ABSTRACT
The licensed HPV vaccines are highly efficacious and induce high levels of neutralizing antibody levels, the assumed mediators of protection. However, a correlate of protection against HPV is lacking, and the evidence is still limited as to long-term persistence of antibodies, especially following reduced dosing schedules. The World Health Organization (WHO) urges immunization of young girls as part of the strategy to eliminate cervical cancer, thus long-lasting protection is required. The current review describes long-term follow-up regarding vaccine-induced seropositivity and antibody level development following the different vaccines and dosing schedules. Implications and opportunities of long-term vaccine-induced immune responses are discussed, such as the gaps in monitoring of long-term immunogenicity, the possibilities of reduced dosing schedules, and the importance of evidence for durable immunity.
Introduction
The human and non-human papillomaviruses can be subdivided into genera. This review focusses on human papillomavirus (HPV) types from the alpha-genus, which are able to infect the human genital tract where they may cause disease.Citation1 Within this genus, high-risk (hr) and low-risk (lr) types can be distinguished; when hrHPV type infections persist, they have the potential to cause the development of cervical (pre-) cancer, whereas lrHPV types are associated with anogenital warts.Citation2,Citation3 However, the vast majority of the infections are asymptomatic and clear spontaneously.
HPV is a very common sexually transmitted infection with an estimated cumulative lifetime risk of 80% in Western countries.Citation4,Citation5 To prevent HPV infection and ultimately preclude the development of cervical cancer, prophylactic HPV vaccines have been developed. The first two were licensed in 2006 and 2007, respectively. The first is the bivalent vaccine (2vHPV), Cervarix®),Citation6 which targets the most important hrHPV types 16 and 18.The second is the quadrivalent vaccine (4vHPV), Gardasil®,Citation7 which targets HPV types 6,11,16, and 18. In 2014, a vaccine targeting these four types plus five additional hrHPV types was licensed: the nonavalent vaccine (9vHPV), Gardasil9®.Citation8 In recent years, vaccine registration has been expanded to protection against non-cervical HPV-associated disease (including other anogenital cancers) and to males as well as females.
High efficacy of the HPV vaccines against cervical infections and lesions was shown by randomized controlled trials (RCTs), indicating protection up to 98% against virological and clinical endpoints caused by the targeted vaccine types.Citation9 These findings were reiterated in observational research following implementation of HPV vaccines in national immunization programs. For example, a large meta-analysis in high-income countries showed an 83% reduction of HPV16/18 prevalence among girls aged 13–19 y comparing pre- and post-vaccination implementation periods up to 8 y following implementation.Citation10 This reduction was likewise observed in field efficacy studies; in Australia, the prevalence of HPV16/18 was flat across age groups following vaccination,Citation11 whereas in Sweden, a substantial risk reduction for cervical cancer was observed among vaccinated women.Citation12 Furthermore, HPV vaccines are known to be highly immunogenic able to provoke a solid systemic immune response, especially through the formation of antibodies.Citation13 Virtually all HPV-vaccinated individuals seroconvertCitation14 and RCTs found peak antibody levels in vaccinated individuals up to 100-fold higher compared to naturally infected individuals.Citation15
HPV vaccines can be provided according to a three-dose schedule (0,1–2, and 6 months), currently recommended for those 15 y and above or two-dose schedule (0, 5–13 months), recommended for those 9–14 y of age. After the initial registration of the HPV vaccines according to three-dose schedules, new evidence implied that two doses induce protection equally well. The underlying concept is known as immunobridging: Efficacy against virological and clinical endpoints was first observed among 15–26-y-old women who were vaccinated three times. Among 9-to-14-y-old girls who were vaccinated twice, non-inferior serum antibody responses were observed as compared to the three-dose-vaccinated women. Immunobridging assumes the same efficacy can be expected in groups where non-inferior antibody responses are found. Hence, from 2014 onwards, two–dose schedules were approved and advised for young vaccine recipients by the Food and Drug Administration (FDA), European Medicines Agency (EMA), and World Health Organization (WHO).Citation6–8
Registered HPV vaccines are prophylactic and thus provide optimal protection among HPV-naïve individuals. Since the prevalence of HPV infection starts to rise from the beginning of sexual activity, vaccination in young adolescence is preferable, feasible, and pursued in most countries where HPV vaccination has been implemented. For optimal benefit, the induced protection should ideally cover the entire life period of sexual activity. As of 2018, the WHO has committed to elimination of cervical cancer as a global health problem. To reach this goal, one of the targets proposed for countries is to have 90% of girls fully vaccinated against HPV by age 15.Citation16 While neutralizing antibodies are the supposed primary mediators of the protection, the minimum level required for protection has not yet been established, nor has the duration of protection.Citation17 In this review, the current knowledge on long-term immunogenicity following HPV vaccines is described and the implications for global HPV reduction are discussed.
Measurement units and assays
We can roughly distinguish three types of assays that are commonly used in RCTs to evaluate HPV antibodies following vaccination: the pseudovirus-based neutralization assay (PBNA), competitive (epitope-specific) immunoassays (cLIA), and VLP-IgG binding assay (ELISA or MIA). The first is considered relevant for measuring the biological activity, whereas the cLIA reflects neutralizing activity with high affinity. ELISA detects all antibodies regardless of neutralization.Citation18 Respectively, the methods require pseudovirions, type-specific monoclonal antibodies, and/or intact VLPs, and their quality affects the individual assay quality. WHO suggests PBNA was as the reference standard for assessing HPV-specific neutralizing antibodies. This method is very time-consuming and costly, but a recently developed high-throughput PBNA assay allows more assays to be processed sequentially and also has increased sensitivity.Citation19 On the other hand, cLIA and ELISA/MIA are fast and suitable for high-throughput but, measure, respectively, a subset of total neutralizing antibodies and the total amount of HPV-specific antibodies.
Variations on these three assays are used in studies because reagents and assay standards are not always available and there are no official guidelines on methods for determining cutoffs. This use of varied techniques gives rise to variation in findings. For example, a comparison study showed that the PBNA is more sensitive than IgG-cLIA for the detection of HPV16- and HPV18-neutralizing antibodies.Citation20 Another study indicated that assays showed reasonable correlation, but that improvement in correlation could be achieved by small alterations.Citation21 To overcome such problems, the International Unit (IU) measure has been established for HPV16 and 18. It should be used to express findings in order to facilitate comparability,Citation18 but not all studies use standardized measurements, and the IU for hrHPV types other than 16 and 18 has yet to be established.
