonance imaging.Citation54
The use of standardized diagnostic criteria, thorough chart review by two investigators including a pediatric neurologist, biologically plausible RIs, use of a national immunization register and use of the SCCS methodology helped minimize variability in diagnostic reliability, recall bias, the impact of confounding time-independent variables and the healthy-vaccinee effect. Only 18% (10/56) of cases were excluded during case-note validation, supporting high specificity of ICD-10 AM coding at these sites using BC criteria categorized ADEM.
Nevertheless, due to the rarity of ADEM, there remain limitations and potential biases, including low case numbers, retrospective case ascertainment and long study length predisposing to altered patterns in diagnosis and management. Other limitations included age limitations due to the use of ACIR, nonspecific vaccine analysis and inability to account for unknown time-dependent variables. The most significant limitation of this study was the low number of overall cases as well as cases identified in the RI. This may be reflected in the wide confidence intervals. As a pilot study with a small number of cases, this study was not able to investigate individual vaccines reliably and findings should be interpreted with caution. The use of ACIR was also a source of several limitations. Although ACIR provided a robust record of routine childhood vaccinations, we had to limit the age of inclusion to 7 years. Routine vaccinations administered outside this age group, for instance the HPV vaccine, or non-routine vaccines such as influenza, were not reliably captured in this study. Finally, additional limitations were the inclusion of multiple vaccinations in the same analyses, changing vaccination schedules and administration of multiple vaccines at the same time point.
In conclusion, our study did not demonstrate a statistically significant increased risk of ADEM following vaccination in children aged under 7 years who received vaccinations within the routine Australian immunization program from the years 2000–2015. Furthermore, the majority of cases experienced a preceding infectious illness. However due to the low numbers of ADEM cases identified, these results should be interpreted with caution. As a potentially devastating acute inflammatory neurological condition, concerns will likely continue to be raised regarding the possible etiological role of vaccines and infectious illnesses in ADEM pathophysiology. Further application of the epidemiological methods used in this study, as well as global and collaborative initiatives utilizing electronic healthcare record databases to increase sample sizes, will enable suitably sized studies to be conducted to address these concerns robustly and rapidly.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Many thanks to staff members from Monash University, Murdoch Children’s Research Institute, The Royal Children’s Hospital, Monash Health and The University of Melbourne who assisted with this research.
Additional information
Funding
References
- Zettl UK, Stuve O, Patejdl R. Immune-mediated CNS diseases: a review on nosological classification and clinical features. Autoimmun Rev. 2012;11:167–73. doi:10.1016/j.autrev.2011.05.008.
- Leake JA, Albani S, Kao AS, Senac MO, Billman GF, Nespeca MP, Paulino AD, Quintela ER, Sawyer MH, Bradley JS, et al. Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr Infect Dis J. 2004;23:756–64. doi:10.1097/01.inf.0000133048.75452.dd.
- Hynson JL, Kornberg AJ, Coleman LT, Shield L, Harvey AS, Kean MJ. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001;56:1308–12. doi:10.1212/WNL.56.10.1308.
- Pohl D, Hennemuth I, Von Kries R, Hanefeld F. Paediatric multiple sclerosis and acute disseminated encephalomyelitis in Germany: results of a nationwide survey. Eur J Pediatr. 2007;166:405–12. doi:10.1007/s00431-006-0249-2.
- Pavone P, Pettoello-Mantovano M, Le Pira A, Giardino I, Pulvirenti A, Giugno R, Parano E, Polizzi A, Distefano A, Ferro A, et al. Acute disseminated encephalomyelitis: a long-term prospective study and meta-analysis. Neuropediatrics. 2010;41:246–55. doi:10.1055/s-0031-1271656.
- Samile N, Hassan T. Acute disseminated encephalomyelitis in children. A descriptive study in Tehran, Iran. Saudi Med J. 2007;28:396–99.
- Torisu H, Kira R, Ishizaki Y, Sanefuji M, Yamaguchi Y, Yasumoto S, Murakami Y, Shimono M, Nagamitsu S, Masuzaki M, et al. Clinical study of childhood acute disseminated encephalomyelitis, multiple sclerosis, and acute transverse myelitis in Fukuoka Prefecture, Japan. Brain Dev. 2010;32:454–62. doi:10.1016/j.braindev.2009.10.006.
- Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59:1224–31. doi:10.1212/WNL.59.8.1224.
- Pellegrino P, Carnovale C, Perrone V, Pozzi M, Antoniazzi S, Clementi E, Radice S. Acute disseminated encephalomyelitis onset: evaluation based on vaccine adverse events reporting systems. PLoS One. 2013;8:e77766. doi:10.1371/journal.pone.0077766.
- Rowhani-Rahbar A, Klein NP, Dekker CL, Edwards KM, Marchant CD, Vellozzi C, Fireman B, Sejvar JJ, Halsey NA, Baxter R, et al. Biologically plausible and evidence-based risk intervals in immunization safety research. Vaccine. 2012;31:271–77. doi:10.1016/j.vaccine.2012.07.024.
