ABSTRACT
Beyond the overall relapse-free survival (RFS) advantage demonstrated in randomized trials (RCT) of adjuvant anti-PD-1 immunotherapy in radically resected stage III–IV melanoma, key issues about subgroups of interest have been raised in recent years, with non-conclusive results when considering single studies. In the present meta analysis, we pooled all RCT data in this setting, analyzing, overall, 3043 patients. The RFS benefit of adjuvant immunotherapy over the comparator (placebo or anti-CTLA-4) was strongly confirmed in the pooled analysis, and it was statistically significant in most subgroups, excluding patients with stage IIIA and stage IV M1c melanoma. Nevertheless, the relative benefit was not statistically significantly different when considering their IIIB-IIIC and M1a-M1b counterparts. Future trials in this setting should consider subgroups of interest for tailoring the adjuvant strategy in terms of duration and drug combination in light of literature data.
Introduction
A few years ago, the therapeutic path of patients undergoing resection of infiltrating melanoma was concluded with complete lymph node dissection (CLND) in cases with metastatic sentinel lymph node.Citation1,Citation2 Only a limited subgroup of patients were candidates to adjuvant therapy with high- or low-dose interferon-α, being the only available systemic intervention to prevent the distant relapse in this disease.Citation3–6 In both cases, the outcome in terms of overall survival (OS) was not improved in the majority of studies, and the benefit in terms of relapse-free survival (RFS) was not consistent across several prospective trials, leading to the current negative recommendation of guidelines in the case of CLND and the frequent abandonment of the adjuvant strategy with interferon-α.Citation7
In 2015, the U.S. Food and Drug Administration (FDA) approved the anti-CTLA-4 ipilimumab as adjuvant therapy for stage III melanoma patients. The approval was granted based on the first pivotal trial in this setting, demonstrating the advantage of ipilimumab over placebo in obtaining longer RFS, higher rates of OS, and distant metastasis-free survival (DMFS) than placebo after surgery.Citation8 Since then, two anti-PD-1 immune checkpoint inhibitors (ICI) have been tested versus placebo or ipilimumab itself, to reduce the risk of recurrence following radical resection of melanoma, in certain cases also including metastatic patients with stage IV disease, rendered disease-free with radical surgery.Citation9–11 To date, the approved ICI drugs in this setting include nivolumab and pembrolizumab, becoming the new standard of therapy, even in the lack of adequate follow-up. Moreover, definite OS results are still missing, suggesting the likely comparable survival gain for the anti-CTLA-4 and the anti-PD-1 strategy, but with a toxicity profile favoring the latter.Citation9,Citation12–14
Beyond the clear overall advantage demonstrated in each trial, key issues about subgroups of interest have been raised in recent years, especially in sight of the radical update of the American Joint Committee on Cancer (AJCC) staging system for this disease, from the 7th version to the 8th, partially reclassifying the stages included in the adjuvant trials.Citation15–17 One of the crucial issues is the inclusion of the current IIIA stages in the adjuvant immunotherapy indication; another is the inclusion of patients with microsatellite only (without nodal involvement); another one is the effectiveness of adjuvant ICI in patients with BRAF-mutated melanoma. In a single trial, the subgroup analyses could underestimate the advantages of experimental therapies due to the limited sample size of the subgroup of interest and the wide margins of uncertainty demonstrated by the confidence intervals.
In this review, we selected all randomized controlled clinical trials investigating the use of ICI immunotherapy in the adjuvant setting for patients with melanoma after surgical radicalization, performing a meta analysis with RFS as the primary endpoint, to offer more robust evidence on the adjuvant indication. Moreover, we performed subgroup meta analysis to improve the statistical power for subgroups of interest, to support with empowered evidence the use of adjuvant immunotherapy in special populations.