Outcomes used to describe the immunogenicity of the HPV vaccines include the geometric mean concentration or titer (GMC/T), or the percentage of seropositives (i.e., number of study participants with an antibody level above a certain cutoff level). Both provide information on the long-term performance of the vaccine regarding stimulation of antibody production. Arbitrary study-specific cutoffs have been applied to determine seropositivity.Citation13 Additionally, antibody avidity can be used as a marker to express affinity maturation, i.e., how well an antibody binds to an antigen. This can for instance be measured with the chaotropic thiocyanate ion method in the ELISA/MIA assays.Citation22,Citation23 Nevertheless, avidity is considered a crude marker for affinity maturation and should therefore be interpreted with caution.
Biological mechanisms underlying HPV vaccination
All three prophylactic HPV vaccines now on the market consist of virus-like particles (VLPs), although these are produced in various expression systems. Also, the vaccines differ in their adjuvant systems (). The VLPs resemble the L1 protein of HPV, the major capsid protein, which is morphologically indistinguishable from real HPV particles.Citation24 All the vaccines contain aluminum salts as an adjuvant to ensure a slow release of the antigen and activation of the innate immune system. However, the 2vHPV vaccine uses the AS04 adjuvants system, which contains both aluminum salt and monophosphoryl lipid A (MPL), which is believed to activate the innate immune response.Citation25
Table 1. Characteristics of the three available HPV VLP vaccines
In animal models, vaccination with L1 VLPs has been shown to induce neutralizing antibody levels and protection against an HPV infection.Citation26 After passive transfer of immune sera, naïve animals were protected against infection.Citation17 These findings were supportive of the general assumption that protection following HPV vaccination is primarily antibody-mediated. Even more supportive were trials in human participants, which showed high and durable, type-restricted titers of VLP antibodies after vaccination.Citation22
Naïve B- and T-cell activation is important in HPV antibody production. Upon entering the body, the VLP antigen part of the vaccine is bound to antigen-presenting cells (APCs). The antigen is then presented to T-cells, which have various functions and can differentiate into one of several T-cell lineages, including cytotoxic, T-helper, or memory T-cells. The T-helper cells in turn stimulate naive B-cells to become either plasma cells or memory B-cells.Citation27 Long-lived plasma cells (LLPC) are generated upon vaccination and secrete antigen-specific antibodies, thereby enabling the persistence of circulating antibodies. However, circulating memory B-cells can be still detected after vaccination and could therefore also assist in a rapid recall when the HPV antigen is encountered again.Citation28 Thus, LLPCs, memory B- and T-cells are essential for establishing long-term protection, i.e., by inducing and maintaining high levels of neutralizing antibodies.
Neutralizing antibodies are the assumed mediators of protection following HPV vaccination. They can bind to a virion and prevent it from binding to a cell, thereby neutralizing the toxin. There are various isotype forms of antibodies, IgG and IgA, which can be further subdivided. After HPV vaccination the subclasses IgG1 and IgG3 are most frequently detected.Citation17 Serum antibodies are thought to arrive at the side of infection via exudate (antibody leak from a damaged blood vessel or membrane) and/or transudate (antibody transfer from the intravascular compartment due to an imbalance of hydrostatic or oncotic pressure or though antibody-transporting receptors) to block HPV binding to the basement membrane.Citation29 To date, no correlate of protection has been established for HPV, as vaccine efficacy after HPV vaccination is high, with few breakthrough infections and hence few vaccinated individuals who are infected with vaccine types. This limits the opportunities to study which antibody levels are needed to give adequate protection. Also, studies might be biased by the difficulties in distinguishing rare breakthrough infections from emergence of prevalent infection at the time of vaccination or reactivation of latent infectionCitation30
Unvaccinated individuals acquiring an HPV infection can likewise develop an immune response. However, detectable antibody levels are not always present after a natural infection (not everyone seroconverts), and it is not known whether a previous infection protects against subsequent exposure to the same HPV type.Citation31 Nonetheless, GMC/Ts reached among naturally infected individuals can provide benchmarks for the evaluation of antibody levels after vaccination.Citation32
Long-term immune responses following three doses of HPV vaccination
Seropositivity rates
Given the initial registration for HPV vaccination, the longest follow-up has been reported in studies adhering to this schedule. In , an overview is given of RCTs conducted for the three different vaccines with follow-up of seropositivity rates. Diverse study populations have been included, both younger and older age groups and women and men. For all three vaccines, follow-up was at least 7.5 y, and the longest was 14 y for the 4vHPV vaccine. ELISA and cLIA were the assays commonly used to assess antibody levels. Although high seroprevalence rates were maintained, a slight decline was observed with increased follow-up, notably for HPV18 after 4vHPV (47.9% seropositive after 4 y).Citation44 Previous research indicated a decline in seropositivity for HPV18 after 4vHPV vaccination, but no breakthrough infections/lesions were reported.Citation48 However, follow-up may have been too short and statistical power too limited to fully examine this.
Table 2. Long-term seropositivity and geometric mean concentration (GMC) following three doses of HPV vaccination (in RCTs).Citation33–47.
Long-term seropositivity appears to be higher when the vaccine is administered at a younger age as compared to older populations, especially those 25 y or older.Citation43 In general, males and females were comparable regarding achieved seropositivity rates, but small differences have been observed.Citation46 This might be due to different populations across studies or minor differences in immune response following vaccination.Citation49 The 2vHPV and 4vHPV vaccine also induce some cross-protection against non-targeted hrHPV types.Citation50 For example, after 2vHPV vaccination, seropositivity rates for HPV31 and HPV45 were slightly lower compared to the 9vHPV vaccine and were maintained until at least 10 y.Citation42 For the 4vHPV vaccine also, cross-protection for these types was observed, although seropositivity rates were slightly lower than for the 2vHPV vaccine.Citation51,Citation52
Similar to RCTs, observational studies have been conducted to monitor the long-term immunogenicity following the implementation of HPV vaccination into national immunization programs. High seroprevalence for HPV16 and HPV18 up to 12 y after 2vHPV vaccination was observed.Citation30,Citation53 Also for cross-protective types HPV31, 33 and 45, persisting seropositivity rates up to 12 y were reported.Citation53 For the 4vHPV vaccine, comparable vaccine-type immunogenicity following three doses was shown in observational studies.Citation54 Regarding cross-reactivity, seropositivity rates were generally lower for non-vaccine types following 4vHPV compared to 2vHPV vaccination.Citation55 A 5-y observational follow-up study of the 9vHPV vaccine indicated long-term seropositivity for all types included in the vaccine, with patterns comparable to those in RCTs.Citation56
Antibody levels over time
Antibody levels against HPV16/18 and cross–protective types over time are reported from an observational cohort study in which 15–16-y-old girls received three doses of 2vHPV vaccination ().Citation30 An initial peak is observed, followed by a rapid, significant decline for the vaccine types between y 2 and 3 post-vaccination. Thereafter a more gradual antibody decline is observed as time progresses. Antibody levels against cross-protective types show a comparable pattern at a lower level. GMCs against HPV16 are higher than for other types from the start and remain so over time.Citation30
Figure 1. Adapted from Hoes et al.Citation30 with 1 additional y of follow-up. Geometric mean concentration (GMC) of HPV16, 18, 31, 33, and 45 IgG before and every year after 3 doses of 2vHPV vaccination (declining number of women due to loss-to-follow–up).