- Pahud BA, Glaser CA, Dekker CL, Arvin AM, Schmid DS. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316–23. doi:10.1093/infdis/jiq066.
- Croft PB. Para-infectious and post-vaccinal encephalomyelitis. Postgrad Med J. 1969;45:392–400. doi:10.1136/pgmj.45.524.392.
- Schattner A. Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine. 2005;23:3876–86. doi:10.1016/j.vaccine.2005.03.005.
- Booss J, Davis LE. Smallpox and smallpox vaccination: neurological implications. Neurology. 2003;60:1241–45. doi:10.1212/01.WNL.0000063319.64515.6B.
- Jackson AC, Mostaco-Guidolin LC, Sinnock H, Bozat-Emre S, Routledge M, Mahmud SM. Pandemic H1N1 vaccination and incidence of acute disseminated encephalomyelitis in Manitoba. Can J Neurol Sci. 2016;43:819–23. doi:10.1017/cjn.2016.291.
- Hung KL, Liao HT, Tsai ML. Postinfectious encephalomyelitis: etiologic and diagnostic trends. J Child Neurol. 2000;15:666–70. doi:10.1177/088307380001501005.
- Shoamanesh A, Traboulsee A. Acute disseminated encephalomyelitis following influenza vaccination. Vaccine. 2011;29:8182–85. doi:10.1016/j.vaccine.2011.08.103.
- Nakayama T, Onoda K. Vaccine adverse events reported in post-marketing study of the Kitasato Institute from 1994 to 2004. Vaccine. 2007;25:570–76. doi:10.1016/j.vaccine.2006.05.130.
- Andrade SD, Andrade MG, Santos PJ, Galvao Mde L, Barros MM, Ramasawmy R, Safe IP, Monteiro WM, Sabidó M, Alecrim MDGC, et al. Acute disseminated encephalomyelitis following inactivated influenza vaccination in the Brazilian Amazon: a case report. Rev Soc Bras Med Trop. 2015;48:498–500. doi:10.1590/0037-8682-0314-2014.
- Van Dam CN, Syed S, Eron JJ, Ostrander M, Engler RJ, Damon I, Montgomery J, Tong S, Adimora A, Kahn K, et al. Severe postvaccinia encephalitis with acute disseminated encephalomyelitis: recovery with early intravenous immunoglobulin, high-dose steroids, and vaccinia immunoglobulin. Clin Infect Dis. 2009;48:e47–9. doi:10.1086/596553.
- Sejvar JJ, Labutta RJ, Chapman LE, Grabenstein JD, Iskander J, Lane JM. Neurologic adverse events associated with smallpox vaccination in the United States, 2002-2004. JAMA. 2005;294:2744–50. doi:10.1001/jama.294.21.2744.
- Ohtaki E, Matsuishi T, Hirano Y, Maekawa K. Acute disseminated encephalomyelitis after treatment with Japanese B encephalitis vaccine (Nakayama-Yoken and Beijing strains). J Neurol Neurosurg Psychiatry. 1995;59:316–17. doi:10.1136/jnnp.59.3.316.
- Bennetto L, Scolding N. Inflammatory/post-infectious encephalomyelitis. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 1):i22–8. doi:10.1136/jnnp.2003.034256.
- Sekiguchi K, Yasui N, Kowa H, Kanda F, Toda T. Two cases of acute disseminated encephalomyelitis following vaccination against human papilloma virus. Intern Med. 2016;55:3181–84. doi:10.2169/internalmedicine.55.5472.
- Hemachudha T, Griffin DE, Giffels JJ, Johnson RT, Moser AB, Phanuphak P. Myelin basic protein as an encephalitogen in encephalomyelitis and polyneuritis following rabies vaccination. N Engl J Med. 1987;316:369–74. doi:10.1056/NEJM198702123160703.
- Ubol S, Hemachudha T, Whitaker JN, Griffin DE. Antibody to peptides of human myelin basic protein in post-rabies vaccine endophalomyelitis sera. J Neuroimmunol. 1990;26:107–11. doi:10.1016/0165-5728(90)90081-W.
- Xiong CH, Yan Y, Liao Z, Peng SH, Wen HR, Zhang YX, Chen S-H, Li J, Chen H-Y, Feng X-W, et al. Epidemiological characteristics of acute disseminated encephalomyelitis in Nanchang, China: a retrospective study. BMC Public Health. 2014;14:111. doi:10.1186/1471-2458-14-111.
- Australian Government. National immunization program schedule. Canberra (ACT): Australian Government; 2020.
- Australian Consortium for Classification Development. ICD-10-AM/ACHI/ACS. Australia, 2017.
- Australian Bureau of Statistics. 3301.0 - Births, Australia, 2016. Canberra (ACT): Australian Bureau of Statistics; 2017.
- Australian Government. Historical coverage data tables for all children. Canberra (ACT): Australia Government; 2020.
- Chin LK, Crawford NW, Rowles G, Buttery JP. Australian immunisation registers: established foundations and opportunities for improvement. Euro Surveill. 2012;17:39.
- Hull BP, Deeks SL, McIntyre PB. The Australian childhood immunisation register-a model for universal immunisation registers? Vaccine. 2009;27:5054–60. doi:10.1016/j.vaccine.2009.06.056.
