ABSTRACT
Booster doses of meningococcal conjugate vaccines induce long-term protection against invasive meningococcal disease. We evaluated the immunogenicity and safety of a booster dose of MenACYW-TT in pre-school children who were primed 3 years earlier with MenACYW-TT or MCV4-TT (Nimenrix®). In this Phase III, open-label, multi-center study (NCT03476135), children (4–5 years old), who received a primary dose of MenACYW-TT or MCV4-TT as toddlers in a previous study, received a booster dose of MenACYW-TT. Titers of antibody against meningococcal serogroups A, C, W and Y were measured by serum bactericidal assay using human (hSBA) and baby rabbit (rSBA) complement in samples collected before (D0) and 30 days after (D30) booster vaccination. Safety was assessed over the 30-day study period. Ninety-one participants received the booster dose. In both study groups, hSBA titers increased from D0 to D30; serogroup C titers [95% confidence interval] were higher in the MenACYW-TT-primed vs MCV4-TT-primed group at D0 (106 [73.2, 153] vs 11.7 [7.03, 19.4], respectively) and D30 (5894 [4325, 8031] vs 1592 [1165, 2174], respectively); rSBA results were similar. Nearly all participants achieved ≥1:8 hSBA and rSBA titers at D30, which were higher or comparable to those observed post-primary dose, suggesting rapid booster responses. At D0, all hSBA and rSBA titers were higher than those observed pre-primary dose, suggesting persistence of immunogenicity. The MenACYW-TT booster dose was well-tolerated and had similar safety outcomes across study groups. These findings suggest that MenACYW-TT elicits robust booster responses in children primed 3 years earlier with MenACYW-TT or MCV4-TT.
Introduction
Invasive meningococcal disease (IMD) is an important cause of mortality and morbidity globally, and a major infectious cause of death in children aged under 5 years.Citation1,Citation2 In Europe, there were 2.5 confirmed cases per 100,000 population in children aged 1–4 years, in 2017.Citation3
Among the 12 meningococcal serogroups that have been identified, 6 (A, B, C, W, X and Y) cause the vast majority of IMD cases worldwide.Citation1,Citation4 The relative importance of each of these serogroups varies geographically and over time.Citation4,Citation5 In Africa, serogroups A and W account for 200 cases per 100,000 population.Citation6,Citation7 In Europe, the Americas and Australia, serogroups B, C and Y together account for a large majority of cases,Citation8 with 67% of confirmed IMD cases in Europe accounted for by serogroups B and C.Citation4,Citation5,Citation9 Increasing numbers of cases caused by serogroups W (17%) and Y (12%) have been reported over recent years in Europe.Citation3–5
Quadrivalent meningococcal conjugate vaccines against serogroups A, C, W and Y (MCV4) are widely used to prevent disease and transmission, particularly in countries where serogroups C, W and Y are responsible for a significant burden of disease.Citation10,Citation11 There are currently four MCV4 vaccines available.Citation12,Citation13 MCV4 conjugated to diphtheria toxoid (MCV4-DT; Menactra®, Sanofi Pasteur) is indicated for prevention of IMD in individuals 9 months through 55 years of age and licensed in over 70 countries including the USA and Asia.Citation14 MCV4 conjugated to the diphtheria protein CRM197 (MCV4-CRM; Menveo®, GlaxoSmithKline) is licensed in the USA for use in individuals aged 2 months up to 55 years and in Europe from 2 years of age with no upper age limit.Citation15,Citation16 MCV4 conjugated to tetanus toxoid (MCV4-TT; Nimenrix®; Pfizer Europe, Belgium) is licensed in Europe as a single dose for individuals aged 6 weeks and older, with no upper age limit.Citation17–19 A new MCV4 conjugated to a tetanus toxoid protein carrier (MenACYW-TT; MenQuadfi®, Sanofi Pasteur) was approved in the USA (April 2020) for use in individuals aged 24 months and olderCitation20 and in Europe (November 2020) for individuals aged 12 months and older.Citation21
The successful implementation of meningococcal vaccination in childhood immunization programs has been shown to be an effective means of controlling IMD.Citation22 Nationwide, including school-based, immunization against serogroup C-induced IMD in England resulted in a reduction of 97% in infection rates over a period of 18 years (2000–2018).Citation23–25 Similarly, a meningococcal C conjugate (MCC) vaccine in Spain resulted in vaccine protection levels exceeding 94% over 4 years in children between the ages of 2 months and 6 years.Citation23,Citation26
However, the bactericidal antibody titers elicited by MCCCitation26 and MCV4 vaccinesCitation27,Citation28 have been shown to wane within 3 to 5 years after primary vaccination; this waning effect is more pronounced in younger children than in older children and adults.Citation26–28 Recent data further showed that after this initial waning, bactericidal antibody titers remained stable between 6 and 10 years after primary vaccination.Citation29 The Advisory Committee on Immunization Practices (ACIP) recommends a single dose of a quadrivalent meningococcal conjugate vaccine at age 11 or 12 years followed by a booster dose administered at age 16 years.Citation30
It has been shown that the recently licensed MenACYW-TT boosts the immune response in adolescents and adults (aged ≥15 years) primed with MCV4 vaccine 4–10 years earlier, irrespective of whether MCV4-DT or MCV4-CRM was used for priming.Citation31 MenACYW-TT was also shown to boost the serogroup C immune response in toddlers 12–23 months of age who received monovalent MCC vaccination (MenC-TT [NeisVac-C®] or MenC-CRM [Menjugate®]) during infancy.Citation32 However, data on the immunogenicity and safety of a booster dose of MenACYW-TT in children previously vaccinated with MenACYW-TT or MCV4-TT are lacking.