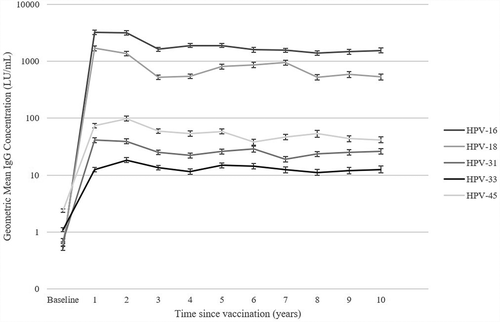
In , GMC/Ts from the RCTs are included if reported. Although various arbitrary measurement units were used, the pattern of antibody level development for all the vaccines and HPV types is comparable to the one described above.Citation42,Citation45–47,Citation57,Citation58 However, the initial decline in HPV18 following both the 4vHPV and 9vHPV vaccination seems more pronounced as measured by cLIA assay (less so in the total binding assay), which could also explain the observed faster decline in seropositivity rates. Furthermore, there were small deviations between the 4vHPV and 9vHPV vaccine that could be related to changes in the VLP concentration. Regarding cross-protective types, the observed responses are higher following 2vHPV compared to 4vHPV vaccination.Citation59 In the first 7 months post-vaccination, the 2vHPV vaccine induced neutralizing HPV31/33/45/52/58 antibodies significantly more often and to higher levels than did the 4vHPV vaccine.Citation52 Neutralizing antibodies remained detectable up to at least 7–12 y post-vaccination, but with expected three- to fourfold higher titers after 2vHPV vaccination than 4vHPV vaccination.Citation55,Citation60 Evidenced by clinical trial data, cross protection against HPV31 and HPV45 and subsequent lesions indeed seems to be higher following 2vHPV compared to 4vHPV vaccination,Citation50 which could be due to the observed higher antibody levels.
Statistical modeling studies indicated that among young girls (10–14 y) receiving timely 2vHPV vaccination, durability of antibody levels above natural infection level was predicted to be 70.1 y for anti-HPV-16 and 78.8 y for anti-HPV–18, or even lifelong, depending on the model used.Citation42 Another modeling study among older women receiving HPV vaccination (15–55 y) indicated that antibody levels after 2vHPV vaccination for vaccine types HPV16 and 18 would remain higher than after natural infection for up to 30 y. However, the age at which participants received vaccination was important, as with older age the predicted prolonged immune response decreased, probably due to lower initial antibody responses.Citation43 For the 4vHPV vaccine, the predicted GMTs up to 20 y after vaccination were also above the level induced by natural infection for anti-HPV-16 antibodies but below the natural infection level for anti-HPV–18 antibodies (among 18–45-y-olds). In general, longer durability of antibodies was predicted following 2vHPV than 4vHPV vaccination.Citation61 This is likely due to the high initial antibody levels after 2vHPV vaccination.
Some RCTs compared the different vaccines directly. In general, they showed that the 2vHPV vaccine induces higher antibody responses for both HPV16 and 18 compared to the 4vHPV vaccine (up to threefold higher as measured by PBNA),Citation62,Citation63 while 9vHPV vaccination induced anti-HPV16/18 responses similar to the 4vHPV vaccine, as measured by cLIA.Citation9,Citation64 Especially, the response against HPV18 differs between the 2vHPV and 4vHPV vaccine, as the 4vHPV vaccine was less immunogenic for HVP18 (shown in lower GMTs in the first year following vaccination).Citation62 This difference persisted with increased follow-up.Citation65 A comparison trial between the 2vHPV and 9vHPV vaccine is ongoing (NCT02834637).
Other immune parameters
Besides seroprevalence and antibody levels, other immune parameters may provide an indication of long-term protection following HPV vaccination. Research showed that avidity levels of antibodies against vaccine types increased with every dose of 2vHPV vaccination, peaked after the third, and remained relatively constant up to 3 y post-vaccination.Citation66 Nevertheless, a correlation between avidity and neutralizing antibody levels could not be established, suggesting that neutralizing activity of antibodies is relatively independent of their avidity (once a threshold level is reached following primary vaccination).Citation66 Furthermore, a study comparing two and three doses of 2vHPV vaccination indicated no differences in avidity up to four and a half years post-vaccination,Citation67 leaving the correlation between avidity and number of doses inconclusive.
Another parameter that is less well studied is cellular immunity following HPV vaccination. Research showed that both the 2vHPV and 4vHPV vaccines give an HPV-specific memory B- and T-cell responseCitation68,Citation69 up to at least 4 y post-vaccination.Citation63 Age at vaccination was found to impact memory B-cell formation, whereas T-cell memory formation influenced by dose number but not by age of vaccination.Citation70 Recipients of the 2vHPV vaccine showed higher numbers of memory B-cells after vaccination compared to those receiving 4vHPV vaccine.Citation63,Citation71 Likewise, for HPV31 and HPV45 numbers of both memory T- and memory B-cells were detected up to 36 months post-vaccination for the 2vHPV vaccine. Again, this level of cross-protection was higher for the 2vHPV compared to the 4vHPV vaccine.Citation72 Generally speaking, although cell-mediated immune effectors provide information on the responsiveness to the vaccine, they do not directly indicate how well the vaccine protects or how long antibodies are maintained (which is due to the production by LLPCs).Citation22 Nevertheless, further research on the long-term persistence of memory B- and T-cells could provide an indication for the sustainability of protection from disease, as is the case in hepatitis B studies.Citation73
Immune responses following fewer than three doses of HPV vaccination
Non-inferiority of the two-dose schedule
To compare immune responses for various dosing schedules, GMT/C ratios are often used (in combination with non-inferiority margins). For all three vaccines, RCTs comparing two (at the required 0-, >5-month interval)- and three-dose schedules have been conducted indicating non-inferiority of the two-dose schedule regarding the vaccine types (among girls aged 9–14 y) according to study-dependent cutoffs, when compared to a three-dose schedule (in women aged 15–26 y).Citation74–76 The longest follow-up with direct comparison of doses was measured for the 4vHPV vaccine (up to 10 y post-vaccination), showing sustained immunogenicity for HPV6/11/16/18 and steady seropositivity rates, following two doses.Citation77 However, a meta–analysis showed that, compared to three doses, two doses of the 4vHPV vaccine could produce an inferior antibody response for HPV18 within 18 months and, likewise, two doses of 2vHPV could produce an inferior responses for HPV16 within 2 y (again, women receiving three doses were older, at 15–26 y, than those receiving two doses, at 9–13 y).Citation78 Moreover, a study by Leung and colleagues compared the 2vHPV and 4vHPV vaccine in a two-dose schedule and found that, as with three doses, the 2vHPV vaccine elicits antibody responses that are up to sixfold higher for the vaccine typesCitation65 compared to 4vHPV.