- Victorian Government. Immunisation schedule Victoria from January 2015. Vol. 2. Melbourne; 2015.
- State Government of Victoria. Vaccine history timeline. Melbourne: State Government of Victoria; 2020.
- Sejvar JJ, Kohl KS, Bilynsky R, Blumberg D, Cvetkovich T, Galama J, Gidudu J, Katikaneni L, Khuri-Bulos N, Oleske J, et al. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5771–92. doi:10.1016/j.vaccine.2007.04.060.
- Whitaker HJ, Hocine MN, Farrington CP. The methodology of self-controlled case series studies. Stat Methods Med Res. 2009;18:7–26. doi:10.1177/0962280208092342.
- Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, Bines J, McIntyre PB. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s National immunization program. Clin Infect Dis. 2013;57:1427–34. doi:10.1093/cid/cit520.
- Weldeselassie Y, Whitaker H, Farrington C. Use of the self-controlled case-series method in vaccine safety studies: review and recommendations for best practice. Epidemiol Infect. 2011;139:1805–17. doi:10.1017/S0950268811001531.
- Crawford NW, Cheng A, Andrews N, Charles PG, Clothier HJ, Day B, Day T, Gates P, Macdonell R, Roberts L, et al. Guillain-Barre syndrome following pandemic (H1N1) 2009 influenza A immunisation in Victoria: a self-controlled case series. Med J Aust. 2012;197:574–78. doi:10.5694/mja12.10534.
- Andrews N, Miller E, Waight P, Farrington P, Crowcroft N, Stowe J, Taylor B. Does oral polio vaccine cause intussusception in infants? Evidence from a sequence of three self-controlled cases series studies in the United Kingdom. Eur J Epidemiol. 2001;17:701–06. doi:10.1023/A:1015691619745.
- Hawken S, Potter BK, Little J, Benchimol EI, Mahmud S, Ducharme R, Wilson K. The use of relative incidence ratios in self-controlled case series studies: an overview. BMC Med Res Methodol. 2016;16:126. doi:10.1186/s12874-016-0225-0.
- Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25:1768–97. doi:10.1002/sim.2302.
- Clothier HJ, Lee KJ, Sundararajan V, Buttery JP, Crawford NW. Human papillomavirus vaccine in boys: background rates of potential adverse events. Med J Aust. 2013;198:554–58. doi:10.5694/mja12.11751.
- Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13:215–24. doi:10.1016/j.autrev.2013.10.003.
- Klein NP, Lewis E, Fireman B, Hambidge SJ, Naleway A, Nelson JC, Belongia EA, Yih WK, Nordin JD, Hechter RC, et al. Safety of measles-containing vaccines in 1-year-old children. Pediatrics. 2015;135:e321–9. doi:10.1542/peds.2014-1822.
- Bolukbasi O, Ozmenoglu M. Acute disseminated encephalomyelitis associated with tetanus vaccination. Eur Neurol. 1999;41:231–32. doi:10.1159/000008056.
- Shibazaki K, Murakami T, Kushida R, Kurokawa K, Terada K, Sunada Y. Acute disseminated encephalomyelitis associated with oral polio vaccine. Int Med (Tokyo, Japan). 2005;45:1143–46. doi:10.2169/internalmedicine.45.6029.
- Mancini J, Chabrol B, Moulene E, Pinsard N. Relapsing acute encephalopathy: a complication of diphtheria-tetanus-poliomyelitis immunization in a young boy. Eur J Pediatr. 1996;155:136–38. doi:10.1007/BF02075768.
- Gold MS, Effler P, Kelly H, Richmond PC, Buttery JP. Febrile convulsions after 2010 seasonal trivalent influenza vaccine: implications for vaccine safety surveillance in Australia. Med J Aust. 2010;193:492–93. doi:10.5694/j.1326-5377.2010.tb04029.x.
- Varricchio F, Iskander J, Destefano F, Ball R, Pless R, Braun MM, Chen RT. Understanding vaccine safety information from the vaccine adverse event reporting system. Pediatr Infect Dis J. 2004;23:287–94. doi:10.1097/00006454-200404000-00002.
- Persson I, Granath F, Askling J, Ludvigsson JF, Olsson T, Feltelius N. Risks of neurological and immune-related diseases, including narcolepsy, after vaccination with Pandemrix: a population- and registry-based cohort study with over 2 years of follow-up. J Intern Med. 2014;275:172–90. doi:10.1111/joim.12150.
- Idrissova ZR, Boldyreva MN, Dekonenko EP, Malishev NA, Leontyeva IY, Martinenko IN, Petrukhin AS. Acute disseminated encephalomyelitis in children: clinical features and HLA-DR linkage. Eur J Neurol. 2003;10:537–46. doi:10.1046/j.1468-1331.2003.00639.x.
- Britton PN, Khoury L, Booy R, Wood N, Jones CA. Encephalitis in Australian children: contemporary trends in hospitalisation. Arch Dis Child. 2016;101:51–56. doi:10.1136/archdischild-2015-308468.