In a previous randomized Phase II exploratory study conducted in Finland (MET54; NCT03205358), 188 meningococcal vaccine naïve toddlers aged 12–23 months were randomized 1:1 to receive a single primary vaccine dose of MenACYW-TT or of the licensed vaccine MCV4-TT; a single dose of the MenACYW-TT vaccine was well tolerated and immunogenic.Citation33 The current Phase III study evaluated the immunogenicity and safety of a booster dose of MenACYW-TT administered to pre-school aged children who were primed 3 years earlier as toddlers with a dose of MenACYW-TT or MCV4-TT as part of the MET54 study. We also describe the immune persistence up to 3 years after the priming dose and the antibody response to the tetanus toxoid carrier protein before and after booster vaccination.
Methods
Study design and participants
This was a Phase III, open-label, multi-center study to describe the immunogenicity and safety of a single booster dose of MenACYW-TT and the immune persistence of the priming dose of MenACYW-TT or MCV4-TT in children (aged 4–5 years) who had been vaccinated three years earlier as toddlers (aged 12–23 months).Citation33 The current study (MET62; NCT03476135) was conducted between 27 February 2018 and 10 September 2018 at eight centers in Finland.
Participants who had received a primary dose of either MenACYW-TT or MCV4-TT 3 years (± 45 days) earlier as part of the MET54 study and had completed that study (attended the final study visit) were invited to participate. Exclusion criteria included participation in another clinical trial; any vaccination in the 4 weeks preceding the study or planned before the final blood sampling (except for influenza vaccination ≥2 weeks before or after study vaccine); previous receipt of any meningococcal vaccine containing serogroups A, B, C, W or Y (with the exception of that administered as part of the MET54 study); a history of meningococcal infection (confirmed either clinically, serologically, or microbiologically); participants at high risk for meningococcal infection during the trial, including those with persistent complement deficiency, with anatomic or functional asplenia, or those traveling to countries with high rates of endemic or epidemic disease; receipt of immunoglobulins, blood, or blood-derived products in the past 3 months, or antibiotic therapy within 72 hours before the first blood sampling; known or suspected congenital or acquired immunodeficiency, or receipt of immunosuppressive therapy within the preceding 6 months; known systemic hypersensitivity or history of a life-threatening reaction to any of the vaccine components; personal history of Guillain–Barre syndrome or an Arthus-like reaction after vaccination with a tetanus toxoid-containing vaccine.
All participants received a single booster dose of MenACYW-TT by intramuscular injection in the deltoid muscle of the arm. MenACYW-TT was presented in 0.5 mL of saline solution containing 10 μg of each of meningococcal capsular polysaccharides of serogroups A, C, W and Y, and approximately 55 μg of tetanus toxoid protein carrier.
For data analyses, the study participants were divided into two groups: those who previously received a primary dose of MenACYW-TT and those who previously received a primary dose of the licensed MCV4-TT vaccine, as part of the MET54 study. The current study was open-label as only the MenACYW-TT vaccine was administered as a booster dose.
Written informed consent was provided by the parents or legal representatives of study participants. The conduct of this study was consistent with standards established by the Declaration of Helsinki and compliant with the International Conference on Harmonization guidelines for good clinical practice as well as with all local and/or national regulations and directives. The study was approved by the National Ethical Committee of Finland prior to the start of the study.
Immunogenicity
Blood samples for immunogenicity assessment were obtained before (on Day 0) and 30–44 days after booster vaccination. Titers of antibody against meningococcal serogroups A, C, W and Y were measured with serum bactericidal assay (SBA) using human complement (hSBA; GCI, Sanofi Pasteur, Swiftwater, PA, USA) and baby rabbit complement (rSBA; Public Health England, Manchester, United Kingdom), both of which were performed by qualified laboratory personnel as described previously.Citation34 The lower limit of quantitation (LLOQ) of both assays was a titer of 1:4.
Antibody persistence over 3 years and the subsequent booster effect were assessed in Day 0 and post-booster sera from this study, paired with data obtained before and 30 days after the primary MenACYW-TT dose administered 3 years earlier in the MET54 study.
Geometric mean concentrations (GMCs) for anti-tetanus antibodies were measured in both pre- and post-booster vaccination blood samples using a multiplex electrochemiluminescent assay (a serological assay which allows for the simultaneous quantification of human antibodies to tetanus toxoid), performed at GCI, Sanofi Pasteur, Swiftwater, PA, USA.