Antibody levels and the development of GMC/T over time are comparable following two doses and three doses of the same vaccine, at least for the first period (up to 36 months) post–vaccination.Citation75,Citation76,Citation79,Citation80 Among young girls (9–14 y) receiving two doses of 2vHPV vaccination, modeling studies predicted lifelong durability of antibody levels above natural infection level, comparable to the three-dose schedule.Citation75 No differences in avidity following a two-dose 2vHPV schedule were observed at months 7, 24 or 48 post-vaccination, suggesting that the quality of the antibody response in terms of avidity was similar in the two-dose recipients compared to three-dose recipients.Citation81 Also following two doses, cross-protection against non-vaccine types can be observed. For the 2vHPV vaccine, similar antibody concentrations against HPV31 and HPV45 were measured up to 5 y after both two and three doses of vaccination.Citation72 For the 4vHPV vaccine, higher antibody concentrations against HPV31 were observed up to 6 y after one-, two-, or three-dose-vaccination as compared to no vaccination, but this was not observed for other cross-protective serotypes.Citation82 This difference in antibody response against cross-protective types between vaccines might be due to the AS04 adjuvant system used in the 2vHPV vaccine, which is claimed to induce a broader immune response and may hence lead to higher antibody levels.Citation51
One-dose HPV vaccination
Besides RCTs, other prospective studies investigated optimal dosing schedules, including one-dose vaccination. This most reduced number of doses makes HPV vaccination cheaper and logistically more accessible, especially for low- and middle-income countries. Nevertheless, one-dose delivery would require sufficiently high efficacy and long-term immunogenicity. Several studies have shown seropositivity for HPV16 and HPV18 following one-dose 2vHPV vaccination (up to 11 y of follow-up), although antibody levels were lower compared to two or three doses.Citation41,Citation83 With one-dose delivery of 4vHPV, avidity was non-inferior, and detectable concentrations of neutralizing antibodies to all four vaccine-targeted HPV types were present, but at much lower concentration after one dose than after two/three doses (up to 6 y of follow-up) and seropositivity rates decreased rapidly; however, protection from infection seemed comparable between dosing schedules.Citation54,Citation84,Citation85 Another study indicated that besides lower antibody levels following one-dose 2vHPV, also lower levels of memory B- and T-cells were measured. Altogether, these findings suggest that one-dose vaccine recipients are at higher risk of waning immunity.Citation86 Despite the potential of the one dose schedule, the trade-off between costs and accessibility on one side and the effectiveness and immunogenicity on the other should be considered carefully, especially regarding long-term protection.
Implications of long-term HPV vaccine-induced immunogenicity
Long–term protection following HPV vaccination is important to prevent people from acquiring an HPV infection throughout their lives. Therefore, protection during sexually active life is desirable, as this reduces lifetime risk of HPV infection and subsequent disease, and supports the WHO goal to eliminate cervical cancer.Citation16 If protection following vaccination persists for a limited amount of time, the peak prevalence of HPV infections may shift toward older age, influencing the outcomes of and the need for secondary prevention (i.e., screening) of cervical cancer. The pattern in which HPV prevalence peaks and thus protection is needed might differ geographically, causing the HPV burden to be unequally distributed across countries. In higher income countries, the HPV infection prevalence peaks between 20 and 25 y of age and is followed by a decline and finally a plateau in prevalence.Citation87 However, a large meta–analysis including 1 million women with normal cytology from all over the world indicated that HPV prevalence in less developed countries remains at a higher level following the initial peak. In some cases, a second peak can be observed around age 45-65, although the actual risk of developing cancer from these infections might be limited.Citation88
While a correlate of protection is still lacking for HPV, protection is assumed to be antibody-mediated. The three vaccines are very immunogenic and induce solid protection against HPV infections and cervical lesions,Citation9 which could indicate that protection will last if there is a high and detectable immune response. In general, long-term follow-up studies align with regard to immunogenicity. Following all three vaccines, seropositivity rates for targeted vaccine types remain high up to at least 7.5 y after vaccination, with few indications of waning. This finding is underscored by modeling studies and the development of GMC/Ts over time. Nevertheless, some decline of 4vHPV-induced antibodies against HPV18 is observed with all dosing schedules.Citation44,Citation78 This could indicate an increased risk of waning immunity, although no supporting evidence in the form of breakthrough cases has been reported.Citation48 The observation does emphasize the importance of continued immunosurveillance and proper comparisons between vaccines, e.g., between 2vHPV, which induces the highest levels of cross protection, and 9vHPV, which provides broad-spectrum protection.
In , the vaccines are summarized as to their dosing schedules and longest reported follow-up of immunogenicity. It shows that the current knowledge gaps mainly concern long-term follow-up of reduced dosing schedules and 9vHPV vaccine, all requiring further research. Despite the benefits of immunosurveillance over clinical surveillance (e.g., less invasive, easier to collect samples, especially from males), vaccine efficacy is often seen as most important outcome, with immunogenicity outcomes considered seperately.Citation9 Accordingly, research is needed to study the linkage between protection and observed immune responses over time, which can aid in our understanding of long-term efficacy following HPV vaccination. However, such research remains challenging due to the low number of breakthrough infections, the large confidence intervals around effectiveness estimates, and the variety in antibody levels among individuals.Citation30 In RCTs, prolonged or incidental follow-up of vaccine recipients with relatively low antibody response reveal the individual level of antibodies that must be achieved for protection.