Methods
Search strategy and inclusion criteria
We followed PRISMA guidelines for this systematic review and meta-analysis. We searched PubMed for randomized controlled trials published in English language from each database’s inception to November 21, 2020. Two investigators (FP and MB) independently searched the databases. The search terms were “adjuvant” AND “melanoma” AND “immune checkpoint inhibitor” OR “anti-PD-1”. We also reviewed the references of the included article for any further potential publication. Eligible studies had to be: (1) randomized trials assessing ICI alone or in combination for the adjuvant treatment of patients with any stage melanoma and (2) had to have available or calculable hazard ratios (HRs) for relapse according to patients’ clinical subgroups (where RFS was compared between treated vs not treated with immunotherapy in any subgroups). We excluded non-randomized trials, non-cutaneous melanoma, and trials having other drugs as experimental arms. Two investigators (FP and MB) independently reviewed the retrieved articles to select the relevant articles, and any disagreement was resolved with the consensus of a third investigator (SB). Three reviewers (MB, SB, and FP) independently extracted data from the studies, and all discrepancies were resolved by consensus with all investigators.
Data extraction and quality assessment
From each study, SB and FP extracted the first author and year of publication, study phase, type of malignancy, number of patients, age, sex, stages, ulceration/nodal status, median follow-up, study arms, HR for RFS according to patients’ characteristics (when available). We included the most updated report of any trials when duplicate publications were identified.
Statistical analysis
The primary endpoint was the difference in patients’ outcome to ICI between different subgroups measured in terms of HR for RFS reported for these subgroups. Depending on available data, we applied subgroup analysis by stage (IIIA, IIIB-C, or IV), nodal status (N0 or N+), age (0–64 vs 65+ years), sex, presence of ulceration, BRAF status, PD-L1 expression. We extracted the HRs for relapse in the intervention group and control group and their 95% CIs from each study, separately for the different subgroups. We calculated the pooled HRs of RFS using the random-effects models. We assessed the heterogeneity between the two estimates using an interaction test to give P for heterogeneity. We did the Q-test to assess between-study heterogeneity and calculated the I2 statistic, which expresses the percentage of the total observed variability due to study heterogeneity. The null hypothesis was that the interaction between the covariates and immunotherapy efficacy is equal across subgroups and was tested with a χ2 test. All reported P values are two-sided. The analyses were performed with Review Manager (RevMan) Version 5.3 (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014).
Results
After selecting the pertinent publications, a total of n = 4 randomized studies were aggregated in the quantitative analysis according to the inclusion criteria (). Overall, n = 3043 patients were analyzed. Among the included studies (), three were Phase III randomized trials, Citation12–14 and one was a Phase II randomized study.Citation11 One of the studiesCitation11 was considered separately for nivolumab plus ipilimumab vs placebo and nivolumab vs placebo, respectively. Three had the placebo as the comparator in the control arm; only oneCitation13 had the anti-CTLA-4 ipilimumab as the control treatment.
Table 1. Studies included in the present review and meta analysis
Figure 1. Flow diagram of the study selection process for the qualitative and quantitative analysis.
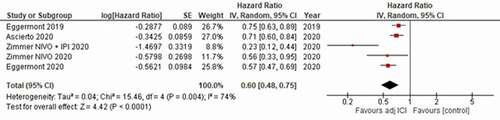
Overall, the pooled analysis showed a significant RFS benefit for adjuvant ICI against the control arms, with a hazard ratio (HR) of 0.60 [95% confidence interval (CI) 0.48–0.75]; p < .0001 (). The heterogeneity of the included studies was significant (P = .004, I2 = 74%).
Figure 2. Forest plot resulting from the meta analysis of the included studies for the primary endpoint of relapse free survival (RFS).
According to subgroup meta analysis, a statistically significant RFS benefit from ICI adjuvant therapy was confirmed across subgroups () considering sex (male vs female), age (elderly vs younger, cutoff 65 years), BRAF mutational status (BRAF mutated vs wild-type), PD-L1 expression (negative vs positive, where available; different cutoff at 1% or 5%), ulceration (present vs absent). None of the tests evidenced a significant difference among these subgroups, demonstrating that the interaction between the covariates and immunotherapy efficacy was equal across subgroups of interest.