Safety
Safety endpoints included the occurrence, time to onset, duration and intensity of adverse events (AEs), including adverse reactions (ARs). Participants were observed for 30 minutes after vaccination to assess the occurrence of any unsolicited systemic AEs. Participants’ parents or legally acceptable representatives were provided with an electronic device (e-diary) that contained an application to record information about the occurrence of solicited injection site and systemic reactions up to 7 days post-booster vaccination and unsolicited AEs up to 30 days post-booster vaccination. Serious AEs (SAEs), including AEs of special interest (AESIs), were assessed by the investigator, collected throughout the trial and recorded through the electronic data capture system; unsolicited non-serious AEs were graded on a 3-point intensity scale as previously described.Citation33 Relationship to vaccination was assessed by the investigator for unsolicited systemic AEs that occurred within 30 minutes of vaccination, unsolicited nonserious AEs occurring between Day 0 and Day 30 post-booster vaccination and SAEs occurring throughout the trial; AEs involving the injection site were presumed to be related to vaccination. AEs were defined using preferred terms from the Medical Dictionary for Regulatory Activities (MedDRA) version 21.1.
Statistical analyses
This was a descriptive study; no hypotheses were tested and no formal sample size calculations were performed. Categorical variables were summarized and presented as frequency counts with 95% confidence intervals (CIs) calculated using the exact binomial distribution (Clopper–Pearson method) for proportions.Citation35 Bactericidal antibody titers and corresponding 95% CIs were calculated on Log10 transformed data assuming normal distribution for the transformed data, with antilog transformations applied to provide GMTs and their 95% CIs. For safety results, the numbers and proportions with 95% CIs were recorded and calculated.
The proportions of participants with hSBA titers ≥1:4 and ≥1:8, and rSBA titers ≥1:8 and ≥1:128 with corresponding 95% CIs were determined. hSBA seroresponse was defined as post-vaccination titers ≥1:8 for participants with pre-vaccination titers <1:8, or a ≥ 4-fold increase in titers from pre- to post-vaccination for participants with pre-vaccination titers ≥1:8. rSBA seroresponse was defined as post-vaccination titers ≥1:32 for participants with pre-vaccination rSBA titer <1:8 or a ≥4-fold increase in titers from pre- to post-vaccination for participants with pre-vaccination titers ≥1:8. GMCs for the samples and the proportions of participants achieving levels ≥0.01, ≥0.1 and ≥1.0 IU/mL of antibody concentrations to tetanus toxoid were determined.
Four analysis sets were used: the full analysis set (FAS), the full analysis set for persistence (FASP), the per-protocol analysis set (PPAS) and the safety analysis set (SafAS). The FAS was defined as participants who received at least one dose of the study vaccine and had a valid post-vaccination blood sample result. The FASP was defined as a subset of participants who had a valid pre-vaccination blood sample result. Persistence of the vaccine immune response was evaluated in the FASP. The PPAS included all participants from the FAS who adhered to the protocol-specific inclusion criteria and did not have the relevant deviations from the study protocol, such as receiving an alternative vaccine to MenACYW-TT, not receiving the vaccine in the proper time window or receiving a protocol-prohibited therapy/medication/vaccine; all immunogenicity analyses were performed on the PPAS. The SafAS was defined as those participants who had received one dose of the study vaccine and had any safety data available. Analyses were conducted using SAS® Version 9.4 or later (SAS Institute Inc.; Cary, NC).
Results
Study participants
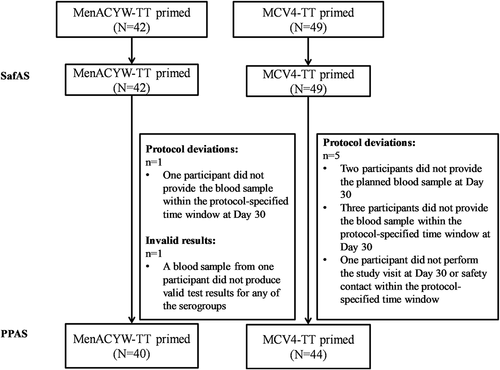
A total of 91 children were enrolled, all of whom received a booster dose of MenACYW-TT: 42 who had previously received a primary dose of MenACYW-TT and 49 who had received a primary dose of MCV4-TT 3 years earlier. All participants completed the study; there were no early terminations. Protocol deviations were reported for 6 participants, one in the MenACYW-TT-primed group and five in the MCV4-TT-primed group. An additional participant in the MenACYW-TT-primed group had a blood sample that did not produce valid test results for any of the serogroups (). The PPAS therefore included 84 participants: 40 (95.2%) in the MenACYW-TT-primed group and 44 (89.8%) in the MCV4-TT-primed group ().
Baseline demographics are summarized in . Mean age was 3.9 years, overall and in each study group. Equal numbers of males and females were included in the study overall, although the MenACYW-TT-primed group had a majority (62%) of males and the MCV4-TT-primed group had a majority (61%) of females.