Figure 2. Longest reported follow-up time concerning antibody levels and/or seropositivity rate. Studies can be both RCT or observational. Stratified for HPV vaccine and dosing schedule.Citation38,Citation47,Citation53,Citation76,Citation77,Citation83,Citation89
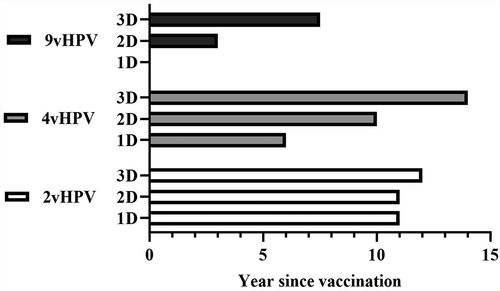
Vaccine uptake, age at vaccination, and the optimal dosing schedule remain critical research areas. Since HPV vaccination was introduced, uptake has remained behind in low- and middle-income countries, although they have the highest burden and minimal screening opportunities.Citation90 Even in high-income countries uptake has been suboptimal, possibly due to negative media attention or parental concerns.Citation91 Thus, increased effort is required to offer timely vaccination to young girls around the globe. Age of vaccination remains important, since vaccination at higher ages increases pre-vaccination HPV exposure risk. Moreover, the number of doses might affect GMT/Cs; more evidence is needed on the non-inferiority and long-term follow-up of a one-dose schedule regarding both immunogenicity and effectiveness.Citation92 Besides RCTs in which some participants received one dose and virological endpoints were evaluated,Citation41,Citation93 there are ongoing comparison studies between two- and one-dose recipients as to both non-inferiority of immune response and protection against clinical outcomes (NCT03180034 and NCT03675256, both focus on 2vHPV and 9vHPV vaccination). Additionally, some early initiated studies will continue their follow-up and frequently report their findings.Citation92 Evidence for or against one-dose vaccination, based on efficacy against persistent infection and immunogenicity as to targeted HPV types, will be available from the RCTs and other studies in the coming years, aiding in the evaluation and formal implementation of a one-dose schedule.
To conclude, long-term immunogenicity following HPV vaccination looks promising, with little indication for a decline. Future studies should focus on establishing a correlate of protection in order to optimize dosing schedules and to realize sustained protection through sexually active life. This will aid in reducing HPV infections and subsequent disease, with the ultimate goal of worldwide elimination of cervical cancer.
Disclosure of potential conflicts of interest
The authors have no conflict of interest to disclose.
Additional information
Funding
References
- De Villiers E-M, Fauquet C, Broker TR, Bernard H-U, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324(1):17–11. doi:10.1016/j.virol.2004.03.033.
- Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health. 2010;46(4):S20–S6. doi:10.1016/j.jadohealth.2010.01.016.
- Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30:F55–F70. doi:10.1016/j.vaccine.2012.06.083.
- Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41(11):660. doi:10.1097/OLQ.0000000000000193.
- Syrjänen K, Hakama M, Saarikoski S, Väyrynen M, Yliskoski M, Syrjänen S, Kataja V, Castren O. Prevalence, incidence, and estimated life-time risk of cervical human papillomavirus infections in a nonselected Finnish female population. Sex Transm Dis. 1990;17(1):15–19. doi:10.1097/00007435-199017010-00004.
- Committee for Medicinal Products for Human Use (CHMP). European public assessment report (EPAR) for Cervarix. UK: European Medicines Agency. 2009.
- Committee for Medicinal Products for Human Use (CHMP). European public assessment report (EPAR) for Gardasil. UK: European Medicines Agency, 2009.
- Committee for Medicinal Products for Human Use (CHMP). European public assessment report (EPAR) for Gardasil9. UK: European Medicines Agency, 2015.
- Harper DM, DeMars LR. HPV vaccines - a review of the first decade. Gynecol Oncol. 2017;146(1):196–204. doi:10.1016/j.ygyno.2017.04.004.
- Drolet M, Bénard É, Pérez N, Brisson M, Ali H, Boily M-C, Baldo V, Brassard P, Brotherton JML, Callander D, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394(10197):497–509. doi:10.1016/S0140-6736(19)30298-3.
- Brotherton JM, Hawkes D, Sultana F, Malloy MJ, Machalek DA, Smith MA, Garland SM, Saville M. Age-specific HPV prevalence among 116,052 women in Australia’s renewed cervical screening program: a new tool for monitoring vaccine impact. Vaccine. 2019;37(3):412–16. doi:10.1016/j.vaccine.2018.11.075.
- Lei J, Ploner A, Elfström KM, Wang J, Roth A, Fang F, Sundström K, Dillner J, Sparén P. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383(14):1340–48. doi:10.1056/NEJMoa1917338.
- Turner TB, Huh WK. HPV vaccines: translating immunogenicity into efficacy. Hum Vaccine Immunother. 2016;12(6):1403–05. doi:10.1080/21645515.2015.1103936.
- Harro CD, Pang -Y-YS, Roden RB, Hildesheim A, Wang Z, Reynolds MJ, Mast TC, Robinson R, Murphy BR, Karron RA, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001;93(4):284–92. doi:10.1093/jnci/93.4.284.
- Petaja T, Pedersen C, Poder A, Strauss G, Catteau G, Thomas F, Lehtinen M, Descamps D. Long-term persistence of systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women. Int J Cancer. 2011;129(9):2147–57. doi:10.1002/ijc.25887.
- World Health Organization. Draft: global strategy towards eliminating cervical cancer as a public health problem. World Health Organization: Geneva, 2020.
- Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol. 2012;10(10):681–92. doi:10.1038/nrmicro2872.
- Pinto LA, Dillner J, Beddows S, Unger ER. Immunogenicity of HPV prophylactic vaccines: serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36(32 Pt A):4792–99. doi:10.1016/j.vaccine.2017.11.089.
- Sehr P, Rubio I, Seitz H, Putzker K, Ribeiro-Müller L, Pawlita M, Müller M. High-throughput pseudovirion-based neutralization assay for analysis of natural and vaccine-induced antibodies against human papillomaviruses. PloS One. 2013;8(10):e75677. doi:10.1371/journal.pone.0075677.
- Brown D, Müller M, Sehr P, Pawlita M, Seitz H, Rubio I, Antonello J, Radley D, Roberts C, Saah A, et al. Concordance assessment between a multiplexed competitive Luminex immunoassay, a multiplexed IgG Luminex immunoassay, and a pseudovirion-based neutralization assay for detection of human papillomaviruse types 16 and 18. Vaccine. 2014;32(44):5880–87. doi:10.1016/j.vaccine.2014.08.004.
- Scherpenisse M, Schepp RM, Mollers M, Mooij SH, Meijer CJ, Berbers GA, van der Klis FR. A comparison of different assays to assess HPV16 and 18-specific antibodies after HPV infection and vaccination. Clin Vaccine Immunol. 2013;CVI:00153–13.
- Schiller J, Lowy D. Explanations for the high potency of HPV prophylactic vaccines. Vaccine. 2018;36(32):4768–73. doi:10.1016/j.vaccine.2017.12.079.
- Scherpenisse M, Schepp RM, Mollers M, Meijer CJLM, Berbers GAM, van der Klis FRM. Characteristics of HPV-specific antibody responses induced by infection and vaccination: cross-reactivity, neutralizing activity, avidity and IgG subclasses. PloS One. 2013;8(9):e74797. doi:10.1371/journal.pone.0074797.
- Schiller JT, Lowy DR Papillomavirus-like particles and HPV vaccine development. Seminars in cancer biology; 1996; Elsevier. p. 373–82.
- Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, et al. AS04, an aluminum salt-and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183(10):6186–97. doi:10.4049/jimmunol.0901474.