Figure 3. Subgroup meta analysis for sex (a), age (b), BRAF mutational status (c), PD-L1 expression (d), ulceration (e), stage (f), M substage (g).

Two subgroups did not reach statistically significant RFS benefit from adjuvant ICI immunotherapy: those of patients with stage IIIA melanoma according to the AJCC 7th ed., Citation9 (HR 0.74 [95% CI 0.47–1.17], p = .20; ) and with stage IV disease M1c (HR 0.58 [95% CI 0.23–1.51], p = .27; ). Nevertheless, the test for subgroup difference was not statistically significant in both cases.
Discussion
This meta-analysis confirmed a significantly improved RFS in patients with radically resected stage IIIA or worse melanoma treated with ICI compared with placebo/comparator. The overall estimate (HR = 0.60) strengthens the encouraging results of the individual trials.Citation8–14 Moreover, the significant efficacy of ICI was confirmed in sub-analysis with relevant effects among both women and men, young and elderly patients, in wild type and mutated BRAF types, in positive and negative PD-L1, for ulcerated and not ulcerated melanomas, in stage IIIB-C, and IV (M1a-b).
Regarding stage IIIA, it was available in two of the analyzed studies and with a relatively limited sample size: overall, 175 treated patients vs 163 controls were pooled in the present analysis. The HR point estimate showed a better RFS for ICI (0.74) but the confidence intervals overlapped the unit. Stage III is expected to have a central role in the clinical debate about translating experimental results on ICI efficacy in the real-world. In fact, all the four analyzed trials included patients according to the 7th version of the AJCC staging system. Unfortunately, the current (8th) AJCC version has a variable agreement with the previous one.Citation15,Citation18 An extremely poor agreement has been documented in real-world populations for stage III (Κ = 8.1%), due to the shift of former IIIB into IIIA.Citation18 The majority of studies’ recurrent pitfall in this setting is the patient selection based on AJCC 7th edition; moreover, the 8th update was based on data gathered when checkpoint inhibitors were not used as adjuvant therapy in stage III melanoma. Of note, recent evidence demonstrated that AJCC-8 staging had a robust prognostic importance for RFS but no predictive importance toward adjuvant immunotherapy.Citation19 Studies involving greater sample sizes are needed to fully understand the real efficacy of ICI in patients with stage IIIA melanoma.
Also, in patients with stage IV M1c melanoma, 56 treated cases and 37 controls were available across three trials, with an extremely limited sample: consequently, even in the pooled analysis, the results for this subgroup are not conclusive. On the other hand, at least one of the RCT included had negative results for this subgroupCitation13 and, moreover, the lack of benefit in the meta analysis could be due to the early discontinuation of ICI treatment after 1 year, as provided by the majority of RCT, probably not enough for such high-risk patients.
The pooled analysis’s usefulness to confirm significant RFS benefit, despite single-trial data not reaching statistically significant subgroup results, emerged for the subgroups of elderly and patients with PD-L1-negative tumors. Previously, at least two of the four trials considered reported non-statistically significant RFS benefit for adjuvant ICI in these subgroups of interest.Citation11,Citation12,Citation14 Our results finally confirm statistically significant and clinically meaningful benefit (absolute decrease of relapse of 28% and 50%, respectively) for the elderly and patients with PD-L1-negative melanoma.
Despite the unreliability of a comparative analysis regarding the safety of different adjuvant ICI regimens, indirect comparison of literature data about immune-related adverse events (irAEs) in these RCTs allows considering anti-PD-1 monotherapy as the best option in terms of tolerability (both over combinations and over single-agent anti-CTLA-4). On the other hand, the statistical strength of the results obtained in each of the analyzed subgroups with the combination of ipilimumab and nivolumab, in a single trial, with a huge improvement of RFS across all patients when compared to placebo, is undoubtedly attractive even in the face of greater toxicity.Citation11
Finally, considering the possibility of different adjuvant treatment choices for patients with BRAF-mutated melanoma, Citation20 the present meta-analysis provides evidence that the expected magnitude of benefit from ICI adjuvant therapy is maintained in this population. The strength of each single-trial subgroup is overcome with 963 patients BRAF-mutated melanoma included in our analysis (see ), confirming the efficacy of the immunotherapeutic strategy in this setting and offering the opportunity of basing the adjuvant treatment choice on the toxicity profile according to the patient comorbidities
The limitations of the current study are represented by the following: significant heterogeneity among RCT included, with various comparator arms (placebo or anti-CTLA-4 active therapy) and different inclusion criteria (i.e., stage III only or stage IV included); relatively limited sample size for specific subgroups; outdated AJCC version used for the trial inclusion criteria; relatively small numerosity of RCT published in this setting for melanoma patients.