Table 1. Baseline demographics in MenACYW-TT primed and MCV4-TT primed study groups – SafAS
Immunogenicity
Pre-booster immune status
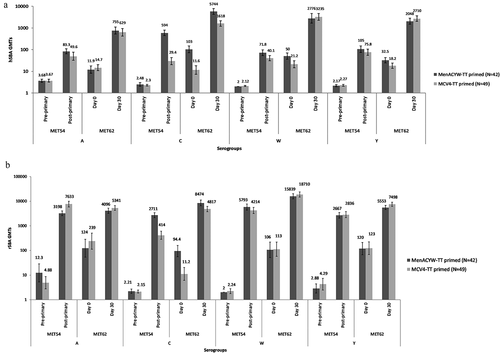
Day 0 (pre-booster) meningococcal hSBA GMTs ranged from 12.1 (serogroup A) to 106 (serogroup C) in the MenACYW-TT-primed group and from 11.7 (serogroup C) to 21.9 (serogroup W) in the MCV4-TT-primed group (); meningococcal hSBA GMTs were higher (non-overlapping 95% CIs) for serogroup C and, to a lesser extent, for serogroups W and Y (non-overlapping 95% CIs), in the MenACYW-TT-primed group than in the MCV4-TT-primed group; serogroup A titers were comparable between groups (). All participants (100%) had hSBA titers ≥1:8 for serogroup C in the MenACYW-TT-primed group at Day 0, compared with 54.5% in the MCV4-TT-primed group; there was a trend toward slightly higher proportions (overlapping 95% CIs) for serogroups W and Y, and toward lower proportions (overlapping 95% CIs) for serogroup A in the MenACYW-TT-primed group compared with the MCV4-TT-primed group ().
Figure 2. GMTs at baseline (Day 0) and Day 30 after MenACYW-TT booster dose as assessed by (a) hSBA and (b) rSBA in both MenACYW-TT primed and MCV4-TT primed study groups – PPAS.
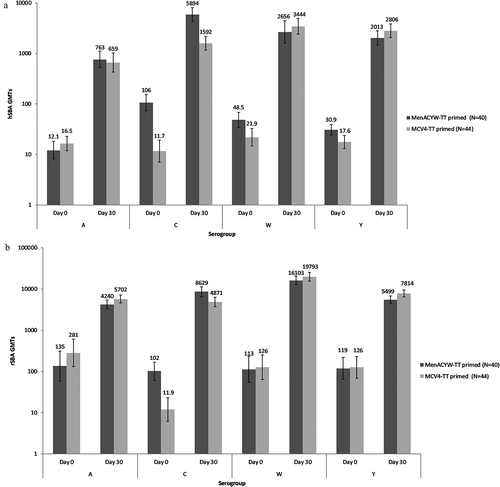
Figure 3. Proportion of participants with hSBA (a) and rSBA (b) titers ≥1:8 at pre-booster dose (Day 0) and Day 30 after MenACYW-TT booster dose in both MenACYW-TT primed and MCV4-TT primed study groups – PPAS.
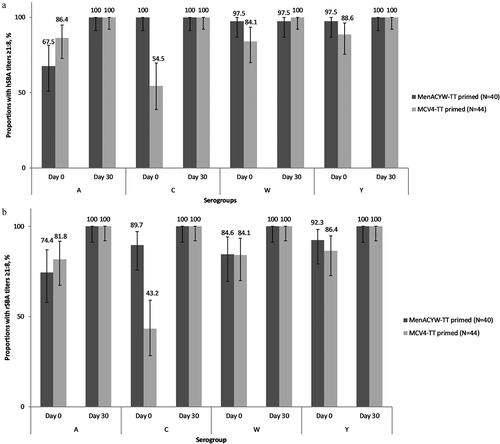
Similar results were observed for GMTs of rSBA against meningococcal serogroup C, with higher levels (non-overlapping 95% CIs) in the MenACYW-TT-primed group than the MCV4-TT-primed group; rSBA titers were comparable between groups for serogroups A, W and Y (). The proportion of participants with rSBA titers ≥1:8 () or ≥1:128 (Supplementary Figure 1) was also higher in the MenACYW-TT-primed group than the MCV4-TT-primed group for serogroup C.
Vaccine response at Day 30 post-booster
At 30 days post-booster, meningococcal hSBA GMTs increased for all four serogroups, with the greatest increase observed for serogroup C among MenACYW-TT-primed participants; Day 30 hSBA GMTs ranged from 763 (serogroup A) to 5894 (serogroup C) in the MenACYW-TT-primed group and from 659 (serogroup A) to 3444 (serogroup W) in the MCV4-TT-primed group (). High rSBA GMTs were also observed at Day 30 post-booster across serogroups, with the highest titers observed for serogroup W ().
All participants (100%) had meningococcal hSBA titers ≥1:8 for the four serogroups at Day 30 post-booster in both study groups, except for serogroup W in the MenACYW-TT-primed group (97.5% of participants; ); all participants (100%) had meningococcal rSBA titers ≥1:8 () and ≥1:128 at Day 30 post-booster dose (Supplementary Figure 1) for all serogroups in both study groups. Most participants had ≥4-fold rise in hSBA and rSBA titers at Day 30 post-booster dose in both groups (≥95.0% in the MenACYW-TT-primed group and ≥95.5% in the MCV4-TT-primed group for hSBA titers; and ≥92.3% and ≥81.8%, respectively, for rSBA titers; ).