- Day PM, Kines RC, Thompson CD, Jagu S, Roden RB, Lowy DR, Schiller JT. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8(3):260–70. doi:10.1016/j.chom.2010.08.003.
- Stanley M. Potential mechanisms for HPV vaccine-induced long-term protection. Gynecol Oncol. 2010;118(1):S2–S7. doi:10.1016/j.ygyno.2010.04.002.
- Siegrist C-A. Vaccine immunology. Vaccines. 2008;5:17–36.
- Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008;109(2):S15–S21. doi:10.1016/j.ygyno.2008.02.003.
- Hoes J, Pasmans H, Knol MJ, Donken R, Van Marm-wattimena N, Schepp RM, King AJ, van der Klis FRM, De Melker HE. Persisting antibody response 9 years after bivalent Human Papillomavirus (HPV) vaccination in a Cohort of Dutch women: immune response and the relation to genital HPV infections. J Infect Dis. 2020;221(11):1884–94. doi:10.1093/infdis/jiaa007.
- Mollers M, Vossen JM, Scherpenisse M, van der Klis FR, Meijer CJ, De Melker HE. Current knowledge on the role of HPV antibodies after natural infection and vaccination: implications for monitoring an HPV vaccination programme. J Med Virol. 2013;85(8):1379–85. doi:10.1002/jmv.23616.
- Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, Poncelet S, Zahaf T, Hardt K, Descamps D, et al. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15–55 years. Vaccine. 2009;27(4):581–87. doi:10.1016/j.vaccine.2008.10.088.
- Petäjä T, Keränen H, Karppa T, Kawa A, Lantela S, Siitari-Mattila M, Levänen H, Tocklin T, Godeaux O, Lehtinen M, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health. 2009;44(1):33–40. doi:10.1016/j.jadohealth.2008.10.002.
- Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, Garland SM, Chatterjee A, Lazcano-Ponce E, Salmerón J, McNeil S, Stapleton JT, Bouchard C, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis. 2016;16(10):1154–68. doi:10.1016/S1473-3099(16)30120-7.
- Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, de Borba PC, Sanchez N, Zahaf T, Catteau G, Geeraerts B, Descamps D. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccine Immunother. 2014;10(8):2147–62. doi:10.4161/hv.29532.
- Giuliano AR, Isaacs-Soriano K, Torres BN, Abrahamsen M, Ingles DJ, Sirak BA, Quiterio M, Lazcano-Ponce E. Immunogenicity and safety of Gardasil among mid-adult aged men (27–45 years)—the MAM study. Vaccine. 2015;33(42):5640–46.
- Hillman RJ, Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Vardas E, Aranda C, Jessen H, Ferris DG, Coutlee F, et al. Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old. Clin Vaccine Immunol. 2012;19(2):261–67. doi:10.1128/CVI.05208-11.
- Kjaer SK, Nygård M, Sundström K, Dillner J, Tryggvadottir L, Munk C, Berger S, Enerly E, Hortlund M, Ágústsson ÁI, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi:10.1016/j.eclinm.2020.100401.
- Castellsagué X, Giuliano A, Goldstone S, Guevara A, Mogensen O, Palefsky JM, Group T, Shields C, Liu K, Maansson R, et al. Immunogenicity and safety of the 9-valent HPV vaccine in men. Vaccine. 2015;33(48):6892–901. doi:10.1016/j.vaccine.2015.06.088.
- Huh WK, Joura EA, Giuliano AR, Iversen OE, de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN, Hirschberg AL, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet. 2017;390(10108):2143–59. doi:10.1016/S0140-6736(17)31821-4.
- Safaeian M, Sampson JN, Pan Y, Porras C, Kemp TJ, Herrero R, Quint W, Van Doorn LJ, Schussler J, Lowy DR, et al. Durability of protection afforded by fewer doses of the HPV16/18 vaccine: the CVT trial. J Natl Cancer Inst. 2018;110(2):205–12. doi:10.1093/jnci/djx158.
- Schwarz TF, Huang LM, Valencia A, Panzer F, Chiu C-H, Decreux A, Poncelet S, Karkada N, Folschweiller N, Lin L, et al. A ten-year study of immunogenicity and safety of the AS04-HPV-16/18 vaccine in adolescent girls aged 10–14 years. Hum Vaccine Immunother. 2019;15(7–8):1970–79. doi:10.1080/21645515.2019.1625644.
- Schwarz TF, Galaj A, Spaczynski M, Wysocki J, Kaufmann AM, Poncelet S, Suryakiran PV, Folschweiller N, Thomas F, Lin L, et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15–55 years of age. Cancer Med. 2017;6(11):2723–31. doi:10.1002/cam4.1155.
- Castellsague X, Munoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, Luna J, Myers E, Mallary S, Bautista OM, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br J Cancer. 2011;105(1):28–37. doi:10.1038/bjc.2011.185.
- Ferris DG, Samakoses R, Block SL, Lazcano-Ponce E, Restrepo JA, Mehlsen J, Chatterjee A, Iversen O-E, Joshi A, Chu J-L, et al. 4-valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140(6):e20163947. doi:10.1542/peds.2016-3947.
- Van Damme P, Olsson SE, Block S, Castellsague X, Gray GE, Herrera T, Huang L-M, Kim DS, Pitisuttithum P, Chen J, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136(1):e28–39. doi:10.1542/peds.2014-3745.
- Olsson S-E, Restrepo JA, Reina JC, Pitisuttithum P, Ulied A, Varman M, Van Damme P, Moreira ED, Ferris D, Block S, et al. Long-term immunogenicity, effectiveness, and safety of nine-valent human papillomavirus vaccine in girls and boys 9 to 15 years of age: interim analysis after 8 years of follow-up. Papillomavirus Res. 2020;10:100203. doi:10.1016/j.pvr.2020.100203.
- Villa L, Costa R, Petta C, Andrade RP, Paavonen J, Iversen O-E, Olsson S-E, Høye J, Steinwall M, Riis-Johannessen G, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95(11):1459–66. doi:10.1038/sj.bjc.6603469.
- Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338–49. doi:10.1016/S1473-3099(10)70049-9.
- Malagón T, Drolet M, Boily M-C, Franco EL, Jit M, Brisson J, Brisson M. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(10):781–89. doi:10.1016/S1473-3099(12)70187-1.
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, Rosen J, Chakhtoura N, Lebacq M, van der Most R, et al. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18–45 years. Hum Vaccine. 2011;7(12):1359–73. doi:10.4161/hv.7.12.18282.
- Mariz FC, Bender N, Anantharaman D, Basu P, Bhatla N, Pillai MR, Prabhu PR, Sankaranarayanan R, Eriksson T, Pawlita M, et al. Peak neutralizing and cross-neutralizing antibody levels to human papillomavirus types 6/16/18/31/33/45/52/58 induced by bivalent and quadrivalent HPV vaccines. NPJ Vaccines. 2020;5:14. doi:10.1038/s41541-020-0165-x.