Conclusion
The benefit of ICI-based adjuvant immunotherapy for radically resected melanoma patients was confirmed in this pooled analysis of all randomized trials in this setting, with no significant differences across subgroups. The prolongation of therapy over 1 year could represent the possible evolution of the adjuvant approach in future trials for radically resected stage IV melanoma, and the selection of high-risk patients suitable to be candidates to anti-PD-1/anti-CTLA-4 combinations instead of a monotherapy. Eventually, an unsolved issue in the field of adjuvant immunotherapy in melanoma is represented by the lack of data about patients who did not undergo radical lymph node dissection in the case of sentinel biopsy positivity, currently dramatically increasing in clinical practice, but still missing in pivotal clinical trials.
Disclosure of potential conflicts of interest
Melissa Bersanelli received research funding from Seqirus UK, Pfizer, Novartis, BMS, Astra Zeneca, Roche S.p.A., and Sanofi Genzyme; honoraria as speaker at scientific events by Bristol-Myers Squibb (BMS), Novartis, Astra Zeneca, and Pfizer and as consultant for advisory role by Novartis, BMS, and Pfizer; she also received fees for copyright transfer by Sciclone Pharmaceuticals.
Sebastiano Buti received honoraria as speaker at scientific events and advisory role by Bristol-Myers Squibb (BMS), Pfizer; MSD, Ipsen, Roche, Eli-Lilly, AstraZeneca and Novartis; he also received research funding from Novartis.
Ignazio Stanganelli received honoraria as speaker at scientific meetings by BMS, Novartis, MSD.
The other authors have no conflict of interest to declare.
References
- Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, Jahkola T, Bowles TL, Testori A, Beitsch PD, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017 June 8;376(23):2211–6. doi:https://doi.org/10.1056/NEJMoa1613210.
- Leiter U, Stadler R, Mauch C, Hohenberger W, Brockmeyer N, Berking C, Sunderkötter C, Kaatz M, Schulte K-W, Lehmann P, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016 June;17(6):757–67. doi:https://doi.org/10.1016/S1470-2045(16)00141-8.
- Cascinelli N, Belli F, MacKie RM, Santinami M, Bufalino R, Morabito A. Effect of long-term adjuvant therapy with interferon alpha-2a in patients with regional node metastases from cutaneous melanoma: a randomised trial. Lancet. 2001 Sept 15;358(9285):866–69. doi:https://doi.org/10.1016/S0140-6736(01)06068-8.
- Garbe C, Radny P, Linse R, Dummer R, Gutzmer R, Ulrich J, Stadler R, Weichenthal M, Eigentler TK, Ellwanger U, et al. Adjuvant low-dose interferon {alpha}2a with or without dacarbazine compared with surgery alone: a prospective-randomized phase III DeCOG trial in melanoma patients with regional lymph node metastasis. Ann Oncol. 2008 June;19(6):1195–201. Epub 2008 Feb 14. doi:https://doi.org/10.1093/annonc/mdn001.
- McMasters KM, Egger ME, Edwards MJ, Ross MI, Reintgen DS, Noyes RD, Martin RCG, Goydos JS, Beitsch PD, Urist MM, et al. Final results of the sunbelt melanoma trial: a multi-institutional prospective randomized phase III study evaluating the role of adjuvant high-dose interferon Alfa-2b and completion lymph node dissection for patients staged by sentinel lymph node biopsy. J Clin Oncol. 2016 Apr 1;34(10):1079–86. doi:https://doi.org/10.1200/JCO.2015.63.3776.