Table 2. Proportion of participants with ≥4-fold rise of hSBA and rSBA titers at Day 30 relative to baseline (Day 0) in both MenACYW-TT primed and MCV4-TT primed study groups – PPAS
Most participants in both study groups demonstrated hSBA and rSBA vaccine seroresponse at Day 30 (Supplementary Table 1).
Antibody persistence
Meningococcal hSBA and rSBA GMTs decreased as expected over the 3 years from 30 days post-primary vaccination in the MET54 study to Day 0 of the current study, for all serogroups and in both study groups. However, hSBA and rSBA GMTs in the FASP (N = 91) were higher at Day 0 of the current study than those observed for paired sera pre-primary vaccination in MET54 (). Most participants retained hSBA titers ≥1:8 for the four serogroups in both groups at Day 0 of the current study (ranging from 66.7% [serogroup A] to 100% [serogroup C] in the MenACYW-TT-primed group and from 57.1% [serogroup C] to 89.8% [serogroup Y] in the MCV4-TT-primed group); similar trends were observed for the proportion of participants with rSBA titers above ≥1:8 and ≥1:128 for serogroups A, W and Y in both study groups and for serogroup C in the MenACYW-TT-primed group. In the MCV4-TT-primed group, for serogroup C, the proportions of participants with rSBA titers above ≥1:8 and ≥1:128 dropped from 98.0% and 93.9% at 30 days post-primary vaccination, respectively, to 42.9% and 22.4% at Day 0 of the current study, respectively (Supplementary Table 2).
Booster effect
The hSBA titers were consistently higher 30 days post booster versus pre-booster dose for all four serogroups in both study groups; this was particularly marked in the MenACYW-TT-primed group relative to the MCV4-TT-primed group for serogroup C (). For rSBA, higher titers were observed post-booster than post-primary vaccination for serogroups C, W and Y; for serogroup A, post-primary and post-booster levels were comparable ().
Tetanus toxoid response
GMCs (95% CIs) of tetanus toxoid antibodies increased from pre- to 30 days post-booster vaccination in both study groups: from 3.12 IU/mL (2.05, 4.75) and 3.02 IU/mL (2.11, 4.31) in MenACYW-TT- and MCV4-TT-primed groups, respectively, to 10.4 IU/mL (8.41, 12.8) and 9.36 IU/mL (7.61, 11.5) for MenACYW-TT- and MCV4-TT-primed groups, respectively.
Pre-booster dose, all participants (100%) in both study groups had tetanus toxoid antibody concentrations ≥0.1 IU/mL and most (82.1% [32/39] in the MenACYW-TT-primed and 81.8% [36/44] in the MCV4-TT-primed group) had concentrations ≥1 IU/mL. At Day 30 post-booster dose, all participants (100%) had tetanus toxoid antibody concentrations ≥1 IU/mL.
Safety
There were no immediate unsolicited AEs or ARs after the booster dose in either study group (). Solicited injection site and systemic reactions reported between Days 0 and 7 are summarized in . The proportions of participants with at least one solicited injection site or solicited systemic reactions were comparable between the two study groups.
Table 3. Summary of safety outcomes from baseline (Day 0) to Day 30 after MenACYW-TT booster dose in MenACYW-TT primed and MCV4-TT primed study groups – SafAS
In both study groups, the majority of injection site reactions were Grade 1 or Grade 2 in intensity, with pain and erythema being the most frequently reported reactions (); all started within 1–3 days post-vaccination and all resolved within 1–7 days. Most solicited systemic reactions were Grade 1 or Grade 2 in intensity, started within 1–3 days post-vaccination and resolved within 1–3 days; myalgia (33.3%) and malaise (31%) were the most frequent reactions ().
Unsolicited AEs and ARs reported between Days 0 and 30 are summarized in . All unsolicited non-serious AEs and ARs were of Grade 1 or Grade 2 intensity and most resolved within 1–3 days. Five participants in the MCV4-TT-primed group (10.2% [5/49]) reported at least one unsolicited non-serious AR: nasopharyngitis, nausea, abdominal pain, and gastroenteritis each in different participants, and urticaria and swelling of the face in another single participant; the urticaria and swelling of the face were both of Grade 1 intensity, started on Day 0 and resolved after 1 day without leading to any SAE.
No SAEs (including deaths) were reported during the study, and there were no study discontinuations due to an AE.
Discussion
This descriptive Phase III study showed for the first time that a booster dose of MenACYW-TT vaccine in children 4–5 years old, who had been vaccinated approximately 3 years earlier with a single dose of MenACYW-TT or MCV-TT, was immunogenic and well tolerated. We observed a robust booster response for all four meningococcal serogroups following a single booster dose of MenACYW-TT in children primed with MenACYW-TT or MCV4-TT. The immunogenicity of a booster dose of MenACYW-TT was previously demonstrated in adults and adolescents (≥15 years old) who were primed with MCV4-DT or MCV4-CRM vaccines 4–10 years earlier.Citation31 The findings of the current study thus support evidence that MenACYW-TT can elicit robust booster responses against meningococcal serogroups A, C, W and Y, irrespective of the meningococcal vaccine used for primary vaccination.