- Artemchuk H, Eriksson T, Poljak M, Surcel HM, Dillner J, Lehtinen M, Faust H. Long-term antibody response to human papillomavirus vaccines: up to 12 years of follow-up in the Finnish maternity cohort. J Infect Dis. 2018;219(4):582–89.
- Sankaranarayanan R, Prabhu PR, Pawlita M, Gheit T, Bhatla N, Muwonge R, Nene BM, Esmy PO, Joshi S, Poli URR, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. 2016;17(1):67–77. doi:10.1016/S1470-2045(15)00414-3.
- Kann H, Lehtinen M, Eriksson T, Surcel H-M, Dillner J, Faust H. Sustained cross-reactive antibody responses after human papillomavirus vaccinations: up to 12 years follow-up in the Finnish maternity cohort. J Infect Dis. 2020. doi:10.1093/infdis/jiaa617.
- Guevara A, Cabello R, Woelber L, Moreira ED, Joura E, Reich O, Shields C, Ellison MC, Joshi A, Luxembourg A, et al. Antibody persistence and evidence of immune memory at 5 years following administration of the 9-valent HPV vaccine. Vaccine. 2017;35(37):5050–57. doi:10.1016/j.vaccine.2017.07.017.
- Schwarz TF, Huang L-M, Medina DMR, Valencia A, Lin TY, Behre U, Catteau G, Thomas F, Descamps D. Four-year follow-up of the immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine when administered to adolescent girls aged 10–14 years. J Adolesc Health. 2012;50(2):187–94. doi:10.1016/j.jadohealth.2011.11.004.
- Reisinger KS, Block SL, Lazcano-Ponce E, Samakoses R, Esser MT, Erick J, Puchalski D, Giacoletti KE, Sings HL, Lukac S, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J. 2007;26(3):201–09. doi:10.1097/01.inf.0000253970.29190.5a.
- Barzon L, Squarzon L, Masiero S, Pacenti M, Marcati G, Mantelli B, Gabrielli L, Pascucci MG, Lazzarotto T, Caputo A, et al. Neutralizing and cross-neutralizing antibody titres induced by bivalent and quadrivalent human papillomavirus vaccines in the target population of organized vaccination programmes. Vaccine. 2014;32(41):5357–62. doi:10.1016/j.vaccine.2014.07.014.
- Godi A, Panwar K, Haque M, Cocuzza CE, Andrews N, Southern J, Turner P, Miller E, Beddows S. Durability of the neutralizing antibody response to vaccine and non-vaccine HPV types 7 years following immunization with either Cervarix® or Gardasil® vaccine. Vaccine. 2019;37(18):2455–62. doi:10.1016/j.vaccine.2019.03.052.
- Einstein MH, Takacs P, Chatterjee A, Sperling RS, Chakhtoura N, Blatter MM, Lalezari J, David M-P, Lin L, Struyf F, et al. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: end-of-study analysis of a phase III randomized trial. Hum Vaccine Immunother. 2014;10(12):3435–45. doi:10.4161/hv.36121.
- Leung TF, Liu AP, Lim FS, Thollot F, Oh HM, Lee BW, Rombo L, Tan NC, Rouzier R, Friel D, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine administered according to 2- and 3-dose schedules in girls aged 9–14 years: results to month 12 from a randomized trial. Hum Vaccine Immunother. 2015;11(7):1689–702. doi:10.1080/21645515.2015.1050570.
- Einstein MH, Levin MJ, Chatterjee A, Chakhtoura N, Takacs P, Catteau G, Dessy FJ, Moris P, Lin L, Struyf F, et al. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: follow-up through month 48 in a phase III randomized study. Hum Vaccine Immunother. 2014;10(12):3455–65. doi:10.4161/hv.36117.
- Vesikari T, Brodszki N, Van Damme P, Diez-Domingo J, Icardi G, Petersen LK, Tran C, Thomas S, Luxembourg A, Baudin M, et al. A randomized, double-blind, phase III study of the immunogenicity and safety of a 9-valent human papillomavirus L1 virus-like particle vaccine (V503) versus Gardasil® in 9–15-year-old girls. Pediatr Infect Dis J. 2015;34(9):992–98. doi:10.1097/INF.0000000000000773.
- Leung TF, Liu AP, Lim FS, Thollot F, Oh HML, Lee BW, Rombo L, Tan NC, Rouzier R, De Simoni S, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and 4vHPV vaccine administered according to two- or three-dose schedules in girls aged 9–14 years: results to month 36 from a randomized trial. Vaccine. 2018;36(1):98–106. doi:10.1016/j.vaccine.2017.11.034.
- Kemp TJ, Safaeian M, Hildesheim A, Pan Y, Penrose KJ, Porras C, Schiller JT, Lowy DR, Herrero R, Pinto LA, et al. Kinetic and HPV infection effects on cross-type neutralizing antibody and avidity responses induced by Cervarix®. Vaccine. 2012;31(1):165–70. doi:10.1016/j.vaccine.2012.10.067.
- Donken R, Schurink-Van’t Klooster TM, Schepp RM, van der Klis FRM, Knol MJ, Meijer CJLM, de Melker HE Immune responses after 2 versus 3 doses of HPV vaccination up to 4½ years after vaccination: an observational study among Dutch routinely vaccinated girls. J Infect Dis. 2017;215(3):359–67. doi:10.1093/infdis/jiw588.
- Matsui K, Adelsberger JW, Kemp TJ, Baseler MW, Ledgerwood JE, Pinto LA. Circulating CXCR5(+)CD4(+) T follicular-like helper cell and memory B cell responses to human Papillomavirus vaccines. PLoS One. 2015;10(9):e0137195. doi:10.1371/journal.pone.0137195.
- Dauner JG, Pan Y, Hildesheim A, Harro C, Pinto LA. Characterization of the HPV-specific memory B cell and systemic antibody responses in women receiving an unadjuvanted HPV16 L1 VLP vaccine. Vaccine. 2010;28(33):5407–13. doi:10.1016/j.vaccine.2010.06.018.
- Smolen KK, Gelinas L, Franzen L, Dobson S, Dawar M, Ogilvie G, Krajden M, Fortuno ES, Kollmann TR. Age of recipient and number of doses differentially impact human B and T cell immune memory responses to HPV vaccination. Vaccine. 2012;30(24):3572–79. doi:10.1016/j.vaccine.2012.03.051.
- Giannini S, Hanon E, Moris P, Vanmechelen M, Morel S, Dessy F, Fourneau M, Colau B, Suzich J, Losonksy G, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24(33–34):5937–49. doi:10.1016/j.vaccine.2006.06.005.