- Eggermont AM, Suciu S, MacKie R, Ruka W, Testori A, Kruit W, Punt CJ, Delauney M, Sales F, Groenewegen G, et al. Post-surgery adjuvant therapy with intermediate doses of interferon alfa 2b versus observation in patients with stage IIb/III melanoma (EORTC 18952): randomised controlled trial. Lancet. 2005 Oct 1;366(9492):1189–96. doi:https://doi.org/10.1016/S0140-6736(05)67482-X.
- National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology (NCCN Guidelines, US). Version 1.2021. Melanoma: Cutaneous; 2020 Nov 25. [accessed 2021 Feb]. www.NCCN.org.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–55. doi:https://doi.org/10.1056/NEJMoa1611299.
- Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–35. doi:https://doi.org/10.1056/NEJMoa1709030.
- Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A, Carlino MS, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–801. doi:https://doi.org/10.1056/NEJMoa1802357.
- Zimmer L, Livingstone E, Hassel JC, Fluck M, Eigentler T, Loquai C, Haferkamp S, Gutzmer R, Meier F, Mohr P, et al. Nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;395(10236):1558–68. doi:https://doi.org/10.1016/S0140-6736(20)30417-7.
- Eggermont AMM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur J Cancer. 2019;119:1–10. doi:https://doi.org/10.1016/j.ejca.2019.07.001.
- Ascierto PA, Del Vecchio M, Mandalá M, Gogas H, Arance AM, Dalle S, Cowey CL, Schenker M, Grob -J-J, Chiarion-Sileni V, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(11):1465–77. doi:https://doi.org/10.1016/S1470-2045(20)30494-0.
- Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson VG, Dalle S, Haydon AM, Meshcheryakov A, Khattak A, Carlino MS, et al. Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk Stage III melanoma: updated results from the EORTC 1325-MG/KEYNOTE-054 trial. J Clin Oncol. 2020;38(33):3925–36. doi:https://doi.org/10.1200/JCO.20.02110.
- Elmore JG, Elder DE, Barnhill RL, Knezevich SR, Longton GM, Titus LJ, Weinstock MA, Pepe MS, Nelson HD, Reisch LM, et al. Concordance and reproducibility of melanoma staging according to the 7th vs 8th edition of the AJCC cancer staging manual. JAMA Netw Open. 2018 May;1(1):e180083. doi:https://doi.org/10.1001/jamanetworkopen.2018.0083.
- Edge SB, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC cancer staging manual. 7th ed. New York (NY): Springer; 2010.
- Amin MB, Edge SB, Greene FL, Byrd DR, eds. AJCC cancer staging manual. 8th ed. New York (NY): Springer; 2017.
- Crocetti E, Stanganelli I, Mancini S, Vattiato R, Giuliani O, Ravaioli A, Balducci C, Falcini F, Pimpinelli N. Evaluation of the agreement between TNM 7th and 8th in a population-based series of cutaneous melanoma. JEADV. 2019;33:521–24. doi:https://doi.org/10.1111/jdv.15285.
- Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson VG, Dalle S, Haydon A, Lichinitser M, Khattak A, Carlino MS, et al. Prognostic and predictive value of AJCC-8 staging in the phase III EORTC1325/KEYNOTE-054 trial of pembrolizumab vs placebo in resected high-risk stage III melanoma. Eur J Cancer. 2019 July;116:148–57. doi:https://doi.org/10.1016/j.ejca.2019.05.020.
- Dummer R, Hauschild A, Santinami M, Atkinson V, Mandalà M, Kirkwood JM, Chiarion Sileni V, Larkin J, Nyakas M, Dutriaux C, et al. Five-year analysis of adjuvant dabrafenib plus trametinib in stage III melanoma. N Engl J Med. 2020 Sept 17;383(12):1139–48. doi:https://doi.org/10.1056/NEJMoa2005493.