Despite the expected waning over the three-year period following primary vaccination, the levels of antibody against meningococcal serogroups A, C, W and Y remained higher at the pre-booster time-point than at the pre-primary time-point, suggesting persistence of the antibody response up to 3 years after primary vaccination. Furthermore, the majority of participants had hSBA titers ≥1:8 at the pre-booster visit, and therefore may already have had protection against IMD at this time. The waning of antibody levels in this study is consistent with observations following primary vaccination with other licensed MCV4 vaccines in children and infants,Citation27,Citation36–38 whereby meningococcal antibody responses waned but remained higher than at baseline over 4 and 10 years following primary vaccination with MCV4-TT in toddlers (12–23 months of age) and children (2–11 years of age),Citation29,Citation39,Citation40 up to 5 years following MCV4-CRM primary vaccination in children (40–60 months of age) and 3–5 years in infants and toddlers.Citation27,Citation41 Evidence of waning antibody responses after primary vaccination in children prompted the recommendation from ACIP (USA) to administer a booster dose for children deemed to be at increased risk of meningococcal infection; for those under the age of 7 years, a booster dose is recommended 3 years after completion of the primary series and for those 7 years old or older, 5 years after primary vaccination, and at regular intervals thereafter.Citation30
The response following MenACYW-TT booster vaccination in this study was particularly marked for serogroup C in MenACYW-TT primed children, regardless of the bactericidal assay (hSBA or rSBA) used. Notably, a strong serogroup C response was also observed after the primary MenACYW-TT dose in the MET54 study, and this was maintained over the 3-year period between the MET54 and MET62 studies. However, it should be noted that the number of participants included in this analysis (in the FASP) was small (42 participants), indicating the need to confirm this observation in a large population of participants.
In Europe, where IMD is most commonly caused by serogroups B and C, successful implementation of the MCC vaccine into national childhood immunization programs led to a significant reduction in annual notification rates of meningococcal C disease by 17.5% between 2004 and 2014.Citation4 Most European countries have introduced a booster dose of meningococcal C vaccines in toddlers to maintain protective antibody levels against serogroup C.Citation4 Furthermore, the increase in the prevalence of serogroup W in Europe over the last decade has led several European countries, such as the UK, Netherlands, Italy and Spain, to introduce an MCV4 into their routine vaccination schedule.Citation5 If the findings from the current study are confirmed in larger datasets, MenACYW-TT could provide an alternative means of boosting immunity not only against serogroup C but also against other serogroups in Europe.
Participants in both study groups demonstrated an increase in GMCs of tetanus toxoid antibodies following the administration of the MenACYW-TT booster dose, due to the presence of tetanus toxoid as a carrier protein. Similar responses were observed in the MET54 study following a primary dose of either MenACYW-TT or MCV4-TT, and are in line with previous observations that TT and other protein carriers (DT and CRM) within conjugated vaccines induce a protective immune response against these carriers (diphtheria toxoid in MCV4-DT [Menactra®], CRM197 in MCV4-CRM [Menveo®]).Citation42 The anti-tetanus toxoid immune response indicates that the use of the tetanus toxoid protein in MenACYW-TT vaccine does not have an adverse effect on the tetanus immunogenicity.
Moreover, booster vaccination with MenACYW-TT in pre-school children, 3 years after the primary dose given as toddlers, revealed no apparent safety concerns. AEs and ARs were of mild (Grade 1) or moderate (Grade 2) intensity.
One of the strengths of the study was the credibility of the antibody titer data since both hSBA and rSBA antibody assays were performed in the same environment (GCI Sanofi Pasteur and Public Health England, respectively) in both MET54 and MET62 studies. However, there were some limitations. Results were only descriptive due to the small sample numbers (N = 91), which may limit the generalizability of these results. Our findings would therefore need to be confirmed in larger datasets. Additionally, there was an imbalance in the gender ratio between the study groups, which arose unintentionally at randomization in the MET54 study. Despite this gender imbalance, no difference was observed between the two groups in terms of the immune response to the priming vaccines.Citation33 Furthermore, there is no published evidence of a potential impact of gender on the immune response to the study vaccine. It is therefore unlikely that the imbalance in gender ratio between the study groups would have affected the booster responses observed in the current study.
This study showed that MenACYW-TT can elicit a robust booster response against all of the meningococcal serogroups contained in the vaccine in MenACYW-TT primed and MCV4-TT primed children, without safety concerns. If confirmed in a larger dataset, the MenACYW-TT vaccine could be considered for use as a booster following primary vaccination with a MCV4 vaccine, including MenACYW-TT, to maintain continued immunity against meningococcal infection in pre-school aged children.
Disclosure of potential conflicts of interest
AA, AF, BU, MP and MV declare no conflict of interest. DN, EJ, FP, JD and MSD are employed by SP. AEJ was an employee of SP at the time of the conduct of the study and the generation of the results. DN reports personal fees from SP outside the submitted work. IS reports receiving funds from SP for an industry-initiated study and travel expenses to investigator meetings.
Author contributions
All authors attest they meet the ICMJE criteria for authorship. AEJ, EJ, JD and MSD were involved in study design. MV, MP, BU, AA, AF and IS were involved in data acquisition. FP, MV, MP, BU, AA, JD, DN, EJ and MSD were involved in data analysis and study interpretation. All authors reviewed and approved submission of the final manuscript.