- Folschweiller N, Behre U, Dionne M, Durando P, Esposito S, Ferguson L, Ferguson M, Hillemanns P, McNeil SA, Peters K, et al. Long-term cross-reactivity against nonvaccine human Papillomavirus types 31 and 45 after 2- or 3-dose schedules of the AS04-adjuvanted human HPV-16/18 vaccine. J Infect Dis. 2019;219(11):1799–803. doi:10.1093/infdis/jiy743.
- Stanley MA, Sudenga SL, Giuliano AR. Alternative dosage schedules with HPV virus-like particle vaccines. Expert Rev Vaccines. 2014;13(8):1027–38. doi:10.1586/14760584.2014.935767.
- Romanowski B, Schwarz TF, Ferguson LM, Ferguson M, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Behre U, et al. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study. Hum Vaccine Immunother. 2014;10(5):1155–65. doi:10.4161/hv.28022.
- Romanowski B, Schwarz TF, Ferguson L, Peters K, Dionne M, Behre U, Schulze K, Hillemanns P, Suryakiran P, Thomas F, et al. Sustained immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine administered as a two-dose schedule in adolescent girls: five-year clinical data and modeling predictions from a randomized study. Hum Vaccine Immunother. 2016;12(1):20–29. doi:10.1080/21645515.2015.1065363.
- Iversen OE, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, Block SL, Skrivanek A, Nur Azurah AG, Fong SM, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. Jama. 2016;316(22):2411–21. doi:10.1001/jama.2016.17615.
- Donken R, Dobson SRM, Marty KD, Cook D, Sauvageau C, Gilca V, Dionne M, McNeil S, Krajden M, Money D, et al. Immunogenicity of 2 and 3 doses of the quadrivalent human Papillomavirus vaccine up to 120 months postvaccination: follow-up of a randomized clinical trial. Clin Infect Dis. 2019;71:1022–1029.
- Donken R, Knol MJ, Bogaards JA, van der Klis FR, Meijer CJ, de Melker HE. Inconclusive evidence for non-inferior immunogenicity of two-compared with three-dose HPV immunization schedules in preadolescent girls: a systematic review and meta-analysis. J Infect. 2015;71(1):61–73. doi:10.1016/j.jinf.2015.02.005.
- Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. Jama. 2013;309(17):1793–802. doi:10.1001/jama.2013.1625.
- Schurink-van’t Klooster TM, Donken R, Schepp RM, van der Klis FR, de Melker HE. Persistence of immune response following bivalent HPV vaccination: a follow-up study among girls routinely vaccinated with a two-dose schedule. Vaccine. 2018;36(49):7580–87. doi:10.1016/j.vaccine.2018.10.018.
- Boxus M, Lockman L, Fochesato M, Lorin C, Thomas F, Giannini SL. Antibody avidity measurements in recipients of Cervarix® vaccine following a two-dose schedule or a three-dose schedule. Vaccine. 2014;32(26):3232–36. doi:10.1016/j.vaccine.2014.04.005.
- Toh ZQ, Kosasih J, Russell FM, Reyburn R, Fong J, Tuivaga E, Ratu FT, Nguyen CD, Matanitobua S, Do LAH, et al. Selective persistence of HPV cross-neutralising antibodies following reduced-dose HPV vaccine schedules. Vaccines. 2019;7(4):200. doi:10.3390/vaccines7040200.
- Kreimer AR, Sampson JN, Porras C, Schiller JT, Kemp T, Herrero R, Wagner S, Boland J, Schussler J, Lowy DR, et al. Evaluation of durability of a single dose of the bivalent HPV vaccine: the CVT trial. J Natl Cancer Inst. 2020;112(10):1038–46. doi:10.1093/jnci/djaa011.
- Toh ZQ, Cheow KWB, Russell FM, Hoe E, Reyburn R, Fong J, Tuivaga E, Ratu FT, Nguyen CD, Matanitobua S, et al. Cellular immune responses 6 years following 1, 2, or 3 doses of quadrivalent HPV vaccine in Fijian girls and subsequent responses to a dose of bivalent HPV vaccine. Open Forum Infect Dis. 2018;5(7):ofy147. doi:10.1093/ofid/ofy147.
- Sankaranarayanan R, Joshi S, Muwonge R, Esmy PO, Basu P, Prabhu P, Bhatla N, Nene BM, Shaw J, Poli URR, et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine. 2018;36(32 Pt A):4783–91. doi:10.1016/j.vaccine.2018.02.087.
- Pasmans H, Schurink-Van’t Klooster TM, Bogaard MJM, Van Rooijen DM, de Melker HE, Welters MJP, van der Burg SH, van der Klis FRM, Buisman A-M. Long-term HPV-specific immune response after one versus two and three doses of bivalent HPV vaccination in Dutch girls. Vaccine. 2019;37(49):7280–88. doi:10.1016/j.vaccine.2019.09.066.
- Cuzick J, Clavel C, Petry KU, Meijer CJLM, Hoyer H, Ratnam S, Szarewski A, Birembaut P, Kulasingam S, Sasieni P, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119(5):1095–101. doi:10.1002/ijc.21955.
- Bruni L, Diaz M, Castellsagué M, Ferrer E, Bosch FX, De Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202(12):1789–99. doi:10.1086/657321.
- Toh ZQ, Russell FM, Reyburn R, Fong J, Tuivaga E, Ratu T, Nguyen CD, Devi R, Kama M, Matanitobua S, et al. Sustained antibody responses 6 years following 1, 2, or 3 doses of quadrivalent human papillomavirus (HPV) vaccine in adolescent Fijian girls, and subsequent responses to a single dose of bivalent HPV vaccine: a prospective cohort study. Clin Infect Dis. 2017;64(7):852–59. doi:10.1093/cid/ciw865.
- Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, De Sanjosé S, Castellsagué X. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Global Health. 2016;4(7):e453–e63. doi:10.1016/S2214-109X(16)30099-7.
- Corcoran B, Clarke A, Barrett T. Rapid response to HPV vaccination crisis in Ireland. Lancet. 2018;391(10135):2103. doi:10.1016/S0140-6736(18)30854-7.
- Basu P, Brisson M, Campos N, Clarke E, Drolet M, Gallagher K, Henão R, Ana M, Howard N, Hutubessy R, et al. Review of the current published evidence on single-dose HPV vaccination. 2nd ed. Single-Dose Seattle, USA: HPV Vaccine Evaluation Consortium; 2019.
- Kreimer AR, Sampson JN, Porras C, Schiller JT, Kemp T, Herrero R, Wagner S, Boland J, Schussler J, Lowy DR, et al. Evaluation of durability of a single-dose of the bivalent HPV vaccine: the CVT trial. J Natl Cancer Inst. 2020;112:1038–46.