Supplemental Material
Download MS Word (76.7 KB)Acknowledgments
The authors would like to thank all participants who volunteered to take part in the study, all study investigators and the staff at the Vaccine Evaluation Unit, Public Health England. The authors also wish to acknowledge and thank the Sanofi Pasteur study team especially Corinne Chalus, Hnin Phyu, Isabelle Lacroix and Jennifer Kinsley for their support during the conduct of this study. Editorial assistance with the preparation of the manuscript was provided by Sam Hijazi PhD and Juliette Gray PhD, of inScience Communications, Springer Healthcare Ltd, UK, and was funded by Sanofi Pasteur.
Data availability statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan and dataset specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1902701
Additional information
Funding
References
- Bosis S, Mayer A, Esposito S. Meningococcal disease in childhood: epidemiology, clinical features and prevention. J Prev Med Hyg. 2015;56:E121–4.
- Acevedo R, Bai X, Borrow R, Caugant DA, Carlos J, Ceyhan M, Christensen H, Climent Y, De Wals P, Dinleyici EC, et al. The global meningococcal initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines. 2019;18:15–30. doi:10.1080/14760584.2019.1557520.
- European Centre for Disease Prevention and Control. Invasive meningococcal disease - annual epidemiological report for 2017. Stockholm, Sweden: ECDC; 2019.
- Whittaker R, Dias JG, Ramliden M, Ködmön C, Economopoulou A, Beer N, Pastore Celentano L, Kanitz E, Richter L, Mattheus W, et al. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004–2014. Vaccine. 2017;35:2034–41. doi:10.1016/j.vaccine.2017.03.007.
- Krone M, Gray S, Abad R, Skoczynska A, Stefanelli P, van der Ende A, Tzanakaki G, Mölling P, João Simões M, Křížová P, et al. Increase of invasive meningococcal serogroup W disease in Europe, 2013 to 2017. Euro Surveill. 2019;24:24. doi:10.2807/1560-7917.ES.2019.24.14.1800245.
- Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Eskola J, Fermon F, Klugman KP, Ramsay M, Sow S, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11:17. doi:10.1186/1478-7954-11-17.
- Borrow R, Alarcón P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echániz-Aviles G, Findlow J, Head C, et al. The global meningococcal initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16:313–28. doi:10.1080/14760584.2017.1258308.
- Prevention CfDCa. Meningococcal disease. 2020 Aug 10. https://www.cdc.gov/meningococcal/global.html.
- European Centre for Disease Prevention and Control. Invasive meningococcal disease: annual epidemiological report for 2017. 2019 Dec 5. https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-invasive-meningococcal-disease.pdf.
- World Health Organization. Meningococcal vaccines: WHO position paper, November 2011. Wkly Epidemiol Rec:No. 47. 2001;86:521–40.
- Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1–20.
- Dretler AW, Rouphael NG, Stephens DS. Progress toward the global control of Neisseria meningitidis: 21st century vaccines, current guidelines, and challenges for future vaccine development. Hum Vaccin Immunother. 2018;14:1146–60. doi:10.1080/21645515.2018.1451810.
- Pizza M, Bekkat-Berkani R, Rappuoli R. Vaccines against meningococcal diseases. Microorganisms. 2020;8(10):1521. doi:10.3390/microorganisms8101521.
- Food and Drug Administration. Menactra. 2020 July 6. https://www.fda.gov/files/vaccines,%20blood%20&%20biologics/published/Package-Insert—Menactra.pdf.
- Food and Drug Administration. Menveo. 2020 July 6. https://www.fda.gov/media/78514/download.
- European Medicines Agency. Menveo. 2020 Oct 29. https://www.ema.europa.eu/en/medicines/human/EPAR/menveo.
- Croxtall JD, Dhillon S. Meningococcal quadrivalent (serogroups A, C, W135 and Y) tetanus toxoid conjugate vaccine (Nimenrix™). Drugs. 2012;72:2407–30.
- Assaf-Casals A, Dbaibo G. Meningococcal quadrivalent tetanus toxoid conjugate vaccine (MenACWY-TT, Nimenrix™): a review of its immunogenicity, safety, co-administration, and antibody persistence. Hum Vaccin Immunother. 2016;12:1825–37. doi:10.1080/21645515.2016.1143157.
- European Medicines Agency. Nimenrix. 2020 Oct 29. https://www.ema.europa.eu/en/medicines/human/EPAR/nimenrix.
- Food and Drug Administration. MenQuadfi. 2020.
- European Medicines Agency. MenQuadfi. 2020 Nov 27. https://www.ema.europa.eu/en/medicines/human/EPAR/menquadfi.
- Cohn AC, Harrison LH. Meningococcal vaccines: current issues and future strategies. Drugs. 2013;73:1147–55. doi:10.1007/s40265-013-0079-2.
- Vuocolo S, Balmer P, Gruber WC, Jansen KU, Anderson AS, Perez JL, York LJ. Vaccination strategies for the prevention of meningococcal disease. Hum Vaccin Immunother. 2018;14:1203–15. doi:10.1080/21645515.2018.1451287.
- Public Health England. Invasive meningococcal disease in England: annual laboratory confirmed reports for epidemiological year 2017 to 2018. 2020 July 27. https://www.gov.uk/government/publications/meningococcal-disease-laboratory-confirmed-cases-in-england-and-wales.
- Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet (London, England). 2004;364:365–67. doi:10.1016/S0140-6736(04)16725-1.
- Larrauri A, Cano R, García M, Mateo S. Impact and effectiveness of meningococcal C conjugate vaccine following its introduction in Spain. Vaccine. 2005;23:4097–100. doi:10.1016/j.vaccine.2005.03.045.
- Baxter R, Keshavan P, Welsch JA, Han L, Smolenov I. Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants. Hum Vaccin Immunother. 2016;12:1300–10. doi:10.1080/21645515.2015.1136040.
- Keyserling H, Papa T, Koranyi K, Ryall R, Bassily E, Bybel MJ, Sullivan K, Gilmet G, Reinhardt A. Safety, immunogenicity, and immune memory of a novel meningococcal (Groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adoles Med. 2005;159:907–13. doi:10.1001/archpedi.159.10.907.
- Vesikari T, Peyrani P, Webber C, Van Der Wielen M, Cheuvart B, De Schrevel N, Aris E, Cutler M, Li P, Perez JL, et al. Ten-year antibody persistence and booster response to MenACWY-TT vaccine after primary vaccination at 1–10 years of age. Hum Vaccin Immunother. 2020;16:1280–91. doi:10.1080/21645515.2020.1746110.
- Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, MacNeil JR. Meningococcal vaccination: recommendations of the advisory committee on immunization practices, United States, 2020. Morb Mortal Wkly Rep. 2020;69:1–41.
- Áñez G, Hedrick J, Simon MW, Christensen S, Jeanfreau R, Yau E, Pan J, Jordanov E, Dhingra MS. Immunogenicity and safety of a booster dose of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in adolescents and adults: a Phase III randomized study. Hum Vaccin Immunother.2020;16:1292–98. doi:10.1080/21645515.2020.1733867.
- Vesikari T, Sandner B, Martinón-Torres F, Muzsay G, van der Vliet D, Bchir S, Neveu D, Jordanov E, Dhingra MS. Safety and immunogenicity of a quadrivalent meningococcal conjugate vaccine (MenACYW-TT) administered in healthy meningococcal vaccine naïve and MenC vaccine primed toddlers (12–23 months). 37th Annual Meeting of the European Society of Pediatric Infectious Diseases (ESPID); 2019; Ljubljana, Slovenia.
- Vesikari T, Borrow R, Forsten A, Findlow H, Dhingra MS, Jordanov E. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in healthy toddlers: a phase II randomized study. Hum Vaccin Immunother. 2020;16:1306–12. doi:10.1080/21645515.2020.1733869.
- Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, Arhin FF, Devi SJ, Frasch CE, Huang JC, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol. 1997;4:156–67. doi:10.1128/CDLI.4.2.156-167.1997.
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–72. doi:10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E.
- Baxter R, Reisinger K, Block SL, Izu A, Odrljin T, Dull P. Antibody persistence and booster response of a quadrivalent meningococcal conjugate vaccine in adolescents. J Pediatr. 2014;164:1409–15.e4. doi:10.1016/j.jpeds.2014.02.025.
- Dhillon S, Pace D. Meningococcal quadrivalent tetanus toxoid conjugate vaccine (MenACWY-TT; Nimenrix®): a review. Drugs. 2017;77:1881–96. doi:10.1007/s40265-017-0828-8.
- Gill CJ, Baxter R, Anemona A, Ciavarro G, Dull P. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo(R)) or Menactra(R) among healthy adolescents. Hum Vaccin. 2010;6:881–87. doi:10.4161/hv.6.11.12849.
- Vesikari T, Forsten A, Bianco V, Van der Wielen M, Miller JM. Immunogenicity, safety and antibody persistence of a booster dose of quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine compared with monovalent meningococcal serogroup c vaccine administered four years after primary vaccination using the same vaccines. Pediatr Infect Dis J. 2015;34:e298–e307.
- Vesikari T, Forsten A, Bianco V, Van der Wielen M, Miller JM. Antibody persistence up to 5 years after vaccination of toddlers and children between 12 months and 10 years of age with a quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine. Hum Vaccin Immunother. 2016;12:132–39. doi:10.1080/21645515.2015.1058457.
- Klein NP, Block SL, Johnston W, Percell S, Han L, Dull PM, Smolenov I. 1085: Persistence of meningococcal bactericidal antibodies and booster response at 60-months of age in children who received infant or toddler doses of MenACWY-CRM conjugate vaccine. Open Forum Infect Dis. 2014;1:S319–S. doi:10.1093/ofid/ofu052.793.
- Bröker M, Berti F, Schneider J, Vojtek I. Polysaccharide conjugate vaccine protein carriers as a “neglected valency” – potential and limitations. Vaccine. 2017;35:3286–94. doi:10.1016/j.vaccine.2017.04.078.